ABSTRACT
Viewing cute images has been reported to promote performance on tasks requiring carefulness, possibly related to an enhanced positive emotional state. However, it is unclear whether viewing infant images also enhances attention control in mothers. Therefore, this experimental study examined whether exposure to images of infants affected mothers’ performance on a visual search task, studying associations with happy facial expressivity. Mothers (N = 101, Mage = 30.88) were randomly assigned to one of two conditions in which they either viewed images of infants or images of adults. Before and after viewing images, mothers performed a visual search task. Mothers’ happy facial expressions at baseline and when viewing images were analysed. Viewing images of infants, in contrast to viewing images of adults, improved task performance indexed by accurateness, but not the number of correct responses. Images of infants elicited happy facial expressivity, which was associated with the number of correct responses on the visual search task. This study showed that viewing images of infants evokes happy facial expressions in mothers and can improve mothers’ performance on a perceptual-cognitive task requiring attention control. Mothers’ responses to infant images may be explained as an attentional preparedness for caregiving.
Cute infant faces capture attention, elicit positive emotional expressions, and trigger caregiving responses (Brosch, Sander, & Scherer, Citation2007; Glocker et al., Citation2009; Hildebrandt & Fitzgerald, Citation1978). Cuteness has been associated with baby schema, described by Lorenz (Citation1943) with features such as a large head, high rounded forehead, large eyes, and rounded cheeks. Responses to baby schema have been proposed to activate the parental care motivational system, consisting of affective and cognitive mechanisms that evolved to regulate behavioural responses, preparing the individual to protect and provide care (Hofer, Buckels, White, Beall, & Schaller, Citation2017; Schaller, Citation2018).
Experimental studies have shown that this parental care motivational system becomes activated when individuals view cute images of infants or baby animals. For example, it has been shown that viewing images of infants or baby animals improves careful behaviour, measured by performance on fine motor dexterity tasks (Nittono, Fukushima, Yano, & Moriya, Citation2012; Sherman, Haidt, & Coan, Citation2009; Sherman, Haidt, Iyer, & Coan, Citation2013). Nittono et al. (Citation2012) have furthermore shown that viewing cute images of baby animals improved performance on visual search tasks. This may be interpreted as improved top-down attention control, because previous research has shown that visual search tasks involve executive functions, requiring individuals to identify and localise objects and to control the disengagement of attention (Han & Kim, Citation2004; Citation2009; Peterson, Beck, & Wong, Citation2008). Cute images may trigger a positive emotional state that is associated with approach motivation and a tendency toward systematic processing, resulting in enhanced attention control that may be part of the individual’s cognitive processes needed to provide care (Nittono et al., Citation2012). Indeed, recent studies have shown that mothers’ executive functioning is a determinant of parenting behaviours (Crandall, Deater-Deckard, & Riley, Citation2015), is related to a correct interpretation of the infants’ mental states (Yatziv, Kessler, & Atzaba-Poria, Citation2018) and is associated with mothers’ curiosity towards their infants’ mental states (Rutherford et al., Citation2018).
Happy facial expressions may be another behavioural manifestation of an activated parental care motivational system, as positive emotional facial expressions are important in mother-infant interaction to facilitate the attachment process (Beebe et al., Citation2016; Bowlby, Citation1969). Individuals who view images of infants respond with increased facial zygomaticus muscle activity, a facial electromyographic response associated with happy facial expressions (Hildebrandt & Fitzgerald, Citation1978). Another study showed that this response was more strongly induced by infant and baby animal images than by neutral images and attractive images unrelated to baby schema (Nittono & Ihara, Citation2017). However, it is still unknown whether happy facial expressivity in response to cute images is associated with attention control. In addition, experimental studies to date on cuteness effects (Nittono et al., Citation2012; Sherman et al., Citation2009; Citation2013) have not yet focused on mothers of young children. Thus, it is unknown how viewing cute images of infants is related to attention control in young mothers, who have a role in nurturing infants.
This experimental study is the first to examine the effects of viewing images of infants, compared to viewing images of adults, on mothers’ performance on a visual search task, additionally examining relations with happy facial expressivity. Mothers were randomly assigned to one of two conditions (adult images, infant images). Before and after viewing images, mothers performed a visual search task. First, effects of viewing images on visual search task performance were tested. Two measures of visual search task performance were examined: the number of correct responses and accurateness of performance (i.e. error rate). Mothers who viewed infant faces were predicted to perform better on the visual search task (i.e. more correct responses and more accurate performance) than mothers who viewed adult faces. Second, effects of viewing images on happy facial expressions were tested. Happy facial expressions before viewing images (baseline) and when viewing images were analysed. Mothers who viewed infant faces were predicted to respond to infant images with stronger increased happy facial expressivity compared to mothers who viewed adult faces. Third, we examined whether increased happy facial expressivity in mothers when viewing infant images was associated with better performance on the visual search task. Increased happy facial expressivity when viewing infant images was expected to be associated with better performance on the visual search task.
Methods
We report how we determined our sample size, all data exclusions (if any), all manipulations, and all measures in the study.
Participants
Mothers were recruited by contacting day-care centres and by asking among acquaintances. Exclusion criteria were hearing problems and insufficient mastery of the Dutch language. The total sample consisted of 101 mothers (Mage = 30.88 years, SDage = 4.23) with an infant up to three years of age (Mage = 13.68 months, SDage = 8.99). The sociodemographic characteristics of the participants are shown in Table S1. A priori power analysis using G*Power 3.1 for repeated measures (within-between interaction) showed that a sample size of 90 is sufficient to detect small to medium effects (f = .15, α = .05, power .80, 2 groups, 2 measurements).
Procedure
Mothers were visited at home by trained undergraduate students as part of a larger study on the perception of infant signals. They were asked to complete an online questionnaire (not used in this study, with the exception of demographic details), one or two weeks before the visit took place. The home visit included tasks for three studies, lasting about 60 min in total. The visit started with fitting electrodes of an ambulatory monitoring system for the measurement of skin conductance for the purpose of other studies. The tasks of the current study were administered first, followed by tasks that were part of other studies.
Mothers participated in the adult images (n = 44) or infant images (n = 57) condition. Data collection started after randomly assigning participant numbers to one of the two conditions, by selecting a random sample of approximately 50% of all cases in SPSS. The design was a 2 (pre-test, post-test) x 2 (adult images, infant images) within-between subjects design. First, mothers performed a visual search task (based on Nittono et al., Citation2012), which was the pre-test of task performance. Next, mothers viewed and ranked the adult or infant images. Finally, mothers performed the visual search task again, which was the post-test of task performance (see Figure S1 for a flow chart of the procedure). Facial expressions were recorded using a webcam during the presentation of the images. Permission for this study was obtained from the local ethics committee (protocol number: EC-2016.38). All mothers signed for consent.
Stimuli
Eight images of adult faces or infant faces were presented on a computer screen, four seconds per image (Sherman et al., Citation2013). Next, mothers were given eight plasticised sheets (paper size A4) with coloured photo images of the same faces. Mothers were asked to rank the images according to kindness (from “least kind” to “most kind”) in the adult images condition or cuteness (from “least cute” to “most cute”) in the infant images condition (Nittono et al., Citation2012). By ranking all images, mothers were to a larger extent exposed to the images than when viewing the images only.
The adult and infant images were selected from a larger set of images tested in a pilot study, with N = 44 women (age M = 28.01 years, SD = 6.01, 79.5% without children). In the pilot study, 20 infant images and 20 adult images were rated on cuteness and kindness on a scale ranging from 1 to 4. Eight infant and eight adult (four women) images with comparable scores on cuteness (M = 3.12, SD = .43) and kindness (M = 3.11, SD = 0.27) were selected for the current study.
Measures
Visual search task performance
An adapted version of the visual search task used by Nittono et al. (Citation2012) was administered. Eighteen different matrices, consisting of 40 digits, were printed on paper (digits 0–9, 4 rows by 10 columns). All digits were distributed randomly. A target digit was shown on the left side of each matrix. The target digit was different for each matrix. Mothers were instructed to search the matrix for this particular digit and to cross out these digits in the matrix. If they had completed a matrix they could proceed to the next one. They were instructed to complete as many matrices as quickly and as accurately as possible in three minutes. They could stop when the test leader said ‘stop’. This instruction was followed by an example matrix, in which the target digits had already been crossed out, and a practice matrix, which was completed together with the test leader. Correct trials ranged from two to six. Mothers performed the visual search task before viewing adult or infant images (pre-test) and directly after viewing adult or infant images (post-test). For both measures, two scores were calculated: the number of correct digits, representing the number of correct responses, and the error rate, representing the accuracy (proportion of incorrect digits plus missed target digits). Missed target digits of the final matrix searched by the participant were not included in the error rate because these digits could have been missed because time was up.
Happy facial expressions
Mothers’ happy facial expressions were recorded and analysed afterwards using Noldus’s FaceReader 7 of the Behavioral Physiology lab (GO-LAB, Tilburg University). This software was trained using the Facial Action Coding System (Ekman, Friesen, & Hager, Citation2002). FaceReader first detects the face, then models the face in 3D by describing over 500 key points in the face and the facial texture of the face entangled by these points, and finally classifies the face, using an artificial neural network to recognise patterns (https://www.noldus.com/facereader/facial-expression-analysis). High accuracy and convergent validity have been reported (Lewinski, den Uyl, & Butler, Citation2014). Happy facial expressions were analysed twice: at baseline during an 8.41-second episode of neutral written instructions and during a 32-second episode in which the mother viewed images of adults or infants that were presented on the computer screen. Intervals of 0.033 s were analysed, resulting in 253 scores at baseline and 960 scores in each condition. Scores represent the strength of an emotional expression, ranging from 0 (absence of emotion) to 1 (full intensity of emotion). The missing data rate was 5.2%. Intact data were averaged to create the happy facial expression scores at baseline and when viewing images.
Statistical analyses
Data were first inspected for missings, outliers, and distributions. Two repeated measures ANOVAs were performed to examine effects of viewing infant images on mothers’ task performance. The first analysis included the number of correct digits as dependent variable, the second analysis included error rate as dependent variable. In both analyses, time (pre, post) was entered as a within-subject factor and condition (adult images, infant images) as a between-subject factor. Next, a repeated measure ANOVA was performed to test effects of viewing infant images on mothers’ happy facial expressions. Again, time (baseline, viewing images) was entered as a within-subject factor and condition (adult images, infant images) as a between-subject factor. Simple effects tests were conducted for the interpretation of effects, based on pairwise comparisons among the estimated marginal means. Finally, to test whether happy facial expressivity was associated with mothers’ task performance, we calculated correlations between the happy facial expressivity difference score (i.e. viewing images minus baseline score) and task performance difference scores (i.e. post-test minus pre-test scores) in both conditions.
Results
Preliminary and descriptive analyses
Visual search task scores of six participants were missing due to procedural errors, and facial expression scores of five participants were missing due to technical errors (n = 3), a procedural error (n = 1), and because no consent was given to be videotaped (n = 1). Outliers were replaced by the most extreme value of the variable in the dataset after excluding the outliers (number of correct digits before viewing images: n = 1, error rate after viewing images n = 5, baseline happiness n = 5, number of correct digits difference score n = 2). Analyses were repeated including outliers, which yielded the same results (not reported). Skewness and kurtosis values of the variables were below 1.53 and 1.50 respectively, with the exception of post-test error rate (skewness: 1.61, kurtosis: 2.37). Skewness and kurtosis of all variables were in an acceptable range (Curran, West, & Finch, Citation1996).
Independent samples t-tests showed that mothers in the adult images condition did not differ from mothers in the infant images condition on age, t(99) = .67, p = .50, Cohen’s d = 0.13, number of children, t(94) = .36, p = .72, Cohen’s d = 0.08, age of the youngest child, t(94) = .67, p = .51, Cohen’s d = 0.14, pre-test number of correct digits, t(93) = −.25, p = .80, Cohen’s d = 0.05, pre-test error rate, t(93) = −1.26, p = .21, Cohen’s d = 0.26, and baseline happy facial expressions, t(92.62) = −1.20, p = .24, Cohen’s d = 0.24. Table S4 presents the descriptive statistics for each condition.
In both conditions, the pre-test number of correct digits was not significantly correlated with the pre-test error rate (adult images condition r = 0.04, p = .79, infant images condition r = −0.11, p = .41). Similarly, the post-test number of correct digits was not significantly correlated with the post-test error rate (adult images condition r = −0.26, p = .10, infant images condition r = 0.06, p = .68).
Visual search task performance
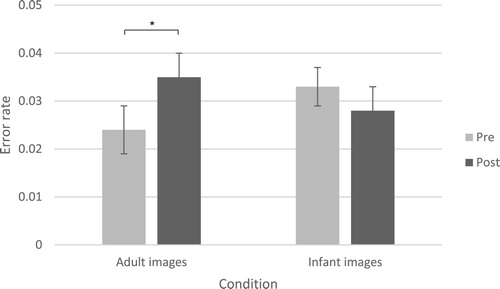
The analysis examining the number of correct digits showed no time X condition effect, F(1, 93) = .05, p = .82, partial ɳ2 = .001, but an effect of time, F(1, 93) = 37.62, p < .001, partial ɳ2 = .29. The number of correct responses improved after viewing the images in both conditions, which may be due to practice (pre-test M = 62.29, 95% CI [58.47, 66.11], post-test M = 69.72, 95% CI [66.02, 73.42]). In contrast, the analysis with the error rate as dependent variable showed no main effect of time, F(1, 93) = .91, p = .34, partial ɳ2 = .01, but a significant time X condition effect, F(1, 93) = 4.29, p = .04, partial ɳ2 = .04. Simple effects tests revealed that mothers showed an increased error rate after viewing adult images, F(1, 93) = 4.02, p = .048, partial ɳ2 = .04 (pre-test M = .02, 95% CI [.01, .03], post-test M = .04, 95% CI [.03, .05]), whereas the error rate of mothers who viewed infant images stayed the same, F(1, 93) = .73, p = .40, partial ɳ2 = .008 (pre-test M = .03, 95% CI [.02, .04], post-test M = .03, 95% CI [.02, .04]). These results are visualised in . See Figure S2 for scatterplots, showing the changes across individuals (according to Weissgerber, Milic, Winham, & Garovic, Citation2015). Thus, viewing images of infants improved mothers’ accurate performance on the visual search task.
Figure 1. Mean (SE) error rate scores for the adult images and infant images conditions, before (pre) and after (post) viewing images.
Notes: Error rate scores represent the proportion of incorrect digits plus missed target digits in the visual search task. A significant result of the simple effects test, indicating a difference between pre- and post-test scores, is marked with a star. *p < .05.
Happy facial expressions
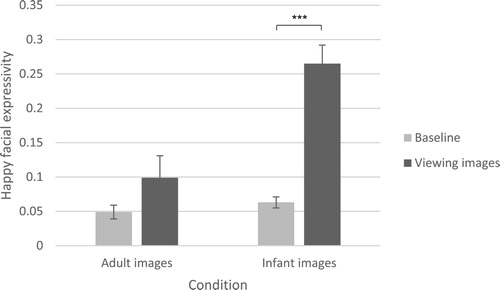
The analysis examining happy facial expressions showed an effect of time, F(1, 94) = 46.33, p < .001 partial ɳ2 = .33, as well as a significant time X condition effect, F(1, 94) = 16.65, p < .001 partial ɳ2 = .15. Simple effects tests showed that, compared to baseline, happy facial expressivity increased when viewing images of infants, F(1, 94) = 69.38, p < .001 partial ɳ2 = .43 (pre-test M = .06, 95% CI [.05, .08], post-test M = .27, 95% CI [.21, .32]), but not when viewing images of adults, F(1, 94) = 3.24, p = .08 partial ɳ2 = .03 (pre-test M = .05, 95% CI [.03, .07], post-test M = .10, 95% CI [.04, .16]). visualises these results. Scatterplots are shown in Figure S3 (Weissgerber et al., Citation2015).
Figure 2 Mean (SE) happy facial expressivity scores for the adult images and infant images conditions, at baseline and when viewing images.
Note: A significant result of the simple effects test, indicating a difference between baseline score and score when viewing images, is marked with a star. ***p < .001.
Correlations between the facial expression difference score and task performance difference scores showed no association in the adult image condition (r = ˗ 0.11, p = .50 for number of correct digits difference score, r = 0.14, p = .40 for error rate difference score). Neither was there a significant association between the facial expression difference score and the error rate difference score in the infant image condition, (r = ˗0.20, p = .15). However, the facial expression difference score was positively associated with the number of correct digits difference score (r = 0.27, p = .049; see Figure S4 for a scatter plot). These findings indicate that increased happy facial expressivity in mothers when viewing images of infants was positively associated with an increased number of correct responses.
Discussion
In support of our hypothesis, this study showed that exposure to images of infants improved mothers’ accurate performance on a visual search task. Mothers’ number of correct responses on the visual search task also improved, but unexpectedly as well in mothers who viewed images of adults. In addition, support was found for a role of happy facial expressions: Infant images elicited happy facial expressivity in mothers, which was positively associated with the number of correct responses on the visual search task. However, no associations were found between induced happy facial expressivity and task accuracy.
These findings extend prior research on effects of cute images in two ways. First, effects were demonstrated in mothers of young children, who performed a visual search task that required attention control. Enhanced attention control may be part of the cognitive processes in mothers needed to provide care (Nittono et al., Citation2012), which corresponds with previous studies have shown that mothers’ executive functioning is related to parenting behaviours and aspects important for parental care, such as an increased attention for and correct interpretation of infants’ mental states (Crandall et al., Citation2015; Rutherford et al., Citation2018; Yatziv et al., Citation2018). Prior research showed that cute images of baby animals improved university students’ performance on a visual search task (Nittono et al., Citation2012). In contrast to our findings, effects were reported on the number of correct matrices, but not the error rate. One explanation for these contrasting results is that task accurateness may rely to a larger extent on attention control than the number of correct responses, which may not only demand executive functions related to the allocation and control of attention (Han & Kim, Citation2004) but also other abilities. For example, it may also rely on motor speed, which may be less relevant in mothers to provide care. Moreover, task accurateness likely relates to some extent to tenderness, which may be especially improved by cuteness in mothers. Indeed, Sherman et al. (Citation2009) found that cute images induced tenderness in students, especially reported by women, which was positively associated with performance on a fine-motor dexterity task. Tenderness, suggested to be aroused when the parental motivational system is activated (Hofer et al., Citation2017; Schaller, Citation2018), may particularly be triggered by cuteness in mothers to help them with their caregiving practices.
Second, increased happy facial expressions when viewing infant images were found associated with better performance on the perceptual-cognitive task, which has not been demonstrated before. Facial expressions are likely a behavioural manifestation of the parental care motivational system (Hofer et al., Citation2017; Schaller, Citation2018). Mothers’ happy facial expressions play an important role in the affective mother–child interchange, eliciting positive emotion expressions in their infants in return (Mendes, Seidl-de-Moura, & de Oliveira Siqueira, Citation2009). The effect of viewing cute pictures is suggested to be related to, at least to some extent, a positive emotional state (Nittono et al., Citation2012). Sherman et al. (Citation2009) did not find positive emotion experience to be responsible for the link between cuteness and carefulness in their study. Future research should elucidate the mechanism among happy facial expressions, a positive emotional state, and activation of the parental care motivational system.
Several limitations of this study should be noted. First, we did not relate individual cuteness ratings of the infant images to mothers’ task performance and happy facial expressions. However, previous study findings suggest that responses to images of infant faces are independent of perceived cuteness ratings (Endendijk, Spencer, van Baar, & Bos, Citation2018; Hildebrandt & Fitzgerald, Citation1978). Second, the visual search task we used was an adapted version of the task used in prior research (Nittono et al., Citation2012). In the original task participants were asked to mentally count the number of target digits, using working memory without using hands. The task difference may relate to differences in study findings. Third, we did not examine possible moderating effects of mothers’ psychological problems, such as depression and anxiety, which may bias the processing of infant images (Webb & Ayers, Citation2015). Lastly, it should be noted that performance on the perceptual-cognitive task may host not only attentional control but also other executive functions, such as response inhibition. However, our assumption that enhanced performance on the visual search task reflects an attentional effect is in line with neuroimaging research suggesting that cute infant stimuli elicit selective attentional biasing by triggering specific and rapid neural responses in affective brain regions, such as the orbitofrontal cortex (Kringelbach, Stark, Alexander, Bornstein, & Stein, Citation2017).
Conclusion
This study showed that viewing images of infants can improve mothers’ accuracy on a perceptual-cognitive task requiring attention control. Further, infant images elicited increased happy facial expressions, which in turn were associated with mothers’ performance on the task. Mothers’ responses to infant images may be explained as a biologically based attentional preparedness for caregiving. Research should unravel the mechanisms through which cuteness elicits attention control and how it supports parental care.
Supplementary_Material
Download Zip (197.7 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Beebe, B., Messinger, D., Bahrick, L. E., Margolis, A., Buck, K. A., & Chen, H. (2016). A systems view of mother-infant face-to-face communication. Developmental Psychology, 52, 556–571. doi:10.1037/a0040085.
- Bowlby, J. (1969). Attachment and loss: Vol. 1. Attachment. New York: Basic Books.
- Brosch, T. B., Sander, D., & Scherer, K. R. (2007). That baby caught my eye … attention capture by infant faces. Emotion, 7, 685–689. doi: 10.1037/1528-3542.7.3.685
- Crandall, A., Deater-Deckard, K., & Riley, A. W. (2015). Maternal emotion and cognitive control capacities and parenting: A conceptual framework. Developmental Review, 36, 105–126. doi: 10.1016/j.dr.2015.01.004
- Curran, P. J., West, S. G., & Finch, J. F. (1996). The robustness of test statistics to nonnormality and specification error in confirmatory factor analysis. Psychological Methods, 1, 16–29. doi: 10.1037/1082-989X.1.1.16
- Ekman, P., Friesen, W. V., & Hager, J. C. (2002). Facial action coding system: An investigator’s guide. Salt Lake City: Research Nexus.
- Endendijk, J. J., Spencer, H., van Baar, A. L., & Bos, P. A. (2018). Mothers’ neural responses to infant faces are associated with activation of the maternal care system and observed intrusiveness with their own child. Cognitive, Affective, & Behavioral Neuroscience, 18, 609–621. doi: 10.3758/s13415-018-0592-6
- Glocker, M. L., Langleben, D. D., Ruparel, K., Loughead, J. W., Gur, R. C., & Sachser, N. (2009). Baby schema in infant faces induces cuteness perception and motivation for caretaking in adults. Ethology, 115, 257–263. doi: 10.1111/j.1439-0310.2008.01603.x
- Han, S.-H., & Kim, M.-S. (2004). Visual search does not remain efficient when executive working memory is working. Psychological Science, 15, 623–628. doi: 10.1111/j.0956-7976.2004.00730.x
- Han, S.-H., & Kim, M.-S. (2009). Do the contents of working memory capture attention? Yes, but cognitive control matters. Journal of Experimental Psychology: Human Perception and Performance, 35, 1292–1302. doi: 10.1037/a0016452
- Hildebrandt, K. A., & Fitzgerald, H. E. (1978). Adults’ responses to infants varying in perceived cuteness. Behavioural Processes, 3, 159–172. doi: 10.1016/0376-6357(78)90042-6
- Hofer, M. K., Buckels, E. E., White, C. J., Beall, A. T., & Schaller, M. (2017). Individual differences in activation of the parental care motivational system: An empirical distinction between protection and nurturance. Social Psychological and Personality Science, 9, 907–916. doi: 10.1177/1948550617728994
- Kringelbach, M. L., Stark, E. A., Alexander, C., Bornstein, M. H., & Stein, A. (2017). On cuteness: Unlocking the parental brain and beyond. Trends in Cognitive Sciences, 20, 545-558. doi: 10.1016/j.tics.2016.05.003
- Lewinski, P., den Uyl, T. M., & Butler, C. (2014). Automated facial coding: Validation of basic emotions and FACS AUs in FaceReader. Journal of Neuroscience, Psychology, and Economics, 7, 227–236. doi: 10.1037/npe0000028
- Lorenz, K. (1943). Die angeborenen Formen möglicher Erfahrung [Innate forms of potential experience]. Zeitschrift für Tierpsychologie, 5, 235–409. doi: 10.1111/j.1439-0310.1943.tb00655.x
- Mendes, D. M. L. F., Seidl-de-Moura, M. L., & de Oliveira Siqueira, J. (2009). The ontogenesis of smiling and its association with mothers’ affective behaviors: A longitudinal study. Infant Behavior and Development, 32, 445–453. doi: 10.1016/j.infbeh.2009.07.004
- Nittono, H., Fukushima, M., Yano, A., & Moriya, H. (2012). The power of kawaii: Viewing cute images promotes a careful behavior and narrows attentional focus. PLoS One, 7, e46362. doi: 10.1371/journal.pone.0046362
- Nittono, H., & Ihara, N. (2017). Psychophysiological responses to Kawaii pictures with or without baby schema. SAGE Open, 7, 1–11. doi: 10.1177/2158244017709321
- Peterson, M. S., Beck, M. R., & Wong, J. H. (2008). Were you paying attention to where you looked? The role of executive working memory in visual search. Psychonomic Bulletin & Review, 15, 372-377. doi: 10.3758/PBR.15.2.372
- Rutherford, H. J. V., Byrne, S. P., Crowley, M. J., Bornstein, J., Bridgett, D. J., & Mayes, L. C. (2018). Executive functioning predicts reflective functioning in mothers. Journal of Child and Family Studies, 27, 944–952. doi: 10.1007/s10826-017-0928-9
- Schaller, M. (2018). The parental care motivational system and why it matters (for everyone). Current Directions in Psychological Science, 27, 295–301. doi: 10.1177/0963721418767873
- Sherman, G. D., Haidt, J., & Coan, J. A. (2009). Viewing cute images increases behavioral carefulness. Emotion, 9, 282–286. doi: 10.1037/a0014904
- Sherman, G. D., Haidt, J., Iyer, R., & Coan, J. A. (2013). Individual differences in the physical embodiment of care: Prosocially oriented women respond to cuteness by becoming more physically careful. Emotion, 13, 151–158. doi: 10.1037/a0029259
- Webb, R., & Ayers, S. (2015). Cognitive biases in processing infant emotion by women with depression, anxiety and post-traumatic stress disorder in pregnancy or after birth: A systematic review. Cognition and Emotion, 29, 1278-1294. doi: 10.1080/02699931.2014.977849
- Weissgerber, T. L., Milic, N. M., Winham, S. J., Garovic, V. D. (2015). Beyond bar and line graphs: Time for a new data presentation paradigm. PLoS Biology, 13, e1002128. doi: 10.1371/journal.pbio.1002128
- Yatziv, T., Kessler, Y., & Atzaba-Poria, N. (2018). What’s going on in my baby’s mind? Mothers’ executive functions contribute to individual differences in maternal mentalization during mother-infant interactions. PLoS ONE, 13, e0207869. doi: 10.1371/journal.pone.0207869
