 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Changes in pupil size can reflect social interest or affect, and tend to get mimicked by observers during eye contact. Pupil mimicry has recently been observed in young infants, whereas it is unknown whether the extent and the speed of infants’ pupil mimicry response are identical to that of adults. Moreover, the question of whether pupil mimicry in infants is modulated by the race of the observed other remains to be explored. In two studies, pupil mimicry was investigated in infants and their parents. In the first study, 6-, 12- and 18-month-olds (n = 194) and their parents (n = 192) observed eyes with dynamically dilating, constricting, or static pupils. Infants mimicked the pupil sizes of the observed eyes like their parents, but responded slower. Study 2 replicated these findings in a new sample of infants (n = 55, 12-month-olds) and parents (n = 64), and further showed that the pupil mimicry response was not significantly modulated by the race of the observed partner neither in infants nor in parents. We conclude that pupil mimicry is an ancient bonding mechanism that helps to connect people.
The ability to understand, empathise and appropriately act upon the emotions of others is a prerequisite for a healthy social life. From the earliest months of life onwards, young infants eagerly orient towards faces and keep their attention on faces longer than on any other object (e.g. Goren, Sarty, & Wu, Citation1975). In humans, the face provides the richest source of social information and reveals important cues about someone’s emotions and intentions (Reid & Striano, Citation2007). From the face, the eye region is particularly expressive and attracts most attention in newborns and adults alike (Farroni, Csibra, Simion, & Johnson, Citation2002; Hood, Willen, & Driver, Citation1998).
Infants learn to recognise and tune in to another’s emotional expressions through interactions with caregivers (e.g. Aktar, Colonnesi, de Vente, Majdandžić, & Bögels, Citation2016) and through observation of caregiver’s emotional expressions to novelty (e.g. Aktar, Majdanžić, De Vente, & Bögels, Citation2013). Following their parents’ eye gaze and reciprocating smiles are two of the earliest and best-studied examples of infant behaviour during face-to-face exchanges of emotional states, and are important markers in the development of an infant’s social behaviour (Als, Tronick, & Brazelton, Citation1979; Messinger & Fogel, Citation2007). Infants begin to mimic facial expressions of emotion shortly after birth and continue to do so throughout their lifetimes (Meltzhoff & Moore, Citation1994). In addition to infants’ tendency to mimic their parents’ facial expressions, there is evidence to suggest that infant-parent dyads also synchronise on the physiological level, a phenomenon that might be related to the development of empathy and important for social bond formation (Feldman, Citation2007). For instance, a study by Waters and colleagues (Citation2014) showed that mothers’ stressful experiences were contagious to their infants and generated high levels of heart-rate covariation (Waters, West, & Mendes, Citation2014).
Another physiological reaction that is, in principle, directly visible to observers is pupil size (Kret, Citation2015). Recent studies showed that, like adults, infants adapt their pupil sizes to the pupil sizes of others (Fawcett, Arslan, Falck-Ytter, Roeyers, & Gredebäck, Citation2017; Fawcett, Wesevich, & Gredebäck, Citation2016). This phenomenon, also dubbed “pupil mimicry” has first been described in adults (Harrison, Singer, Rotshtein, Dolan, & Critchley, Citation2006; Hess, Citation1975; Kret & de Dreu, Citation2017; Kret, Fischer, & De Dreu, Citation2015; Van Breen, de Dreu, & Kret, Citation2018) and has been observed in chimpanzees as well (Kret, Tomonaga, & Matsuzawa, Citation2014), suggesting that pupil mimicry not only has an early ontogenic onset, but it is also a phylogenetically old, robust phenomenon.
Pupillary alterations can reflect different cognitive and emotional processes including arousal, attention, and social interest (Bradley, Miccoli, Escrig, & Lang, Citation2008; Hepach & Westermann, Citation2016; Hess & Polt, Citation1960, for a review, see Kret & Sjak-Shie, Citation2019). Precisely because pupillary changes are unconscious and beyond control, they provide a direct reflection of a person’s inner state and are a relevant source of information for observers. Recent studies suggest that the mimicry of pupil size is important for the establishment of trust. First, Kret and colleagues (Citation2015) showed that when participants mimicked a partner’s dilating but not constricting pupils, this promoted trust. The positive relationship between pupil dilation mimicry and trust was bound to interactions with partners that belonged to the same ethnic group as the participants (Kret et al., Citation2015), and fell apart in a competitive context (Van Breen et al., Citation2018). Another study confirmed these findings and showed that this pupil mimicry-trust linkage was further modulated by oxytocin, an evolutionary ancient neuropeptide that acts as hormone and neurotransmitter and is important for the formation of social bonds (Kret & de Dreu, Citation2017, for a review, see De Dreu & Kret, Citation2016). Together, this research suggests that pupil mimicry facilitates social bonding.
The finding that in both studies pupil dilation mimicry positively correlated with trust in partners from the same racial group as their own, but not in partners from another racial group, is very interesting from a developmental perspective (Kret et al., Citation2015; Kret & de Dreu, Citation2017). An important open question is whether this reflects different levels of experience with the own group compared to the other race group. From infancy research, it is known that young infants already process own-race faces differently than other-race faces (Kelly et al., Citation2007; Sangrigoli & de Schonen, Citation2004). Although such a difference is not apparent in newborns, infants already show a visual preference for own (vs. other) race by 3-months of age (Kelly et al., Citation2005). This early sensitivity to own-race faces along with environmental exposure to own-race faces between 4 and 9 months contributes to an advantage in infants’ processing of own-race as compared to other-race faces (Kelly et al., Citation2007).
The findings suggest that facial input from the infant’s visual environment is crucial for shaping the face-processing system early in infancy, possibly resulting in differential recognition accuracy for faces of different races in adulthood. However, regardless of recognition, whether a similar advantage to own-race eyes is already observed at this age in differential physiological patterns or mimicry responses is hitherto unknown. Findings from a recent fNIRS study investigating 9-month-olds’ brain responses to dynamic own-race versus other-race eyes provide evidence for an own-race advantage in infants’ brain responses to dilating pupils. This study showed that infants’ brains are sensitive to the dilating pupils of own-race, but not other-race eyes, indexed by an enhanced activity in the right superior temporal cortex (Kelsey, Krol, Kret, & Grossmann, Citation2019). Moreover, infants were found to allocate greater cognitive effort to the processing of other-race faces at the neural level, evidenced by activity in the dorsolateral prefrontal cortex. The current study investigates whether a similar advantage to own race eyes is directly observed in infants’ pupil mimicry responses.
In the present study, we investigated pupil mimicry in 6-, 12-, and 18-month-old infants and their parents. Participants were shown the eye region of adult models with static or dynamically constricting, or dilating pupils, whilst their own pupil size was being measured by eye-tracking equipment. The current study builds on earlier work and aims to extend this in several ways. The first aim is to investigate whether the findings observed by Fawcett and colleagues (Citation2016) are replicable in infants’ pupil responses by using more naturalistic stimuli. Instead of pupillary contagion in static pictures with small or large pupils, the current study uses images of eyes with pupil sizes that change dynamically, as they do in real life. Based on earlier work by Fawcett and colleagues (Citation2016, Citation2017), we predicted that infants, regardless of age, would mimic the pupil sizes observed in a dynamic pair of naturalistic eyes.
A second aim is to take a closer look at the developmental trajectory of pupil mimicry. If infants indeed show pupil mimicry, do they, compared to their parents, mimic pupil sizes to a similar extent, show similar pupillary patterns over time and mimic as fast? To that extent, we tested not only 6-, 12- and 18-month-old infants, but also included their parents for a direct comparison of mimicry across infants and parents. These age groups were of special interest for the current study for several reasons. First, Fawcett and colleagues (Citation2016, Citation2017) observed pupil mimicry already in 4–6-month-olds. Between the 5th and the 7th months, infants also start to differentiate between positive and negative facial expressions (Vaish, Grossmann, & Woodward, Citation2008) and between the 10th and the 14th months, they start to modify their behaviour in accordance with these expressions (Aktar et al., Citation2013; De Rosnay, Cooper, Tsigaras, & Murray, Citation2006; Feinman, Citation1982). In that realm, it is interesting to investigate not only whether the tendency to mimic changes over the course of infancy, but also whether the propensity to mimic dilating versus constricting pupils changes in the first years of life.
Third, we aimed to explore possible differential effects in infants’ compared to adults’ tendency to mimic pupil sizes of partners of own vs. other-race faces. Although latest research has shown that young infants have an own-race bias at the neural level to dilating pupils, it is currently unknown whether they show a similar bias in pupil mimicry. The question of whether they will differentially mimic own- versus other-race partners, is therefore of exploratory nature.
Methods
Study 1
Participants
The infant sample consisted of 194 infants of three age groups 6-month-olds (n = 70, M age = 6.11, range = 4.97–7.01, SD = 0.50, 33 girls), 12-month-olds (n = 62, M age = 12.01, range = 10.66–12.99, SD = 0.66, 38 girls), and 18-month-olds (n = 62, M age = 17.89, range = 16.55–18.95, SD = 0.66, 32 girls,) and the parents (n = 192 parents, 51 fathers, see Table S1 for demographic information). In 94.23% of the participating families the partners were co-habiting (mother’s report). Data from an additional 34 children who visited the lab were missing due to child fussiness/fatigue/movement (n = 18), calibration or tracking failures (n = 12) or experimenter errors (n = 4). An additional 23 infants were excluded from the analysis during data processing (see Data Reduction). For the infants, the drop-out rate is comparable to the earlier study with similar stimuli for the infants (see Fawcett et al., Citation2017). In cases where the testing did not succeed with the infant, the procedure was terminated without testing the parent (n = 47). An additional number of 12 parents were excluded from the analyses during data processing (see Data Reduction)
Families who participated were recruited via invitation letters sent by the municipality to a random sample of families with babies. The study was approved by the ethical committee of the University of Amsterdam. Parents gave written informed consent for their own and for their child’s study participation.
Stimuli
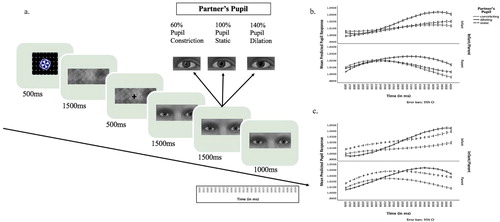
The stimuli were black and white dynamic videos (1280 × 1024 pixels) depicting the eye regions of 10 male and 10 female Dutch models exhibiting constricting, static or dilating pupils (640 × 250 pixels) on the centre of a 1280 × 1024 screen. There were three unique stimuli per model, two videos showing constricting, or dilating pupils, and one picture with static pupils, presented for 4000 ms. For dynamically constricting or dilating pupils, pupils were static for the first 1500 ms, followed by a dynamic constriction or dilation of the pupil that reached the maximum after 1500 ms, and stayed at maximum for another 1000 ms (see (a)). Two sets of 10 partners were counter-balanced across participants and the order of trials was random across families.
Procedure
Infants’ and parents’ pupillary reactions were measured via an EyeLink eye-tracker in a dimly illuminated room. Before the start of the experiment, both infants’ and parents’ gaze were calibrated with a 5-point procedure. The experimental setup is presented in . Infants were placed in a car seat, approximately 60 cm away from the screen. The parents sat on a chair on the side of the infant during testing, and they were instructed not to intervene unless the infant became fussy or was seeking attention. In cases where the child tried to get their attention, parents were instructed to respond as they would normally do.
The experiment consisted of 30 trials. Each trial started with a 500 ms attention-getter followed by 500 ms of blank screen. Next, a scrambled grey screen (with equal luminance to dynamic stimuli) was presented first without (1500 ms) and next with a fixation cross (500 ms), followed by the stimuli. A random order was generated for each infant, and repeated during the testing of the parent. This way, the order was randomised across families, while it was fixed within-family level. Using the same order aimed to make the experimental procedure as similar as possible within families to be able to detect a potential association between the parent and infant pupil mimicry responses. The experimenter monitored infants’ attention during the experiment and repeated the presentation of the attention getters at the beginning of the trials, if necessary. Parents were seated in front of the eye-tracker and completed the task following infants. Parents additionally reported infants’ temperament, and their own negative emotions, and empathy (not used in the current study).
Data Reduction
All the available pupil data from parents and children were included in the analyses, and were pre-processed identically to maximise comparability. The pupil data were first restricted to the last 2500 ms of the stimulus presentation because only then did the changes in partner pupil size occur (see also Kret et al., Citation2015; Kret & de Dreu, Citation2017). Outlying scores (>|3| SD) and blinks were removed and missing data points (maximally 500 ms) were replaced via linear interpolation (Jackson & Sirois, Citation2009). Following the interpolation, the pupil data were down-sampled to 100 ms intervals. The 500 ms just before the partners’ pupils started to change (1000–1500 ms after stimulus onset) in each trial were used as a baseline. Subsequent data points were divided by the average pupil size during these 500 ms. Subsequent data points were divided by the average pupil size during these 500 ms (Kret & de Dreu, Citation2017). The following exclusion criteria were applied at the trial and subject level: Trials in which the data were available for at least 500 ms within the 4 s stimulus presentation time were included in the analyses. The data from 7 infants and 4 parents were excluded at this stage. Finally, infants and parents with less than 10 trials were excluded from the analyses. The data from 16 infants and 8 parents were excluded at this stage. The pupil data were available on average for 23.85 trials (SD = 5.32) per infant, and for 29.31 trials (SD = 2.31) per parent.
Outcome variables
Baseline-corrected pupil size was the outcome measure in the analyses testing whether infants’ and parents’ mimicked partners’ pupil sizes. The pupil data were analysed in 3-level multi-level regression models consisting of repeated observations of time (level 1) nested in trials (level 2), nested in participants. The sample size at the highest level of the current hierarchical data structure (i.e. participants, N = 194 infants and 192 parents) allows sufficient power for multi-level analyses of pupil responses (Maas & Hox, Citation2005; Snijders, Citation2005).
A first-order auto-regressive covariance structure was used for repeated effects of time within trials. Maximum likelihood was the estimation method. We also tested random effects of intercept, partner ID and linear, quadratic, and cubic terms of time in each model, and kept the significant random effects. All relevant interactions were included in the initial models. To reach the best fitting and most parsimonious final model, non-significant interaction effects that did not significantly improve the fit (computed using log-likelihood ratios) were one-by-one excluded from the initial model starting with higher-order interactions and higher p’s. The backward exclusion of interactions was more favourable than the alternative approach of starting with the simple main effects and one-by-one addition of the interactions of interest to the model in a forward fashion, as the former not require decisions about the sequence of inclusion for separate interactions.
For fixed effects, multi-level regression models generate F-scores and B estimates, (see below). Similar to regular ANOVAs, F-scores are used in the testing of overall group differences for the main effect of fixed categorical variables (such as family member), as well as of higher-order interactions including these variables. Inclusion/exclusion of initially tested interactions in the final model was based on the significance of F-scores.
Multi-level models additionally generate the coefficient estimates B. Similar to linear regression analyses, these provide the test for continuous and dichotomous variables. In the current analyses, B estimates were used to interpret the fixed effects of dichotomous categorical variables and continuous variables in the final models.
The initial multi-level model for pupil responses of infants and parents consisted of the fixed effects of partner’s pupil (dilating, and constricting versus static), Infant/Parent (infant versus parent), and the two-way interaction between of partners’ pupil and Infant/Parent (see Table S2). Additional analyses were conducted in the infant sample alone to explore age effects in place of Infant/Parent. In the next analysis, we modeled the slope of participants’ pupil sizes over time by including linear, quadratic and cubic polynomial terms of time, and tested all the two and three-way interactions between Infant/Parent, Partner’s Pupil, and polynomial terms of time, except for the interactions between the polynomials (see Tables S3 and S5).
Study 2
Participants
The sample for Study 2 included 55 infants with a mean age of 12 months (M age = 12.55, range = 9.15–14.9, SD = 0.02, 24 girls), and 64 parents (17 fathers, see Table S1 for demographic information). In 92.18% of the participating families, the partners were co-habiting (mother’s report). Data from an additional 8 children who visited the lab were missing due to child fussiness/fatigue/movement (n = 6), or experimenter errors (n = 2). An additional number of 8 infants were excluded from the analysis during data processing (see Data Reduction). Data from 6 parents were missing from this sample as the procedure was terminated early due to child-related reasons, and one parent was excluded during data processing. Families who participated were recruited in the same way as in Study 1. The study was approved by the ethical committee of the University of Amsterdam. Parents gave written informed consent for study participation.
Stimuli
The stimuli were black and white dynamic videos depicting the eye regions of 2 male and 2 female North European models for own-race and of 2 male and 2 female Asian Japanese models for the other-race category. The videos exhibited dynamically constricting, or dilating pupils, or static pupils (640 × 250 pixels) on the centre of a 1280 × 1024 screen (see (a), for more information on stimuli, also see Kret & de Dreu, Citation2017). The time flow of the trial was identical to Study 1.
Figure 2. (a) The time flow of the experiment. For dynamically constricting or dilating pupils, pupils were static for the first 1500 ms, followed by a dynamic constriction or dilation of the pupil that reached the maximum after 1500 ms, and stayed at maximum for another 1000 ms. Static pupils remained the same size in the static condition throughout the trial. (b) Infants’ and parents’ predicted pupil responses (on the right) over the time interval (1600–4000 ms) investigated within stimulus presentation time in Study 1. (c) Infants’ and parents’ predicted pupil responses (on the right) over the time interval (1600–4000 ms) investigated within stimulus presentation time in Study 2.
Procedure
The procedure was the same as Study 1. The experiment consisted of 24 trials. Just like in Study 1, a random order was generated for each infant, and repeated during the testing of the parent. The time flow of the trials is identical to Study 1.
Data Reduction
To enable comparability with Study 1, the data reduction of Study 2 followed identical steps. No subject was excluded due to missingness within trials, whereas a sample of 8 infants and 1 parent with less than 10 trials were excluded from the analyses at this stage. The pupil data were available on average for 20.42 trials (SD = 3.56) from infants, and for 23.72 trials (SD = 1.20) from parents.
Outcome variables
Like in Study 1, baseline-corrected pupil sizes were the outcome measure. The hierarchical structure and steps to analyse the dataset were the same as in Study 1. The sample size at the highest level of the current hierarchical data structure (i.e. participants) was 119 (55 infants and 64 parents)
The main difference from Study 1 in the analyses was that the multi-level models for differential pupil responses of infants and parents additionally included the fixed effects of partner’s group as well as its two and three-way interactions with Partner’s pupil (dilating, and distracting versus static), and Infant/Parent (infant versus parent). Like in Study 1, we modeled the slope of participants’ pupil sizes over time in the next step, by including the polynomial terms of time.
Results
Study 1
In a linear mixed model including Infant/Parent (Infant, Parent) and Partner Pupil Size (Constricting, Static, Dilating) as fixed factors (N = 386, 194 infants and 192 parents), a significant main effect of Partner Pupil Size was observed, F (2, 10239.17) = 6.85, p = .001 (see Table S2) showing that participants’ pupil sizes were larger when observing a partners’ dilating as compared to static pupils, B = .01, SE = <.01, 95% CI [.003, .013], p = .002, while the pupil sizes did not differ during the observation of constricting (vs. static) pupils, p = .934. Importantly, the interaction between Partner Pupil Size and Infant/Parent was not significant p = .456, showing that infants, like adults, showed pupil mimicry (see (b)). In an additional step where we restricted the model to infants only (N = 194) to detect differences related to infant age, we found that pupil mimicry did not change across as a function of age (the interaction between infant age * Partner’s Pupil was not significant, p = .380, and the main effect of infant age was not significant in the final model, p = .130). In a control analysis with the percentage of missing data per trial as the outcome variable, we inspected age group differences in infants’ attention to partner’s pupil: The missing percentage of infants’ data did not differ as a function of Partner’s Pupil (p = .128) and the two-way interaction between partner’s pupil and age group (6- and 12-months vs. 18-months) was not significant (p = .299).
To investigate potential differences between the dynamic changes in parents’ and infants’ pupillary changes over time, we included linear, quadratic and cubic polynomial terms to the full model (N = 386, 194 infants and 192 parents, see Table S3). An interaction between the linear polynomial and Partner Pupil Size, F (2, 35809.84) = 14.18, p < .001 showed that the linear increase in participants’ pupil sizes over time was faster when observing partners with dilating as compared to static pupils, B = .02, SE = .01, 95% CI [.008, .039], p = .003. Similarly, as seen in , whereas participants’ pupils slightly increased in size during the observation of partners with static pupil sizes, their pupils were constricting relatively slower when those of the partners constricted, which resulted in different linear slopes, B = −.02, SE = .01, 95% CI [−035, −.003], p = .017. An interaction between the cubic polynomial and Partner’s Pupil Size, F (2, 194892.32) = 6.71, p = .001 showed that the slope of participants’ pupillary response had a weaker cubic trend when observing partners with dilating, as compared to static pupils, B = −.01, SE < 0.01, 95% CI [−.019, −.005], p = .001, but did not differ between constricting and static pupils (p = .583).
Interestingly, we observed two-way interactions between the polynomials and Infant/Parent, linear: F (1, 440.75) = 29.38, B = .05, SE = .01, 95% CI [.030, .065], p < .001; quadratic: F (1, 389.51) = 21.14, B = .02 SE < .01, 95% CI [.012 to .029], p < .001; cubic: F (2, 195027.82) = 13.85, B = −.01, SE < .01, 95% CI [−.017, – .005], p < .001. Infants’ pupillary responses over time (see (b)) show high similarities to the pattern of results reported by Fawcett and colleagues (Citation2016), who did not analyse the dynamic effects statistically. Specifically, the results show that adults have a pronounced peak in their pupil size between 800 and 1500 ms after the partner’s pupils started to change in size, whereas infants’ pupils keep dilating linearly without reaching a peak during the course of stimulus presentation. In order to test this observation statistically, we measured the peak in pupil size in each trial and confirmed that parents’ pupils peaked earlier than the pupils of infants, F (1,10500) = 258.43, p < .001.
The direct link between infants’ and parents’ pupil mimicry
The lack of significant differences in the pupil mimicry response between infants and parents raises the question of whether a direct association exists between pupil mimicry responses of infants and their parents. To test this direct association, we first aggregated participants’ pupil responses across time within each trial via averaging, and we tested parents’ pupil responses as a predictor of infants’ responses (N = 172). All the two and three-way interactions between Partner Pupil Size (Constricting, Static, Dilating), infant age and parents’ pupil were also included in this model. None of the tested interactions were significant, p’s .329. Findings revealed no direct association between parents’ and infants’ pupil responses (p = .922) in the final model.
Study 2
The linear mixed model including Infant/Parent (Infant, Parent), Partner Group (Own Race, Other Race) and Partner Pupil Size (Constricting, Static, Dilating) as fixed factors, showed a significant main effect Partner Group, F (1, 3135.72) = 7.42, p = .006 (N = 119, 55 infants and 64 parents, see Table S4). Participants’ pupil sizes were smaller during the observation of own-race as compared to other-race partners, B = −.01, SE = .01, 95% CI [−.020, .003], p = .006, possibly indicating heightened arousal for the outgroup faces. The three-way interaction between Infant/Parent (Infant, Parent), Partner Group and Partner Pupil Size (p = .237) or the two-way interaction between Partner Group and Partner Pupil Size (p = .876) were not significant, suggesting no significant advantage for own-race eyes in the case of pupil mimicry and/or for parents (vs. infants).
A significant main effect of Partner Pupil Size was observed in the final model (presented in Table S4), F (2, 3137.14) = 3.75, p = .024. However, differently from Study 1 where we only observed a significant dilation mimicry, only constriction mimicry was significant in Study 2: Participants’ pupil sizes were smaller when observing a partners’ constricting as compared to static pupils, B = −.01, SE = .01, 95% CI [−.023, −.003], p = .010. The difference between dilating and static pupils did not reach significance, although the means are numerically consistent with Study 1 (p = .659). Concerning the comparison of pupillary responses across parents and infants, the findings were in line with the findings from Study 1: there was no significant two-way interaction between Partner Pupil Size and Infant/Parent, p = .863 (see (c)). In fact, none of the two and three-way interactions between Partners’ Group, Partner’s Pupil Size and Infant/Parent were significant in this model ((p’s ≥ .237), reducing this model to main effects presented in Table S4).
In an additional step where we restricted the model to infants only (N = 55) to detect age differences, we found that pupil mimicry was not modulated by age and/or partner race (the three-way interaction between age, Partner Group, and Partner Pupil Size, p = .588, or the two-way interaction between age * Partner’s Pupil was not significant, p = .264. In line with the findings of Study 1, the main effect of age was not significant in the final model, p = .628). In a control analysis with the percentage of missing data per trial as the outcome variable, we found that the missing percentage per trial in infants’ data did not differ as a function of Partner’s Pupil (p = .798).
In the next step, we included linear, quadratic and cubic polynomial terms to the full model (N = 119, 55 infants and 64 parents, see Table S5). Like in Study 1, an interaction between the linear polynomial and Partner Pupil Size F (2, 9322.85) = 8.46, p < .001 showed that the slope of the linear increase in participants’ pupil sizes was steeper when observing partners with dilating as compared to static pupils, B = .05, SE = .02, 95% CI [.016, .079], p < .003. No significant differences were observed with constricting as compared to static pupils, p = .300. An interaction between the cubic polynomial and Partner’s Pupil, F (2, 52466.01) = 3.75, p = .023 showed that the slope of participants’ pupillary responses had a weaker cubic trend when observing partners with dilating as compared to static pupils, B = −.02, SE = .01, 95% CI [−.031, −.004], p = .014, but did not differ between constricting and static pupils (p = .857), consistent with the findings of Study 1.
Importantly, again replicating Study 1, we observed significant two-way interactions between the polynomials and Infant/Parent. The two-way interactions between the linear and quadratic polynomials and Infant/Parent (linear: F (1, 106.86) = 4.19, B = .03, SE = .02, 95% CI [.001, .063], p = .043; quadratic: F (1, 31911.25) = 11.16, B = .03, SE = .01, 95% CI [.011, .043], p = .001) reflect the pattern observed in the Fawcett study and in Study 1 of this article. As presented in (c), there was a clear peak in adults’ pupil responses between 800 and 2000 ms after the partner’s pupils started to change in size, while infants’ pupils linearly increased without reaching a clear peak over stimulus presentation time. Additional analyses confirmed that the timing of the peak in pupil size came earlier in parents compared to infants, F (1, 2674) = 126.36, p < .001, thus replicated the findings of Study 1.
The direct link between infants’ and parents’ pupil mimicry
To test the direct association between pupil mimicry responses of infants and their parents, we tested parents’ pupil responses as a predictor of infants’ responses (N = 51). None of the two and three-way interactions between Partner Pupil Size (Constricting, Static, Dilating), infant age and parents’ pupil were significant, ps > .799. Findings revealed no direct association between parents’ and infants’ pupil responses, in the final model, p = .104, revealing no significant direct link between parents’ and infants’ pupil mimicry in Study 2.
Exploratory analyses
The current findings reveal significant pupil mimicry in both studies, whereas the specific subtype of mimicry differed between Study 1 and 2, with a significant dilation (but not constriction) mimicry observed in Study 1 and a significant constriction (but not dilation) mimicry observed in Study 2. Although the means across the two studies were numerically consistent with our à priori predictions (dilating > static > constricting), the statistical outcomes were different. To gain further insight into this matter, we repeated the same model across the two studies, after including “Study” as an additional factor, and excluding other-race faces. None of the two and three-way interactions between Study, Partner’s Pupil, Infant/Parent were significant in the initial model (p’s .148). The main effect of Study was also not significant in the final model (p = .484). Taken together, these findings reveal no significant differences across the samples of Study 1 versus Study 2.
Regarding the mimicry response, the findings resonated with Study 1 with a significant main effect of Partner’s Pupil F (2, 11537.90) = 7.33, p = .001 revealing a significant dilation, but not constriction mimicry: Participants’ pupil sizes were larger when observing a partners’ dilating as compared to static pupils, B = .01, SE = < 0.01, 95% CI [.002, .011], p = .003, while the pupil sizes did not differ during the observation of constricting (vs. static) pupils, p = .524. Just like in Study 1 and Study 2, the two-way interaction between Infant/Parent and Partner’s Pupil was not significant in this model across the two studies, p = .279.
Discussion
The eyes are extremely important for communication. From the first days of life on, human infants’ attention is grabbed by others’ eyes and their signals are readily processed (Farroni et al., Citation2002; Hood et al., Citation1998). The importance of the eyes is underscored by a recent finding that 4-to-6-month-old infants already mimic the pupil sizes of observed eyes (Fawcett et al., Citation2016, Citation2017). Large pupil sizes may reflect positive social interest, and their mimicry has been shown to foster trust and social approach (Brambilla, Biella, & Kret, Citation2019; Kret et al., Citation2015). In contrast, small pupils may be indicative of fatigue and boredom, and trigger avoidance tendencies. Both signals are important to detect, and may be perceived differently by infants and adults, as well as from own- versus other-race faces. The key finding of the current study is that infants mimicked observed pupil sizes regardless of race, but that their mimicry response is slower than that of their parents, suggesting that this behaviour might not yet be fully mature. Below we discuss these results in the light of the existing literature.
The current study encompasses two experiments, both confirming pupil mimicry in infants and parents and both showing a faster response in the parents than in infants. We also observed some unexpected differences between Study 1 and 2. In Study 1, there was significant mimicry to partner’s dilating, but not constricting pupils, while the mimicry response only followed partner’s constricting pupils in Study 2. Although the significant constriction mimicry observed in Study 2 may in fact, be related to the inclusion of other-race faces, for which a stronger constriction mimicry was reported earlier in an adult study (Kret et al., Citation2015), the findings did not reveal a significant moderation of the pupil mimicry response to own versus other-race faces in this study. Considering relatively small effect sizes coming from working with pupillary responses with luminance-controlled stimuli, we suspect that the sample of Study 2 was underpowered to detect pupil dilation mimicry. Indeed, the means were numerically consistent with Study 1 and when the datasets of both studies own-race faces were included in one analysis, pupil dilation mimicry was observed and there was no interaction effect with study.
The current study shows that parents’ and infants’ pupil mimicry was overall similar, whereas the dynamic unfolding of the pupillary reactions over time differed between parents and infants. Supporting earlier findings by Kret and colleagues (Citation2015), parents had a clear peak in their pupil response. In contrast to parents, infants’ pupil sizes kept increasing over time, suggesting they might still have to learn to regulate their increasing level of arousal. Note that this linear increase in infants’ pupil responses was also previously observed in the visualisation of the data by Fawcett and colleagues (Citation2016), who investigated pupil mimicry in 6- and 9-month-olds. Moreover, similar to this earlier study, the pupil mimicry response in this study did not differ as a function of age. The current study additionally revealed no synchrony in infant and parent dyads’ mimicry responses, as no significant direct link appeared between parents’ and infants’ responses to dilating, constricting and static pupils at the trial level. Taken together, the findings suggest that pupil mimicry is a robust phenomenon and is present from birth, or develops during the first six months irrespective of the perceptual narrowing observed in infants’ emotion processing of other race (vs. own race) faces between 4 and 9 months of age in typical development (Kelly et al., Citation2007). Moreover, unlike heart-rate indices (Feldman, Citation2007), no physiological synchrony was detected in the current study in parent-infant dyads’ mimicry responses in the first 18 months of life.
Despite well-established evidence showing that infants start to develop an advantage in emotion processing for in-group members, and discriminate the faces from their own race better than other races between 6 and 9 months (e.g. Ge et al., Citation2009; Kelly et al., Citation2007; Liu et al., Citation2015), Study 2 showed no such advantage for pupil mimicry. Infants and parents had overall larger pupils during the observation of other-race faces, while pupil mimicry did not differ across own and other-race faces. In a previous investigation in a student sample, we observed that pupil dilation mimicry was enhanced during interactions with in-group members, yet pupil constriction mimicry was stronger with out-group members (Kret et al., Citation2015). In that study, partners either showed a happy or an angry expression. In a second study, we used neutral faces and did not find a modulation of group membership on either type of mimicry (Kret & de Dreu, Citation2017). However, in both studies, pupil dilation mimicry associated with higher investments in a trust game, but this positive relationship only held during interactions with in-group partners. Obviously, in the current study we did not use a trust game but a passive viewing experiment (Kret et al., Citation2014). Pupil mimicry was observed, but independent of partner group, which is in line with Kret and de Dreu (Citation2017). Thus, we conclude that during emotionally neutral interactions, pupil mimicry is not affected by partner’s group membership. The current findings revealed no other-race effect in pupil mimicry in infants vs. parents, suggesting that this automatic response may be less affected by environmental exposure to in-group members. Overall larger pupil sizes during the observation of other-race faces may be related to greater cognitive effort allocated to the processing of other-race faces as compared to own-race, and are in line with earlier findings by Kelsey and colleagues (Citation2019) who observed greater cognitive effort to other-race faces on the neural level in 9-month-olds.
The findings of the current study must be seen in the light of the following limitations: First, the comparison of the infant and adult pupil mimicry responses in the current dataset were based on the data from infants and adults from the same families. Despite this, we have treated each family member independently in the first set of analyses to be able to compare adult and infant patterns of mimicry. Although our statistical approach was not optimal in this respect, the findings revealing no statistical association between infants’ and parents’ pupil mimicry suggest that disregarding the dependency coming from families may not have an influence in the current dataset. Second, although the study provides preliminary evidence for some continuity in the pupil mimicry response across the first 18-months, future studies investigating this question in longitudinal designs will be necessary to reach a firm conclusion on the developmental trajectories of pupil mimicry. Third, since the current analyses did not include looking analyses, we cannot directly exclude that attention played a role in the observed findings in pupil mimicry response. However, in the light of exploratory analyses that revealed no significant difference in the percentage of missing data at the trial level between constricting, dilating vs. static stimuli, and of earlier evidence that revealed no main effect of race or partner’s pupil on infants’ looking behaviour (Kelsey et al., Citation2019), we conclude that attention is not a potential confound for the observed dynamic changes in pupil mimicry (see also Prochazkova et al., Citation2018, for an analysis of looking behaviour, ruling out that looking patterns modulated the pupil mimicry response). Finally, the current dataset did not include infants who have become fussy during the experiment, and it remains to be investigated whether infants who were dropped due to fussiness differed systematically in their pupil mimicry. Future studies using alternative physiological measurements that are relatively less affected by movement may shed light on this question.
The current study was the first one to demonstrate pupil mimicry in infancy using naturalistic, dynamic stimuli, and to directly compare infants’ pupil mimicry to their parents’. It shows that infants are sensitive to changes in partners’ pupil sizes, and mimic dilating pupils just like their parents. Two important aspects of this phenomenon await attention in future studies. First, individual differences in infants’ or their parents’ personality and how these modulate the tendency to mimic another’s pupil size remains to be investigated in future studies. Second, future studies should investigate parenting or broader social and emotional factors that may enhance the pupil mimicry response in infants.
Supplementary_Material
Download MS Word (35.9 KB)Acknowledgements
The authors are grateful to parents and infants who contributed to the study.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Data Availability
The data for the current study are available by request to the corresponding author.
Additional information
Funding
References
- Aktar, E., Colonnesi, C., de Vente, W., Majdandžić, M., & Bögels, S. M. (2016). How do parents’ depression and anxiety, and infants’ negative temperament relate to parent–infant face-to-face interactions? Development and Psychopathology, 29, 1–14.
- Aktar, E., Majdanžić, M., De Vente, W., & Bögels, S. M. (2013). The interplay between expressed parental anxiety and infant behavioural inhibition predicts infant avoidance in a social referencing paradigm. The Journal of Child Psychology and Psychiatry, 54, 144–156.
- Als, H., Tronick, E., & Brazelton, T. B. (1979). Analysis of face-to-face interaction in infant-adult dyads. In M. E. Lamb, S. J. Suomi, & G. R. Stephenson (Eds.), Social interaction analysis: Methodological issues (pp. 33–77). Madison, WI: University of Wisconsin Press.
- Bradley, M. M., Miccoli, L., Escrig, M. A., & Lang, P. J. (2008). The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology, 45, 602–607.
- Brambilla, M., Biella, M., & Kret, M. E. (2019). Looking into your eyes: Observed pupil size influences approach-avoidance responses. Cognition and Emotion, 33(3), 616–622.
- De Dreu, C. K. W., & Kret, M. E. (2016). Oxytocin conditions intergroup relations through up-regulated in-group empathy, cooperation, conformity, and defence. Biological Psychiatry, 79, 165–173. S0006-3223(15)00259-0.
- De Rosnay, M., Cooper, P. J., Tsigaras, N., & Murray, L. (2006). Transmission of social anxiety from mother to infant: An experimental study using a social referencing paradigm. Behaviour Research and Therapy, 44, 1165–1175.
- Farroni, T., Csibra, G., Simion, F., & Johnson, M. H. (2002). Eye contact detection in humans from birth. Proceedings of the National Academy of Sciences, 99, 9602–9605.
- Fawcett, C., Arslan, M., Falck-Ytter, T., Roeyers, H., & Gredebäck, G. (2017). Human eyes with dilated pupils induce pupillary contagion in infants. Scientific Reports, 7, 9601.
- Fawcett, C., Wesevich, V., & Gredebäck, G. (2016). Pupillary contagion in infancy: Evidence for spontaneous transfer of arousal. Psychological Science, 27, 997–1003. 0956797616643924.
- Feinman, S. (1982). Social referencing in infancy. Merrill-Palmer Quarterly, 28, 445–470.
- Feldman, R. (2007). Parent–infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry, 49, 329–354.
- Ge, L., Zhang, H., Wang, Z., Quinn, P. C., Pascalis, O., Kelly, D., … Lee, K. (2009). Two faces of the other-race effect: Recognition and categorisation of Caucasian and Chinese faces. Perception, 38, 1199–1210.
- Goren, C. C., Sarty, M., & Wu, P. Y. (1975). Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics, 56, 544–549.
- Harrison, N. A., Singer, T., Rotshtein, P., Dolan, R. J., & Critchley, H. D. (2006). Pupillary contagion: Central mechanisms engaged in sadness processing. Social Cognitive and Affective Neuroscience, 1, 5–17.
- Hepach, R., & Westermann, G. (2016). Pupillometry in infancy research. Journal of Cognition and Development, 17, 359–377.
- Hess, E. H. (1975). Role of pupil size in communication. Scientific American, 233, 110–119.
- Hess, E. H., & Polt, J. M. (1960). Pupil size as related to interest value of visual stimuli. Science, 132, 349–350.
- Hood, B. M., Willen, D., & Driver, J. (1998). Adult's eyes trigger shifts of visual attention in human infants. Psychological Science, 9(2), 131–134.
- Jackson, I., & Sirois, S. (2009). Infant cognition: Going full factorial with pupil dilation. Developmental Science, 12, 670–679.
- Kelly, D. J., Quinn, P. C., Slater, A. M., Lee, K., Ge, L., & Pascalis, O. (2007). The other-race effect develops during infancy. Evidence of perceptual narrowing. Psychological Science, 18(12), 1084–1089.
- Kelly, D. J., Quinn, P. C., Slater, A. M., Lee, K., Gibson, A., Smith, M., … Pascalis, O. (2005). Three-month-olds, but not newborns, prefer own-race faces. Developmental Science, 8(6), F31–F36.
- Kelsey, C. M., Krol, K. M., Kret, M. E., & Grossmann, T. (2019). Infants’ brain responses to pupillary changes in others are affected by race. Scientific Reports, 9(1), 4317.
- Kret, M. E. (2015). Emotional expressions beyond facial muscle actions. A call for studying autonomic signals and their impact on social perception. Frontiers in Psychology, 6, 711.
- Kret, M. E., & de Dreu, C. K. W. (2017). Pupil-mimicry conditions trust in exchange partners: Moderation by oxytocin and group membership. The Royal Society B-Biological Sciences, 284, E7265–E7274. 20162554. doi:10.1098/rspb.2016.2554
- Kret, M. E., Fischer, A. H., & De Dreu, C. K. (2015). Pupil mimicry correlates with trust in in-group partners with dilating pupils. Psychological Science, 26, 1401–1410.
- Kret, M. E., & Sjak-Shie, E. E. (2019). Preprocessing pupil size data: Guidelines and code. Behavior Research Methods, 51(3), 1336–1342.
- Kret, M. E., Tomonaga, M., & Matsuzawa, T. (2014). Chimpanzees and humans mimic pupil-size of conspecifics. PloS One, 9(8), e104886. doi:10.1371/journal.pone.0104886
- Liu, S., Xiao, W. S., Xiao, N. G., Quinn, P. C., Zhang, Y., Chen, H., … Lee, K. (2015). Development of visual preference for own-versus other-race faces in infancy. Developmental Psychology, 51, 500–511.
- Maas, C. J., & Hox, J. J. (2005). Sufficient sample sizes for multilevel modeling. Methodology, 1, 86–92.
- Meltzhoff, A. N., & Moore, M. K. (1994). Imitation, memory, and the representation of persons. Infant Behavior and Development, 17, 83–99.
- Messinger, D., & Fogel, A. (2007). The interactive development of social smiling. Advances in Child Development and Behavior, 35, 328–366.
- Prochazkova, E., Prochazkova, L., Giffin, M. R., Scholte, H. S., De Dreu, C. K., & Kret, M. E. (2018). Pupil mimicry promotes trust through the theory-of-mind network. Proceedings of the National Academy of Sciences, 115(31), E7265–E7274.
- Reid, V. M., & Striano, T. (2007). The directed attention model of infant social cognition. European Journal of Developmental Psychology, 4, 100–110.
- Sangrigoli, S., & de Schonen, S. (2004). Recognition of own-race and other-race faces by three-month-old infants. The Journal of Child Psychology and Psychiatry, 45(7), 1219–1227.
- Snijders, T. A. B. (2005). Power and sample size in multilevel linear models. In B. S. Everitt, & D. C. Howell (Eds.), Encyclopedia of statistics in behavioral science, 3 (pp. 1570–1573). Chichester: Wiley.
- Vaish, A., Grossmann, T., & Woodward, A. (2008). Not all emotions are created equal: The negativity bias in social-emotional development. Psychological Bulletin, 134, 383–403.
- Van Breen, J. A., De Dreu, C. K., & Kret, M. E. (2018). Pupil to pupil: The effect of a partner's pupil size on (dis) honest behavior. Journal of Experimental Social Psychology, 74, 231–245. doi:10.1016/j.jesp.2017.09.009
- Waters, S. F., West, T. V., & Mendes, W. B. (2014). Stress contagion: Physiological covariation between mothers and infants. Psychological Science, 25(4), 934–942.

