ABSTRACT
Sensitivity to others’ emotional signals is an important factor for social interaction. While many studies of emotional reactivity focus on facial emotional expressions, signals such as pupil dilation which can indicate arousal, may also affect observers. For example, observers’ pupils dilate when viewing someone with dilated pupils, so-called pupillary contagion. Yet it is unclear how pupil size and emotional expression interact as signals. Further, examining individual differences in emotional reactivity to others can shed light on its mechanisms and potential outcomes. In the current study, adults’ (N = 453) pupil size was assessed while they viewed images of the eye region of individuals varying in emotional expression (neutral, happy, sad, fearful, angry) and pupil size (large, medium, small). Participants showed pupillary contagion regardless of the emotional expression. Individual differences in demographics (gender, age, socioeconomic status) and psychosocial factors (anxiety, depression, sleep problems) were also examined, yet the only factor related to pupillary contagion was socioeconomic status, with higher socioeconomic status predicting less pupillary contagion for emotionally-neutral stimuli. The results suggest that while pupillary contagion is a robust phenomenon, it can vary meaningfully across individuals.
Introduction
Being able to recognise and appropriately react to others’ emotions is a fundamental social ability for humans. It can allow group members to be alerted to important situations or to feel empathy for and comfort each other. Previous research on attention to emotion has shown that people detect and preferentially attend to emotional over neutral stimuli (Yiend, Citation2010) and that they may also be particularly biased toward detecting certain emotions, such as anger (Fox & Damjanovic, Citation2006; Hansen & Hansen, Citation1988) or happiness (Becker et al., Citation2011). However, which emotional expressions stand out most in a visual array may come down to the intensity of the particular emotion displayed (Lundqvist et al., Citation2014) or low-level perceptual features of the stimuli such as having an open mouth or visible teeth (Savage et al., Citation2013). Thus, moving beyond perceptual detection and instead assessing physiological arousal responses to others’ emotions and arousal levels may give a more accurate and nuanced picture of the impact of different emotions on an observer. For example, studies using pupil dilation, which not only results from changes in light but is also an indicator of arousal (Laeng et al., Citation2012), show that observers respond with greater arousal to others’ expressions of anger compared to other emotions (Carsten et al., Citation2019; Kret et al., Citation2013). Together, this suggests that while both happy and angry faces might pop out perceptually (Becker et al., Citation2011; Fox & Damjanovic, Citation2006; Hansen & Hansen, Citation1988), they do not seem to have the same effect on the observer’s own emotional state.
While most research on emotion perception focuses on facial expressions, emotions are conveyed through other signals as well, such as vocalizations, body posture, blushing, and pupil dilation. Moreover, pupil dilation on its own can be a subtle signal of arousal (Bradley et al., Citation2008; Partala & Surakka, Citation2003) and in combination with facial expressions or other cues, it could potentially be a signal for variation in emotional intensity (Kret, Citation2015). Yet how exactly pupil size affects facial emotion perception for observers is not clear. Pupils dilate when one experiences either positively- or negatively-valenced arousal (Partala & Surakka, Citation2003), yet people tend to associate angry faces with smaller pupils than happy faces (Hess, Citation1975; Kret, Citation2018) and rate sad faces with higher sadness intensity when they have smaller pupils (Harrison et al., Citation2007). Further, studies of other types of autonomic nervous system activity, such as heart rate and skin conductance, show that there may be distinctive patterns of activation for specific emotions (Kreibig, Citation2010), suggesting potential for variability beyond the continua for valence and intensity.
One way to examine how observers respond to others’ signals of emotion and arousal on a physiological level is through measuring their pupil dilation. When an observer’s pupils dilate in response to another individual’s dilated pupils, it is known as pupillary contagion and is proposed to be a sign of sensitivity to and sharing of others’ arousal (Fawcett et al., Citation2016, Citation2017; Kret et al., Citation2015). Note that mimicry of pupillary constriction does not occur as reliably as dilation mimicry (Aktar et al., Citation2020; Kret et al., Citation2014), and when it does, it is not related to social factors (Kelsey et al., Citation2019; Kret et al., Citation2015; Prochazkova, Prochazkova, Giffin, Scholte, et al., Citation2018), supporting that it is pupil dilation and the underlying arousal reactions which are being shared across individuals in pupillary contagion. Most research on pupillary contagion has used faces with neutral expressions and the few studies that have examined emotional expressions have had somewhat mixed results. Two studies suggested that ratings of others’ sadness, but not other emotions, were affected by observed pupil sizes, though interestingly it was faces with smaller pupils that were rated to be more sad (Harrison et al., Citation2006, Citation2007). In a small subsample of nine participants, Harrison et al. (Citation2006) also showed that only sad faces elicited pupillary contagion. However a more recent study with a larger sample size, more robust statistical methods, and a revised task to encourage attention to the eyes showed comparable pupillary contagion across neutral, sad, happy, and angry facial expressions with participants’ pupils dilating more to images with large and medium pupils than to small pupils (Carsten et al., Citation2019).
While group-level effects for emotion perception and reactivity can be informative, it is also critical to explain inter-individual differences in responding. Why might some participants show stronger responses than others and what might that tell us about the underlying mechanisms for emotional reactivity and arousal sharing? Previous research on pupillary contagion has examined whether the trait of empathy predicts one’s degree of pupillary contagion. In one case, participants’ self-reported empathy predicted how much they were influenced by the model’s pupil size when rating sadness intensity (Harrison et al., Citation2007), while two larger studies found no link between pupillary contagion and self-reported empathy (Axelsson & Fawcett, Citation2021; Carsten et al., Citation2019). However, there are many more potential individual difference factors that could modulate pupillary contagion, including both demographic factors, such as age, gender, and socioeconomic status, as well as psychosocial factors, such as anxiety, depression, and sleep problems. How each of these factors might relate to the processing of and reaction to others’ emotions and arousal is described below.
Demographic factors
When it comes to gender differences, women have been consistently shown to be more skilled at emotion perception than men (Olderbak et al., Citation2019; Thompson & Voyer, Citation2014) and both women’s self-ratings of emotional experience and their facial emotional expressions are more affected by others’ emotions than men’s are (Dimberg & Lundquist, Citation1990; Doherty et al., Citation1995; Sonnby-Borgström et al., Citation2008; Stellar et al., Citation2012). However, when emotional stimuli are presented outside of conscious awareness, this difference can disappear (Sonnby-Borgström et al., Citation2008), suggesting an important role for socialisation and learned responses in these gender differences. Further, physiological measures of arousal, such as skin conductance, heart rate, and startle responses, to emotional stimuli show mixed results with some revealing gender differences (Bianchin & Angrilli, Citation2012) and others not (Codispoti et al., Citation2008; Partala & Surakka, Citation2003). Finally, when participant gender differences were examined in previous studies of pupillary contagion, none were found (Axelsson & Fawcett, Citation2021; Kret & De Dreu, Citation2017).
While emotion perception abilities decline with age (Olderbak et al., Citation2019; Ruffman et al., Citation2008), emotional reactivity shows more complex development. For example, compared to younger adults, older adults report greater emotional reactivity to negative images and show greater startle responses to negative images, but also less heart rate deceleration than younger adults (Smith, Hillman, et al., Citation2005). Older adults also attribute less negative emotionality to others’ expressions (Riediger et al., Citation2011). Interestingly, a study in which participants were asked to draw pupil sizes onto happy and angry faces to examine whether larger pupils were associated with positive emotion, showed that the difference between the pupil sizes for the two types of faces increased across adulthood (Kret, Citation2018).
Socioeconomic status (SES), a factor that encompasses income level, education, and occupation, has also been shown to be related to emotion perception and reactivity. Individuals with lower SES show stronger brain activation in areas devoted to attending to others’ mental states (Muscatell et al., Citation2012) and are more accurate when judging others’ emotions (Kraus et al., Citation2010). Lower SES relates in particular to feelings of compassion and physiological responses to others’ distress (Stellar et al., Citation2012). While SES cannot be experimentally manipulated directly, manipulations of increased social power and status have been found to negatively affect both perspective taking and emotion identification (Galinsky et al., Citation2006).
Psychosocial factors
Anxiety is associated with a heightened attentional bias toward threatening stimuli, including emotional faces (Bar-Haim et al., Citation2007) and with stronger emotional reactivity to elicited emotions (Macatee & Cougle, Citation2013). In contrast, depression has been linked to dampened emotional reactivity, for both positive and negative stimuli (Bylsma et al., Citation2008), but also heightened attention to sad faces and reduced attention to happy faces (Duque et al., Citation2014; Lazarov et al., Citation2018). Further, some studies fail to find relations between depression and pupil dilation responses to emotional stimuli (Yrttiaho et al., Citation2021).
Sleep loss can contribute to poorer emotion regulation and mood, for example evaluating stimuli more negatively and reacting more strongly to negative stimuli when sleep deprived (Tempesta et al., Citation2018). In one study using pupillometry, sleep-deprived participants showed greater pupil dilation in expectation of negative stimuli when compared to non-sleep deprived participants (Franzen et al., Citation2009). When examining the processing of emotional faces specifically, sleep-deprived participants have more difficulty accurately identifying sad faces and show greater neural reactivity to angry and fearful faces (Cote et al., Citation2014).
The current study
We aimed to clarify the role of emotional expressions in pupillary contagion and to examine potential individual differences in the phenomenon. Thus, we examined pupil dilation in response to the eye regions of faces with neutral or emotional expressions and variations in displayed pupil sizes. The emotional expressions included happiness, sadness, fear, anger, and neutral and each was shown with small, medium, or large static pupils. The task allowed us to examine not only pupillary contagion across neutral and emotionally expressive eyes, but also overall dilation effects to the different expressions. We included both male and female models in the stimuli to increase generalizability and ecological validity. Participants were parents taking part in a longitudinal study of early-life distress and child development in Finland. In order to examine individual differences in pupil responses, we also assessed a variety of demographic and psychosocial factors.
We chose to show only the eye region in our stimuli – from eyebrows to the top of the nose – for several reasons. First, we wanted to focus attention on the eyes without giving participants a task which could lead to pupil dilation confounds due to individual differences in cognitive effort and would make the task less comparable to previous research on pupillary contagion (Aktar et al., Citation2020; Axelsson & Fawcett, Citation2021; Fawcett et al., Citation2017). Further, we expected that emotion perception would be reliable even with only the eye region visible, given that emotion recognition relies significantly more on the eye region than the mouth, as evidenced in eye tracking research (Wells et al., Citation2016), particularly for sadness, anger, and fear (Smith, Hillman, et al., Citation2005). Finally, while a few recent studies have shown that surgical masks covering the lower half of the face impair emotion recognition, accuracy is still high: typically around 80%, with the most common mistakes being to confuse fearful and sad expressions with each other (Marini et al., Citation2021; Parada-Fernández et al., Citation2022). Further, the eye region alone has been shown to elicit biases in emotion detection (Fox & Damjanovic, Citation2006).
We expected to replicate the pupillary contagion phenomenon that has been robustly demonstrated for neutral expressions (Axelsson & Fawcett, Citation2021; Fawcett et al., Citation2017; Kret & De Dreu, Citation2017), as well as with somewhat more mixed results for emotional expressions (Carsten et al., Citation2019; Harrison et al., Citation2006, Citation2007). We also expected to see greater overall pupil dilation in response to angry eyes compared to other emotions (Carsten et al., Citation2019). For the individual difference measures, we did not have a priori hypotheses. While there are theoretical reasons to propose differences in reaction to others’ emotions for any of the variables we examined, there have been few consistent relationships and there is very little research looking at individual differences in pupillary contagion specifically.
Method
Participants
Participants included 453 parents (59.6% women and 40.4% men), a subsample of those who were already involved in the longitudinal FinnBrain Birth Cohort Study (www.finnbrain.fi). An additional 6 participants participated in the data collection, but were excluded for poor data quality (see “Pupil data” below). The main aim of the FinnBrain study is to examine the effects of early-life distress on the children’s brain development and mental health. Originally, N = 3808 expecting mothers and N = 2624 fathers/spouses were recruited to the study during the first ultrasound visit at gestational week (gwk) 12 between 2011 and 2014 (Karlsson et al., Citation2018). Around N = 1000 families were invited to a more intensive follow-up including, for example, child and parent neuropsychological visits, pediatric visits, brain imaging, and collection of biological samples.
When children were 5.5 years of age, between 2018 and 2021, those parents who had been actively participating with their child in previous study visits were invited to take part in a neuropsychological assessment as part of the FinnBrain Child Development and Parental Functioning Lab visits. This visit included neuropsychological tests (Cogstate test battery, Stroop, WAIS-IV digit span), verbal IQ tasks (WAIS-IV, verbal comprehension tasks), three different eye tracking tasks assessing attention to emotional faces, and completion of questionnaires about their mental health and distress (depressive and anxiety symptoms assessed with the Edinburgh Postnatal Depression Scale and Symptom Check List 90 anxiety subscale) and their sleep (the Athens Insomnia Scale and the Basic Nordic Sleep Questionnaire). The research was performed in accordance with the Declaration of Helsinki. The Joint Ethics Committee of Turku University Hospital and University of Turku approved the study protocol. Written informed consent was obtained from all participants.
Altogether, N = 525 mothers and fathers participated in the study visit between 2018 and 2021, and N = 459 of these provided eye-tracking data. A power analysis indicated that this sample size would be more than sufficient to for the models planned to test the current research questions. That is, G*Power (version 3.1.9.6; Faul et al., Citation2007) suggests a sample size of 253 for linear multiple regression with power of .80, alpha of .05, estimated effect size of f2 = 0.10, and 26 predictors (i.e. model pupil size, emotion, and all of the individual difference factors and their interactions that are included in the models). The demographic characteristics of the participants of the current study are presented in .
Table 1. Descriptive statistics.
Stimuli
Pupil data was collected from the participant’s left eye with a sampling frequency of 500 Hz using an eye tracker (EyeLink1000+; SR Research, Canada) while the participant viewed stimuli on a computer monitor. The researcher sat in the same, dimly lit room as the participant but was separated by a curtain to avoid interference. The researcher used an independent computer to manage the measurement.
The original images used for the pupillary contagion stimuli were obtained from the set of Karolinska Directed Emotional Faces (Lundqvist et al., Citation1998). The images showing emotionally neutral eyes included six different models (BF19NES, BF13NES, BF01NES, AM14NES, AM10NES and AM08NES) and the images showing emotionally expressive eyes included two models, each displaying the emotions anger, fear, happiness, and sadness (AM05ANS, AM05ANS, AM05HAS, AM05SAS, BF01AFS, BF01ANS, BF01HAS, BF01SAS). It was originally intended to include four individuals in the emotion trials, however due to a programming error, only two (one man and one woman) were used in the final procedure. As in similar studies (Axelsson & Fawcett, Citation2021; Fawcett et al., Citation2017), each image was edited in Adobe Photoshop to black and white and showed only the eye region, including the eyebrows and the upper part of the nose at approximately life size on screen. Then, each iris and pupil were replaced with a standard iris and one of three standard-size pupils. The small pupil was 40% smaller and the large pupil was 40% larger than the medium pupil.
Questionnaires
Data from the following questionnaires were used in the current study. Depressive symptoms were screened with The Edinburgh Postnatal Depression Scale (EPDS) which is a widely used questionnaire (e.g. Gibson et al., Citation2009), sensitive to both pre- and postnatal depression, and consists of 10 questions scored on a 4-point Likert scale from 0 to 3 (Cox et al., Citation1987). The total sum scores range from 0 to 30 and a continuous total sum score was used (Cronbach’s alpha = 0.872; data missing from 74 participants). General anxiety symptoms were assessed with the anxiety subscale of the Symptom Checklist-90 (SCL-90; Derogatis et al., Citation1973; Holi et al., Citation1998), with reportedly good psychometric properties (Prinz et al., Citation2013). This subscale consists of 10 items scored on a 5-point Likert scale from 0 to 4 and the range of the total sum score is 0–40. In this study, a continuous sum score was used (Cronbach’s alpha = 0.871; data missing from 74 participants). Two questions derived from the Basic Nordic Sleep Questionnaire (BNSQ; Partinen & Gislason, Citation1995) were used to measure subjective sleep complaints. Specifically, questions regarding sleep duration (i.e. “How many hours do you typically sleep per night?”) and night wakings (i.e. “During the past month, on how many nights have you woken during the night?” with five options ranging from “1. never or less than once a month” to “5. daily or almost daily”) were used in the analyses. Sleep data was missing for 12 participants. Descriptive statistics for the questionnaire variables are presented in .
Procedure
As part of a longer eye tracking session, (i.e. three tasks lasting altogether around 30 min with pre-preparations), the pupillary contagion task was presented as the first task and took a total of approximately six minutes. Participants first saw the 18 trials with emotionally neutral eyes (six individuals with three pupil sizes each) to avoid any priming of reactivity or sensitivity to arousal cues from the emotional faces and then saw the 48 trials with emotionally expressive eyes (two individuals displaying each of four emotions with three pupil sizes, each image shown twice). The trials were presented in one of two semi-randomized orders. In each trial, there was first a black fixation cross on a grey background for 1000 ms and then the eye image was displayed with the same grey background for 5000 ms. Participants were instructed to simply view the images.
Data processing and analysis
Pupil data
Raw gaze data files (.edf) were exported from EyeLink and then the files were reformatted and converted from .edf to .asc and from .asc to .csv format using the EyeLink library from the Fieldtrip toolbox (version 20190922; Oostenveld et al., Citation2011) and a custom script so that they could be processed in TimeStudio (Nyström et al., Citation2016), an open-source programme based in MATLAB (Timestudio version 3.19; timestudioproject.org; MATLAB, Citation2015). Eyelink records pupil sizes in arbitrary units. In Timestudio, individual samples of pupil size were rejected if they were outside of the range 1000–3500, to remove blinks which often include recordings of partial pupils as the eye closes and opens. This range was selected after visual inspection of the full sample indicated that samples outside that range were brief spikes and not sustained or gradual peaks as would be expected for true pupil measurements. Samples with high acceleration (i.e. a change of more than 6 pupil size units per length of sample (i.e. 2 ms) squared) and 5 samples (i.e. 10 ms) before and after the high acceleration samples were also excluded for being likely outliers, as it would be unnatural for a pupil to change size that quickly. Gaps in data 20 samples or fewer were interpolated linearly and then a moving average filter over 50 samples was employed. To obtain the dependent variable of pupil size change from baseline, the baseline was calculated during a screen with a fixation cross (specifically, from 1000 to 500 ms before the stimulus image appeared) and that was subtracted from the average pupil size during 2000–5000 ms of the stimuli being shown. The first 2000 ms of the stimuli viewing were excluded from analysis due to variation in pupil size for the light reflex response that adds noise to the data. Trials with less than 50% of data samples recorded were excluded (n = 888 trials; 2.59%). Six participants out of the original 459 who participated in the task were excluded for having five or fewer included trials, leaving 453 in the final sample who together had an average of only 1.69% of trials excluded. Pupil size change scores with values more than 2.5 standard deviations from the mean for all included participants were replaced with the next closest non-outlying value. In all, 763 trial scores (2.59%) were replaced for being outliers.
Demographics
Gender and age in years were reported by participants at the first questionnaire during pregnancy (gwk 24; age is not reported for one participant). Information about monthly income in euros was initially collected with eight levels at gwk 14, but given the uneven distribution of scores, it was collapsed into four levels with approximately equal numbers of participants (i.e. ≤1500 euros, 1501–2500 euros, 2501–3500 euros, > 3500 euros) at each level (income data is missing for 18 participants). Education was originally assessed with 6 levels at gwk 14, but given uneven distribution was collapsed into 3 roughly equal ones (High School or Vocational education, Polytechnic education, and University-level education or higher; education data is missing for 18 participants). Occupation was rated as one of four levels based on official Finnish registries (salaried, intermediate, working class, or not classified/never worked/unemployed; occupation data is not reported for 1 participant). Following previous research on SES using objective measures (Kraus et al., Citation2009; Stellar et al., Citation2012), we examined whether it was possible to create one score for SES by combining the three measures of income, education, and occupation. In a confirmatory factor analysis, these three variables were found to load significantly onto one factor (see Table A1 in the Supplementary Results; https://osf.io/r6eg3/), thus they were standardised and combined into a single continuous SES score. Descriptive statistics for the demographic variables are presented in .
Analyses
Given that the stimuli for the neutral and emotional conditions varied in the number of individual models shown and in the number of trials, data from each condition was analysed separately. The first analysis step for each condition was to conduct a group-level analysis to determine overall responses to the stimuli. Following that, analyses were carried out to assess individual differences in demographic variables and in psychosocial variables. All of the regression analyses were run on trial-level data using linear mixed-effects modelling in jamovi (The jamovi project, Citation2021) with the GAMLj module 2.0.1 (Galluci, Citation2019) which was developed in R (R Core Team, Citation2020) and includes R’s lme4 package (Bates et al., Citation2015). The factor analysis for SES was run using the lavaan package within jamovi (Rosseel, Citation2012). All models included a random intercept for participant and trial number. Statistical models included all variables of interest and their interactions with the main variables of model pupil size (for both conditions), and model emotion (for the emotion condition). Significant main effects were followed up with Bonferroni-adjusted post hoc tests and significant interactions were followed up with analyses of simple effects. Tables in the manuscript present the results of the fixed effect omnibus tests for the key models. Full model results and the results of the preliminary and follow-up analyses are presented in the Supplementary Results on OSF (https://osf.io/r6eg3/).
Results
Emotionally neutral eyes
Group level analysis
In the model predicting pupil size change from model pupil size (see supplementary Table A2), model pupil size was a significant predictor (F(2, 596.63) = 23.68, p < .001) and there was more dilation to models’ large than medium (t(517.28) = 3.09, p = .006) and large than small (t(3215.66) = 6.43, p < .001), but not medium than small (t(325.86) = 1.51, p = .397) pupils.
Demographic predictors
To assess individual differences in pupillary contagion for emotionally neutral eyes due to demographic factors, we added to the group level model the variables of age, participant gender, SES score, and their interactions with model pupil size (see and supplementary Table A3). There was a significant effect of model pupil size (F(2, 973.36) = 23.09, p < .001), with overall more dilation to large than medium (t(540.90) = 3.07, p = .007) and large than small (t(3303.23) = 6.36, p < .001) pupils, but not medium than small pupils (t(337.98) = 1.51, p = .394). There was also a significant interaction between model pupil size and continuous SES score (F(2, 7263.63) = 4.72, p = .009, see ). Simple effects analyses examining the effect of SES for each model pupil size show that pupil dilation responses to large pupil stimuli decrease with increasing SES (t(1157.10) = −2.53, p = .012), while there were no significant relations between SES and responses to either medium or small pupil stimuli.
Figure 1. Variation in pupillary contagion in the neutral expression trials by socioeconomic status. Pupil size changes are shown using the arbitrary units recorded by the eye tracker. SES score is a continuous, standardised variable. Shaded areas indicate 95% confidence intervals.
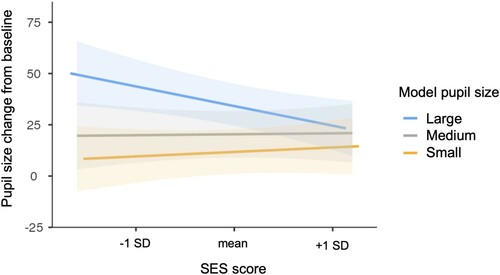
Table 2. Model results for neutral trials, analysis of demographic factors.
Psychosocial predictors
To assess individual differences in pupillary contagion for neutral expressions due to psychosocial factors, we added to the group level model the additional variables of sleep duration, night waking, depression, and anxiety, and their interactions with model pupil size (see and supplementary Table A4). Only model pupil size was significant in this model (F(2, 740.65) = 12.32, p < .001), with greater dilation to large than small pupil images (t(3109.97) = 4.79, p < .001). None of the psychosocial factors or their interactions with model pupil size had a significant effect on reactions to eyes with neutral expressions.
Table 3. Model results for neutral trials, analysis of psychosocial factors.
Emotionally expressive eyes
Group level analysis
The initial model predicting pupil size change in response to emotionally expressive eyes included the variables model pupil size, emotion, and their interaction (see and supplementary Table A5). There were significant effects for model pupil size (F(2, 1593.78) = 33.57, p < .001) with greater dilation to large than medium and medium than small pupils, and for emotion (F(3, 1389.48) = 23.53, p < .001), with greater dilation to angry eyes than any other emotion, greater dilation to sad and happy than fearful eyes, and no significant difference in dilation for happy and sad eyes. There was no significant interaction between model pupil size and emotion, suggesting that the pupillary contagion effect did not differ across the four emotions (see ).
Figure 2. Pupillary contagion across emotional expressions. Pupil size changes are shown using the arbitrary units recorded by the eye tracker. Bars indicate 95% confidence intervals.
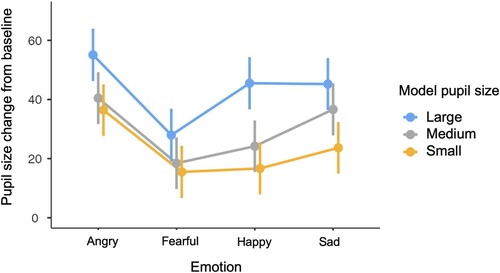
Table 4. Model results for emotion trials, group-level analysis.
Demographic predictors
To assess individual differences in pupillary contagion to emotionally expressive eyes due to demographic factors, the model from the group level analysis was run with the additional variables of SES score, age, participant gender, and their interactions with model pupil size and with emotion (see and supplementary Table A6). A significant effect of size (F(2, 1630.25) = 29.92, p < .001) revealed greater dilation to images with large than medium (t(1308.77) = 5.03, p < .001) and medium than small pupils (t(1919.35) = 2.53, p = .034). A significant effect of emotion (F(3, 1410.81) = 18.84, p < .001) revealed greater dilation to angry than fearful (t(1429.63) = 7.12, p < .001) or happy (t(2017.76) = 4.33, p < .001) eyes and greater dilation to happy (t(1132.62) = 2.86, p = .026) and sad (t(1959.11) = 4.78, p < .001) than fearful eyes. Further, participants’ pupils dilated less overall with increasing age (F(1, 435.64) = 5.22, p = .023) and men overall had greater pupil dilation than women (F(1, 435.38) = 5.87, p = .016).
Table 5. Model results for emotion trials, analysis of demographic factors.
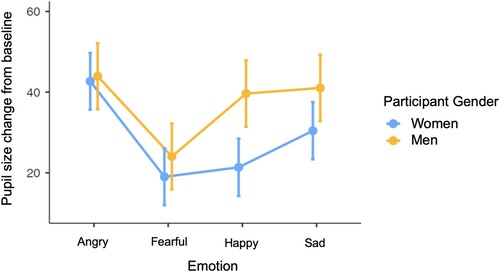
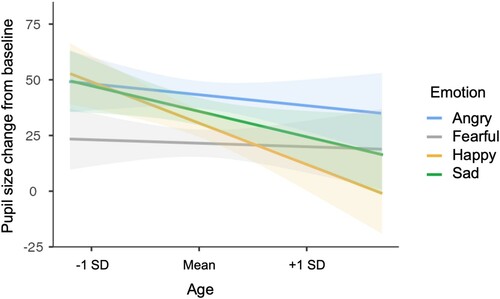
There was a significant interaction between emotion and participant gender (F(3, 20020.01) = 4.59, p = .003; see ). Simple effects analyses indicated that men showed greater dilation than women for both happy (t(1213.71) = 3.89, p < .001) and sad (t(1217.39) = 2.25, p = .024) eyes. There was also a significant interaction between emotion and participant age (F(3, 19984.47) = 3.93, p = .008; see ) with simple effects analyses indicating decreasing dilation responses to happy (t(1208.41) = −3.60, p < .001) and sad (t(1210.06) = −2.21, p = .027) eyes with increasing age.
Psychosocial predictors
To assess individual differences in pupillary contagion to emotionally expressive eyes due to psychosocial factors, the model resulting from the group level analysis was run with the additional variables of anxiety, depression, sleep duration, night waking, and their interactions with model pupil size and with emotion (see and supplementary Table A7). There were significant effects of model pupil size (F(2,1792.80) = 21.02, p < .001) and emotion (F(3,1494.12) = 15.36, p < .001) with the same pattern of results as in the demographics analysis. None of the psychosocial predictors or their interactions had a significant effect in the model (all p’s > .05).
Table 6. Model results for emotion trials, analysis of psychosocial factors.
Discussion
Being sensitive and responsive to others’ emotions and subtle cues of arousal can be an important factor for social interaction. In the current study, we examined how adults respond to others’ eye regions when they vary in both emotional expression – neutral, sad, angry, happy, and fearful – and in pupil size, a marker of arousal. Participants’ own pupil dilation varied with the dilation of the observed individuals, both for neutral and emotionally expressive eye regions, in line with previous research demonstrating this phenomenon of pupillary contagion (Aktar et al., Citation2020; Carsten et al., Citation2019; Fawcett et al., Citation2016, Citation2017; Kret et al., Citation2015). In addition, we uncovered group-level effects of emotional expression, such that participants responded with greatest pupil dilation to angry eyes, greater dilation to sad than fearful eyes, and similar dilation for happy compared to either sad or fearful eyes. The pattern of results for the group-level effects of emotional expression on pupil dilation and pupillary contagion are in line with results from a similar large study published recently by Carsten et al. (Citation2019). They found comparable levels of pupillary contagion using images of full faces across the emotions of anger, happiness, sadness, and neutral expressions, and overall greater pupil dilation in response to angry facial expressions than any other emotion. Two earlier studies examining how pupillary contagion relates to emotional expression found effects only for sad facial expressions (Harrison et al., Citation2007, Citation2009), but they were limited by small sample sizes and lack of control over gaze scanning patterns that could have influenced the pupil results.
Beyond the group-level effects, we also examined individual differences in pupillary contagion and pupil dilation responses to different emotional expressions. These individual difference factors included demographics: gender, age, and SES, as well as psychosocial factors: anxiety, depression, and sleep problems. While previous research on emotional reactivity suggests that these factors could influence pupillary contagion and pupil dilation to others’ emotions, the predicted effects were not always clear and thus we did not have a priori hypotheses for these effects.
The only demographic factor that had an impact on the degree of pupillary contagion shown by participants was SES. Higher SES was associated with lower degree of pupillary contagion for neutral, though not for emotionally expressive eyes. The effect was particularly seen in increased reactivity to others’ large pupils with decreasing SES. Together, this suggests that SES might be more related to reactivity to others’ subtle emotional signals – noticing pupils that indicate higher arousal in the context of neutral face, rather than noticing pupil size variation in the context of a clear emotional expression.
Consistent with the current results, previous research on SES has shown that individuals with higher SES consistently perform lower on cognitive tasks related to emotion (Kraus et al., Citation2010), as well as on low-level emotional reactivity tasks (Stellar et al., Citation2012), which can be seen as more comparable to pupillary contagion. Thus, our findings are in line with others showing that higher SES is related to lower sensitivity to others’ emotions. In contrast, one recent study showed that in South African mothers, lower SES was related to less difference in pupil reactivity for images of distressed relative to happy infants (Yrttiaho et al., Citation2021). It is important to note several key differences between that study and ours that could lead to different findings. First, our stimuli displayed adults while their stimuli displayed infant faces and it could be that the effect of SES on emotional reactivity differs by age of target. Second, the SES levels in their samples were much lower than ours, potentially making them difficult to compare. It could be that both very high and very low SES lead to dampened emotional reactivity, though possibly for different reasons. Third, there could be cultural differences between South Africa and Finland that contribute to different patterns of responses in the different studies.
As to why the SES and pupillary contagion relationship might exist, it could be that higher levels of SES shift one’s attention away from other people and their subtle emotional signals, similar to the effects that manipulating people’s feelings of social power has on their perspective-taking (Galinsky et al., Citation2006). When a person has control over others and does not need to rely on them, due to their status, perspective-taking and empathy may become less important. In contrast, being more reliant on others and more stressed about one’s economic situation could also create a greater need to tune in to others’ emotional states to maintain social support networks. On the other hand, given the automaticity of the pupillary contagion response, another explanation could be that people who are already more emotionally reactive to others (due to genetics, early-life environmental factors and/or upbringing) are drawn toward careers in education or caring fields, which tend to be associated with fewer years of education and lower salaries. Studies to disentangle the causal chain between pupillary contagion and factors related to SES will be important for future research.
When it comes to gender, both women and men demonstrated pupillary contagion to a similar degree. However, we found that in the emotion trials, men had overall greater dilation than women and an interaction effect revealed that this was due to men reacting to happy and sad expressions with greater pupil dilation than did women. This finding contrasts previous research suggesting greater reactivity to emotion in women than men, however these effects tend to be found in tasks that involve more cognitive processing, such as identifying emotions (Olderbak et al., Citation2019; Thompson & Voyer, Citation2014) or giving ratings of one’s own emotional experiences (Dimberg & Lundquist, Citation1990; Doherty et al., Citation1995; Sonnby-Borgström et al., Citation2008; Stellar et al., Citation2012). In contrast, it is not uncommon for there to be no gender differences in initial physiological responses to others’ emotions (Codispoti et al., Citation2008; Partala & Surakka, Citation2003). In addition, greater overall dilation effects could also be related to physiological differences (e.g. Fan et al., Citation2009) or cognitive factors, such as taking more effort to process the stimuli.
With increasing age, participants reacted to the emotionally expressive stimuli with less pupil dilation overall, and particularly so for happy and sad expressions. While previous research has shown effects of age on emotional reactivity, these have been complex with different measures indicating greater or lesser arousal with age (Smith, Hillman, et al., Citation2005). The current results show that for both a positive (happy) and negative (sad) emotional expression, physiological reactivity appears to decrease with age, at least within our sample of primarily middle-aged adults. Effects of age were not significant for angry or fearful expressions, however. Given that anger and fear were the expressions with the highest and lowest overall dilation responses, it could be that ceiling and floor effects played a role in their not showing age effects.
When it comes to the psychosocial factors examined in relation to pupillary reactions to others’ emotions, there were surprisingly no effects. Neither participants’ pupillary contagion, nor their overall pupil dilation responses to images of others’ neutral and emotionally expressive eyes varied based on their symptoms of anxiety or depression, or their sleep problems. It could be that there was not sufficient variation in these variables for differences to be revealed, though failure to find effects of psychosocial variables on pupil response to infant facial expressions has also been reported (Yrttiaho et al., Citation2021). It may thus be possible that automatic physiological reactions to emotion cues are not as affected by these factors as are aspects of emotion processing that are further downstream in cognition.
Together, the results revealed few individual differences in pupillary contagion, which suggests that the phenomenon is a robust and fundamental one which may underlie other emotional processes. That is, it could be that factors such as anxiety and depression affect emotional responding and attention further downstream in processing such that they affect the cognition around emotion more so than the initial physiological responses to it. This suggestion is further supported by the fact that pupillary contagion develops early in life (Fawcett et al., Citation2016, Citation2017) and does not appear to have significant developmental shifts (Aktar et al., Citation2020).
A few limitations of the current study should also be noted. First, the sample was recruited from only one country, which could limit generalizability. However, in comparison to many studies in the field which recruit participants from university student populations, the current sample was more diverse in terms of age and SES. Second, the individual difference measures may not all have had sufficient variability to detect relations. Focusing on samples that are already known to be high on certain factors, such as anxiety and depression, could allow more effects to be revealed. Finally, the stimuli used posed emotional expressions and static pupil sizes which, while high in experimental control, can be criticised for having lower ecological validity. That is, we cannot be certain that participants’ responses to the current stimuli mirror what occurs in natural social interaction. Future studies should consider using more naturalistic emotional facial expressions and dynamic pupil sizes.
The current study demonstrated that pupillary contagion is a robust and social phenomenon. Whether participants were observing others’ emotionally neutral or emotionally expressive eyes, their own pupil size tended to increase with increases in the pupil sizes of the observed individuals, suggesting a sharing of arousal. Further, this pattern was found across ages and genders and was not significantly impacted by sleep problems or symptoms of anxiety or depression. In fact, the only individual difference measure that was related to participants’ degree of pupillary contagion was their SES, with higher SES participants showing less pupillary contagion for emotionally neutral eyes. This modulating factor underscores that pupillary contagion is more than a reaction to light and instead has a social-cognitive basis (Prochazkova, Prochazkova, Giffin, Steven Scholte, et al., Citation2018). Together, the findings show that pupillary contagion is a robust and largely automatic process, though individual differences do occur and can give insight into the roots of this social phenomenon.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aktar, E., Raijmakers, M. E. J., & Kret, M. E. (2020). Pupil mimicry in infants and parents. Cognition and Emotion, 34(6), 1160–1170. https://doi.org/10.1080/02699931.2020.1732875
- Axelsson, E. L., & Fawcett, C. (2021). Humans’ pupillary contagion extends to cats and dogs. Social Cognitive and Affective Neuroscience, 16(1–2), 153–166. https://doi.org/10.1093/scan/nsaa138
- Bar-Haim, Y., Lamy, D., Pergamin, L., Bakermans-Kranenburg, M. J., & Van Ijzendoorn, M. H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin, 133(1), 1–24. https://doi.org/10.1037/0033-2909.133.1.1
- Bates, D., Mächler, M., Bolker, B. M., & Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. https://doi.org/10.18637/jss.v067.i01
- Becker, D. V., Anderson, U. S., Mortensen, C. R., Neufeld, S. L., & Neel, R. (2011). The face in the crowd effect unconfounded: Happy faces, not angry faces, are more efficiently detected in single- and multiple-target visual search tasks. Journal of Experimental Psychology: General, 140(4), 637–659. https://doi.org/10.1037/a0024060
- Bianchin, M., & Angrilli, A. (2012). Gender differences in emotional responses: A psychophysiological study. Physiology and Behavior, 105(4), 925–932. https://doi.org/10.1016/j.physbeh.2011.10.031
- Bradley, M. M., Miccoli, L., Escrig, M. a., & Lang, P. J. (2008). The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology, 45(4), 602–607. https://doi.org/10.1111/j.1469-8986.2008.00654.x
- Bylsma, L. M., Morris, B. H., & Rottenberg, J. (2008). A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review, 28(4), 676–691. https://doi.org/10.1016/j.cpr.2007.10.001
- Carsten, T., Desmet, C., Krebs, R. M., & Brass, M. (2019). Pupillary contagion is independent of the emotional expression of the face. Emotion, 19(8), 1343–1352. https://doi.org/10.1037/emo0000503
- Codispoti, M., Surcinelli, P., & Baldaro, B. (2008). Watching emotional movies: Affective reactions and gender differences. International Journal of Psychophysiology, 69(2), 90–95. https://doi.org/10.1016/j.ijpsycho.2008.03.004
- Cote, K. A., Mondloch, C. J., Sergeeva, V., Taylor, M., & Semplonius, T. (2014). Impact of total sleep deprivation on behavioural neural processing of emotionally expressive faces. Experimental Brain Research, 232(5), 1429–1442. https://doi.org/10.1007/s00221-013-3780-1
- Cox, J. L., Holden, J. M., & Sagovsky, R. (1987). Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry, 150(JUNE), 782–786. https://doi.org/10.1192/bjp.150.6.782
- Derogatis, L. R., Lipman, R. S., & Covi, L. (1973). SCL-90: An outpatient psychiatric rating scale – preliminary report. Psychopharmacogy Bulletin, 9(1), 13–28.
- Dimberg, U., & Lundquist, L. O. (1990). Gender differences in facial reactions to facial expressions. Biological Psychology, 30(2), 151–159. https://doi.org/10.1016/0301-0511(90)90024-Q
- Doherty, R. W., Orimoto, L., Singelis, T. M., Hatfield, E., & Hebb, J. (1995). Emotion contagion: Gender and occupational differences. Psychology of Women Quarterly, 19(3), 355–371. https://doi.org/10.1111/j.1471-6402.1995.tb00080.x
- Duque, A., Sanchez, A., & Vazquez, C. (2014). Gaze-fixation and pupil dilation in the processing of emotional faces: The role of rumination. Cognition & Emotion, 28(8), 1347–1366. https://doi.org/10.1080/02699931.2014.881327
- Fan, X., Hearne, L., Lei, B., Miles, J. H., Takahashi, N., & Yao, G. (2009). Weak gender effects on transient pupillary light reflex. Autonomic Neuroscience: Basic and Clinical, 147(1–2), 9–13. https://doi.org/10.1016/j.autneu.2008.12.010
- Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. https://doi.org/10.3758/BF03193146
- Fawcett, C., Arslan, M., Falck-Ytter, T., Roeyers, H., & Gredebäck, G. (2017). Human eyes with dilated pupils induce pupillary contagion in infants. Scientific Reports, 7(1), 9601. https://doi.org/10.1038/s41598-017-08223-3
- Fawcett, C., Wesevich, V., & Gredebäck, G. (2016). Pupillary contagion in infancy: Evidence for spontaneous transfer of arousal. Psychological Science, 27(7), 997–1003. https://doi.org/10.1177/0956797616643924
- Fox, E., & Damjanovic, L. (2006). The eyes are sufficient to produce a threat superiority effect. Emotion, 6(3), 534–539. https://doi.org/10.1037/1528-3542.6.3.534
- Franzen, P. L., Buysse, D. J., Dahl, R. E., Thompson, W., & Siegle, G. J. (2009). Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biological Psychology, 80(3), 300–305. https://doi.org/10.1016/j.biopsycho.2008.10.010
- Galinsky, A. D., Magee, J. C., Ena Inesi, M., & Gruenfeld, D. H. (2006). Power and perspectives not taken. Psychological Science, 17(12), 1068–1074. https://doi.org/10.1111/j.1467-9280.2006.01824.x
- Galluci, M. (2019). GAMLj: General analyses for linear models. [jamovi module]. https://gamlj.github.io/
- Gibson, J., McKenzie-Mcharg, K., Shakespeare, J., Price, J., & Gray, R. (2009). A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatrica Scandinavica, 119(5), 350–364. https://doi.org/10.1111/j.1600-0447.2009.01363.x
- Hansen, C. H., & Hansen, R. D. (1988). Finding the face in the crowd: An anger superiority effect. Journal of Personality and Social Psychology, 54(6), 917–924. https://doi.org/10.1037/0022-3514.54.6.917
- Harrison, N. A., Gray, M. A., & Critchley, H. D. (2009). Dynamic pupillary exchange engages brain regions encoding social salience. Social Neuroscience, 4(3), 233–243. https://doi.org/10.1080/17470910802553508
- Harrison, N. A., Singer, T., Rotshtein, P., Dolan, R. J., & Critchley, H. D. (2006). Pupillary contagion: Central mechanisms engaged in sadness processing. Social Cognitive and Affective Neuroscience, 1(1), 5–17. https://doi.org/10.1093/scan/nsl006
- Harrison, N. A., Wilson, C. E., & Critchley, H. D. (2007). Processing of observed pupil size modulates perception of sadness and predicts empathy. Emotion, 7(4), 724–729. https://doi.org/10.1037/1528-3542.7.4.724
- Hess, E. H. (1975). The role of pupil size in communication. Scientific American, 233(5), 110–119. https://doi.org/10.1038/scientificamerican1175-110
- Holi, M. M., Sammallahti, P. R., & Aalberg, V. A. (1998). A Finnish validation study of the SCL-90. Acta Psychiatrica Scandinavica, 97(1), 42–46. https://doi.org/10.1111/j.1600-0447.1998.tb09961.x
- Karlsson, L., Tolvanen, M., Scheinin, N. M., Uusitupa, H. M., Korja, R., Ekholm, E., Tuulari, J. J., Pajulo, M., Huotilainen, M., Paunio, T., & Karlsson, H. (2018). Cohort profile: The FinnBrain Birth Cohort Study (FinnBrain). International Journal of Epidemiology, 47(1), 15–16j. https://doi.org/10.1093/ije/dyx173
- Kelsey, C. M., Krol, K. M., Kret, M. E., & Grossmann, T. (2019). Infants’ brain responses to pupillary changes in others are affected by race. Scientific Reports, 9(1), 1–10. https://doi.org/10.1038/s41598-019-40661-z
- Kraus, M. W., Côté, S., & Keltner, D. (2010). Social class, contextualism, and empathic accuracy. Psychological Science, 21(11), 1716–1723. https://doi.org/10.1177/0956797610387613
- Kraus, M. W., Piff, P. K., & Keltner, D. (2009). Social Class, sense of control, and social explanation. Journal of Personality and Social Psychology, 97(6), 992–1004. https://doi.org/10.1037/a0016357
- Kreibig, S. D. (2010). Autonomic nervous system activity in emotion: A review. Biological Psychology, 84(3), 394–421. https://doi.org/10.1016/j.biopsycho.2010.03.010
- Kret, M. E. (2015). Emotional expressions beyond facial muscle actions. A call for studying autonomic signals and their impact on social perception. Frontiers in Psychology, 6(May), 1–10. https://doi.org/10.3389/fpsyg.2015.00711
- Kret, M. E. (2018). The role of pupil size in communication. Is there room for learning? Cognition and Emotion, 32(5), 1139–1145. https://doi.org/10.1080/02699931.2017.1370417
- Kret, M. E., & De Dreu, C. K. W. (2017). Pupil-mimicry conditions trust in partners: Moderation by oxytocin and group membership. Proceedings of the Royal Society B: Biological Sciences, 284(1850), 1–10. https://doi.org/10.1098/rspb.2016.2554
- Kret, M. E., Fischer, A. H., & De Dreu, C. K. W. (2015). Pupil mimicry correlates with trust in in-group partners with dilating pupils. Psychological Science, 26(9), 1401–1410. https://doi.org/10.1177/0956797615588306
- Kret, M. E., Roelofs, K., Stekelenburg, J. J., & de Gelder, B. (2013). Emotional signals from faces, bodies and scenes influence observers’ face expressions, fixations and pupil-size. Frontiers in Human Neuroscience, 7(DEC), 1–9. https://doi.org/10.3389/fnhum.2013.00810
- Kret, M. E., Tomonaga, M., & Matsuzawa, T. (2014). Chimpanzees and humans mimic pupil-size of conspecifics. PloS One, 9(8), e104886. https://doi.org/10.1371/journal.pone.0104886
- Laeng, B., Sirois, S., & Gredebäck, G. (2012). Pupillometry: A window to the preconscious? Perspectives on Psychological Science, 7(1), 18–27. https://doi.org/10.1177/1745691611427305
- Lazarov, A., Ben-Zion, Z., Shamai, D., Pine, D. S., & Bar-Haim, Y. (2018). Free viewing of sad and happy faces in depression: A potential target for attention bias modification. Journal of Affective Disorders, 238(February), 94–100. https://doi.org/10.1016/j.jad.2018.05.047
- Lundqvist, D., Flykt, A., & Öhman, A. (1998). The Karolinska Directed Emotional Faces – KDEF. CD ROM from Department of Clinical Neuroscience, Psychology section, Karolinska Institutet.
- Lundqvist, D., Juth, P., & Öhman, A. (2014). Using facial emotional stimuli in visual search experiments: The arousal factor explains contradictory results. Cognition and Emotion, 28(6), 1012–1029. https://doi.org/10.1080/02699931.2013.867479
- MATLAB. (2015). version 8.5.0 (R2015a). Natick, Massachusetts: The MathWorks Inc.
- Macatee, R. J., & Cougle, J. R. (2013). The roles of emotional reactivity and tolerance in generalized, social, and health anxiety: A multimethod exploration. Behavior Therapy, 44(1), 39–50. https://doi.org/10.1016/j.beth.2012.05.006
- Marini, M., Ansani, A., Paglieri, F., Caruana, F., & Viola, M. (2021). The impact of facemasks on emotion recognition, trust attribution and re-identification. Scientific Reports, 11(1), 1–14. https://doi.org/10.1038/s41598-021-84806-5
- Muscatell, K. A., Morelli, S. A., Falk, E. B., Way, B. M., Pfeifer, J. H., Galinsky, A. D., Lieberman, M. D., Dapretto, M., & Eisenberger, N. I. (2012). Social status modulates neural activity in the mentalizing network. NeuroImage, 60(3), 1771–1777. https://doi.org/10.1016/j.neuroimage.2012.01.080
- Nyström, P., Falck-Ytter, T., & Gredebäck, G. (2016). The TimeStudio project: An open source scientific workflow system for the behavioral and brain sciences. Behavior Research Methods, 48(2), 542–552. https://doi.org/10.3758/s13428-015-0616-x
- Olderbak, S., Wilhelm, O., Hildebrandt, A., & Quoidbach, J. (2019). Sex differences in facial emotion perception ability across the lifespan. Cognition and Emotion, 33(3), 579–588. https://doi.org/10.1080/02699931.2018.1454403
- Oostenveld, R., Fries, P., Maris, E., & Schoffelen, J. M. (2011). FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011.
- Parada-Fernández, P., Herrero-Fernández, D., Jorge, R., & Comesaña, P. (2022). Wearing mask hinders emotion recognition, but enhances perception of attractiveness. Personality and Individual Differences, 184(March 2021), 111195. https://doi.org/10.1016/j.paid.2021.111195
- Partala, T., & Surakka, V. (2003). Pupil size variation as an indication of affective processing. International Journal of Human-Computer Studies, 59(1–2), 185–198. https://doi.org/10.1016/S1071-5819(03)00017-X
- Partinen, M., & Gislason, T. (1995). Basic Nordic Sleep Questionnaire (BNSQ): A quantitated measure of subjective sleep complaints. Journal of Sleep Research, 4, 150–155. https://doi.org/10.1111/j.1365-2869.1995.tb00205.x
- Prinz, U., Nutzinger, D. O., Schulz, H., Petermann, F., Braukhaus, C., & Andreas, S. (2013). Comparative psychometric analyses of the SCL-90-R and its short versions in patients with affective disorders. BMC Psychiatry, 13(1), 1–9. https://doi.org/10.1186/1471-244X-13-104
- Prochazkova, E., Prochazkova, L., Giffin, M. R., Scholte, H. S., De Dreu, C. K. W., & Kret, M. E. (2018). Pupil mimicry promotes trust through the theory-of-mind network. Proceedings of the National Academy of Sciences of the United States of America, 115(31), E7265–E7274. https://doi.org/10.1073/pnas.1803916115
- Prochazkova, E., Prochazkova, L., Giffin, M. R., Steven Scholte, H., De Dreu, C. K. W., & Kret, M. E. (2018). Reply to Mathôt and Naber: Neuroimaging shows that pupil mimicry is a social phenomenon. Proceedings of the National Academy of Sciences of the United States of America, 115(50), E11566–E11567. https://doi.org/10.1073/pnas.1815545115
- R Core Team. (2020). R: A Language and environment for statistical computing (Version 4.0) [Computer software]. https://cran.r-project.org
- Riediger, M., Voelkle, M. C., Ebner, N. C., & Lindenberger, U. (2011). Beyond “happy, angry, or sad?”: Age-of-poser and age-of-rater effects on multi-dimensional emotion perception. Cognition and Emotion, 25(6), 968–982. https://doi.org/10.1080/02699931.2010.540812
- Rosseel, Y. (2012). Lavaan: An R package for structural equation modeling. Journal of Statistical Software, 48(2), https://doi.org/10.18637/jss.v048.i02
- Ruffman, T., Henry, J. D., Livingstone, V., & Phillips, L. H. (2008). A meta-analytic review of emotion recognition and aging: Implications for neuropsychological models of aging. Neuroscience and Biobehavioral Reviews, 32(4), 863–881. https://doi.org/10.1016/j.neubiorev.2008.01.001
- Savage, R. A., Lipp, O. V., Craig, B. M., Becker, S. I., & Horstmann, G. (2013). In search of the emotional face: Anger versus happiness superiority in visual search.. Emotion, 13(4), 758–768. https://doi.org/10.1037/a0031970
- Smith, D. P., Hillman, C. H., & Duley, A. R. (2005). Influences of age on emotional reactivity during picture processing. Journals of Gerontology – Series B Psychological Sciences and Social Sciences, 60, 1. https://doi.org/10.1093/geronb/60.1.P49
- Sonnby-Borgström, M., Jönsson, P., & Svensson, O. (2008). Gender differences in facial imitation and verbally reported emotional contagion from spontaneous to emotionally regulated processing levels: Cognition and neurosciences. Scandinavian Journal of Psychology, 49(2), 111–122. https://doi.org/10.1111/j.1467-9450.2008.00626.x
- Stellar, J. E., Manzo, V. M., Kraus, M. W., & Keltner, D. (2012). Class and compassion: Socioeconomic factors predict responses to suffering. Emotion, 12(3), 449–459. https://doi.org/10.1037/a0026508
- Tempesta, D., Socci, V., De Gennaro, L., & Ferrara, M. (2018). Sleep and emotional processing. Sleep Medicine Reviews, 40, 183–195. https://doi.org/10.1016/j.smrv.2017.12.005
- The jamovi project. (2021). jamovi (Version 1.6) [Computer software]. https://www.jamovi.org
- Thompson, A. E., & Voyer, D. (2014). Sex differences in the ability to recognise non-verbal displays of emotion: A meta-analysis. Cognition and Emotion, 28(7), 1164–1195. https://doi.org/10.1080/02699931.2013.875889
- Wells, L. J., Gillespie, S. M., Rotshtein, P., & Key, A. (2016). Identification of emotional facial expressions: Effects of expression, intensity, and sex on eye gaze. PLoS ONE, 11(12), e0168307. https://doi.org/10.1371/journal.pone.0168307
- Yiend, J. (2010). The effects of emotion on attention: A review of attentional processing of emotional information. Cognition and Emotion, 24(1), 3–47. https://doi.org/10.1080/02699930903205698
- Yrttiaho, S., Bruwer, B., Zar, H. J., Donald, K. A., Malcolm-Smith, S., Ginton, L., Hoffman, N., Vuong, E., Niehaus, D., Leppänen, J. M., & Stein, D. J. (2021). Pupillary and attentional responses to infant facial expressions in mothers across socioeconomic variations. Child Development, 92(3), e236–e251. https://doi.org/10.1111/cdev.13503