?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Salient landmarks enhance route learning. We hypothesised that semantically salient nostalgic landmarks would improve route learning compared to non-nostalgic landmarks. In two experiments, participants learned a route through a computer-generated maze using directional arrows and wall-mounted pictures. On the test trial, the arrows were removed, and participants completed the maze using only the pictures. In the nostalgia condition, pictures were of popular music artists and TV characters from 5 to 10 years ago. In the control condition, they were recent pictures of these same artists and characters. In Experiment 1, in the test trial, participants in the nostalgia condition completed the maze faster than controls. Experiment 2 conceptually replicated these findings and extended them by exploring boundary conditions. Participants had to learn two mazes sequentially. In Maze 1, we placed nostalgic/control landmarks only at non-decision points (whereas we placed them at decision points in Experiment 1). In Maze 2, we placed nostalgic/control landmarks at decision points during acquisition but removed them in the test trial (whereas they were present in the test trial in Experiment 1). In both mazes, participants in the nostalgia (compared to control) condition completed the test trial faster.
The salience of a stimulus increases the rate at which it acquires associative strength, due to the elevated attention paid to more salient stimuli (Mackintosh, Citation1976). In typical associative learning paradigms, more salient stimuli become associated with the neutral stimulus faster than less salient ones by acquiring the finite available associative strength at a faster rate (Redhead, Citation2007; Redhead & Pearce, Citation1995). For example, food will become associated with a loud bell faster than it would with a quiet bell. Likewise, in a spatial learning paradigm, larger landmarks are more likely to facilitate learning the position of a hidden goal than smaller ones (Chamizo et al., Citation2006). Davis et al. (Citation2017) demonstrated that brightly coloured landmarks facilitated performance on route-learning tasks. Specifically, a salient landmark at a junction where participants have to learn to go either left or right will more readily become associated with the correct response than will less salient landmarks. However, the salience of a stimulus is not determined exclusively by its physical attributes, like size or colour. Salience is also a function of the personal, cultural, or historical meaning of a stimulus (i.e. semantic salience; Caduff & Timpf, Citation2008; Seetharaman et al., Citation2021). The aim of the current research is to explore whether, by virtue of their high personal meaning, nostalgic stimuli can enhance route learning.
The role of semantic salience
Caduff and Timpf (Citation2008) proposed that the salience of a landmark used in a navigation task depends on three factors: (1) The visual salience of the landmark, such as size, contrast, luminance, and colour. For example, colour pictures of objects facilitated route learning compared to black-and-white pictures of the same objects (Davis & Therrien, Citation2012). (2) The structural salience of a landmark, such as its proximity to decision points within a maze. For instance, landmarks that were placed at decision points (intersections within a maze) were more readily remembered than landmarks that were placed at non-decision points (e.g. simple turns; Kessels et al., Citation2011). (3) The landmark’s semantic salience to the wayfinder (i.e. salience due to personal, cultural, or historical meaning). For example, people living with dementia rated the salience of a landmark as partly due to meaningfulness, which included subjective factors of personal and emotional significance that linked the landmarks to participants’ pasts (Seetharaman et al., Citation2021). Further, installing personalised memory boxes (e.g. pictures, personal memorabilia) outside the rooms of people living with dementia increased these individuals’ ability to locate their rooms by 45% (Nolan et al., Citation2002).
The relative importance of semantic and visual salience of landmarks has been assessed by using eye tracking to map fixation points on a scene. Henderson et al. (Citation2019) created a visual-salience map and a semantic-salience map of a single scene. They created the visual-salience map by analysing each section of the scene to identify images with, for example, high luminance and contrast. They created the semantic-salience map by presenting sections of the scene to participants, who then rated how much meaning each section contributed to the scene as a whole. For instance, a section depicting bricks within a wall of a building would be rated less semantically salient than a section depicting the intersection between two buildings. The researchers found that, when participants looked at the scene as a whole, they were more likely to fixate on sections that were high (compared to low) in visual salience as well as on sections that were high (compared to low) in semantic salience. However, when the positive correlation between the sections’ visual and semantic salience was statistically controlled, only semantic salience uniquely predicted increased attention (i.e. fixation points).
Nostalgia and semantic salience
We propose that nostalgia entails a high level of semantic salience owing to its connection to unique and meaningful personal memories. Nostalgia has been defined as “a sentimental longing or wistful affection for the past” (The New Oxford Dictionary of English, Citation1998, p. 1266). Empirical evidence dovetails with this dictionary definition. Nostalgia typically refers to fond and personally meaningful memories of childhood and/or close relationships (Hepper et al., Citation2012, Citation2014). It is a predominantly positive and low-arousal emotion (Sedikides & Wildschut, Citation2016; Van Tilburg et al., Citation2018) but includes a tinge of sadness, giving rise to its distinctive, bittersweet affective signature (Frankenbach et al., Citation2021; Leunissen et al., Citation2021). The emotion is usually evoked by events that are appraised as temporally distant, unique, and pleasant, yet irretrievably lost (Hepper et al., Citation2021; Van Tilburg et al., Citation2019). As a result, one typically feels tender, content, and happy but with a sense of longing. The relevance of nostalgic memories to unique, bittersweet, and meaningful experiences of the self with close others accounts for its social, self-oriented, existential, and future-oriented psychological benefits (Sedikides et al., Citation2015; Wildschut & Sedikides, Citation2020). In light of the emotion’s high degree of semantic salience (i.e. personal meaning), nostalgic landmarks should enhance performance on route-learning tasks. We tested this hypothesis in two experiments. Both were approved by the University of Southampton’s Ethics Committee.
Experiment 1
In Experiment 1, we instructed participants to learn a specific route through a virtual maze. During acquisition, participants followed directional arrows to the end of the maze. There were local landmarks on the walls of the maze and four large distal landmarks in cardinal directions beyond the walls, which could be seen from most points in the maze. After three acquisition trials, the directional arrows were removed, and participants followed the learned route through the maze using the local and distal landmarks (test trial).
We completed Experiment 1 in 2021. The experiment used a between-subjects design: nostalgia versus control. In both conditions, the landmarks in the virtual maze depicted popular music artists and TV characters. In the nostalgia condition, the landmarks depicted music artists and TV characters who were popular during the participants’ youth. For example, the pictures included Doctor Who, a British TV character, as portrayed by the actor Matt Smith between 2011 and 2014. In the control condition, the landmarks depicted these same music artists and TV characters but with more recent pictures. For example, the pictures included Dr. Who as portrayed by the actor Jodie Whittaker between 2017 and 2022 (). We hypothesised that participants in the nostalgia condition would complete the test trial faster (i.e. have shorter latencies) than those in the control condition.
Figure 1. Example of nostalgic versus control pictures.
Note. Left image illustrates a nostalgic picture. Right image illustrates a control picture.

By manipulating nostalgia with matched pictures of the same familiar artists and TV characters, we intended to provide landmarks that differed in evoked nostalgia but were equally familiar and recognisable for participants in both conditions (Davis & Therrien, Citation2012). By carefully matching the nostalgic images with parallel control images, we also sought to rule out potential alternative explanations in terms of visual and structural salience (Caduff & Timpf, Citation2008), or to at least render such alternative explanations less plausible. We controlled for visual salience by using colour images with identical dimensions in both conditions. We controlled for structural salience by placing the matched images in the same position within the maze in both conditions.
Method
Participants
We based the sample size on an a priori power analysis. Our effect-size estimate was informed by an experiment testing the effect of emotional landmarks on spatial memory (Palmiero & Piccardi, Citation2017).Footnote1 We predicated our power analysis on this study’s effect size (d = 0.78). Detecting an effect of this magnitude with power = .80 and two-tailed α = .05 requires 54 participants (G*Power 3.1; Faul et al., Citation2007).
We exceeded this target to hedge against attrition. Specifically, we recruited 60 participants (39 women, 21 men) via the crowdsourcing website Prolific Academic (https://www.prolific.co). We established eligibility criteria based on age (18–26 years) and location (U.K.). All participants were compensated £2.00 for taking part in the 25-minute experiment. We randomly assigned participants to conditions: nostalgia (n = 29; 20 women, 9 men) and control (n = 31; 19 women, 12 men). Participant age ranged from 18 to 26 years (M = 21.17, SD = 2.16).
Materials and procedure
We posted a link to the experiment on Prolific Academic. First, we assessed participants’ prior experience with video games (“How much experience do you have with playing computer games?”; 1 = no experience at all, 7 = a lot of previous and recent experience; M = 5.57, SD = 1.63) and virtual reality environments (“How much experience do you have with playing computer games which involve a virtual environment technology [e.g. flight simulation]?”; 1 = no experience at all, 7 = a lot of previous and recent experience; M = 3.55, SD = 1.77). We did so to ascertain the absence of pre-existing differences between the randomised nostalgia and control conditions on these control variables.
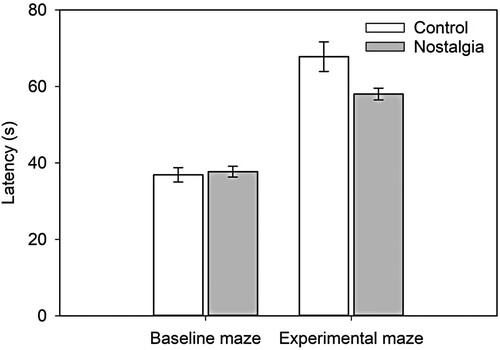
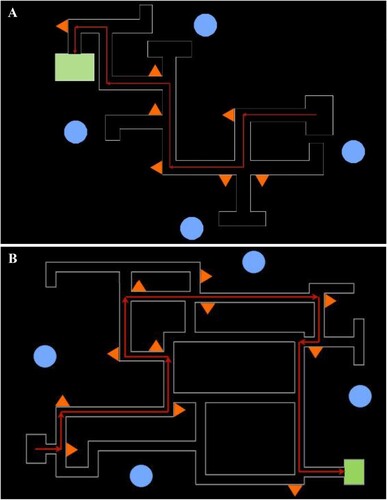
Next, we trained participants on a maze containing only neutral local and distal landmarks, to obtain a baseline measure of spatial ability ( and A). Before participants completed the baseline test trial, they completed three acquisition trials. On these acquisition trials, we instructed them to follow the directional arrows in order to find the end of the maze, and to look at the wall-mounted local landmarks in order to aid navigation. Following the acquisition trials, we removed the directional arrows and instructed participants to complete the baseline test trial (Mtest trial 1 = 37.30 s, SDtest trial 1 = 9.09 s). We then placed participants in the experimental maze with either nostalgic or control (i.e. recent) landmarks located at decision points (e.g. intersections; B). After three acquisition trials, which included the directional arrows, we again instructed participants to follow the route without the arrows (Mtest trial 2 = 63.08 s, SDtest trial 2 = 17.06 s). The route through the baseline maze was shorter than that of the experimental maze, which accounts for the shorter mean latency on the baseline test trial.
Figure 2. Virtual maze presentation from participants’ perspective.
Note. Participants’ perspective of the maze including a neutral wall-mounted image functioning as a local landmark, with a distal landmark located beyond the maze, and a directional arrow positioned at a junction.

Figure 3. Route layout for the baseline and experimental maze in Experiment 1.
Note. Schematic of the baseline maze (A) and experimental maze (B) including layout, route, and landmark positions. The red arrows illustrate the specified route and the green box indicates the end destination. The blue circles mark the position of neutral distal landmarks. The orange triangles indicate the position of the neutral/nostalgic/control local landmarks.
Following completion of the test trial in the experimental maze, we showed participants six pictures, five of which had been randomly selected from the six images presented at decision points and one of which had not been presented in the maze. For each picture, participants indicated whether they had seen it in the maze and, if so, which direction (left, right, or straight on) they took at the landmark. We summed the number of correct responses (M = 4.08, SD = 1.13). Our procedure was similar to those used by O’Malley et al. (Citation2018), who showed participants four landmarks that had been presented at decision points in the maze, and Grzeschik et al. (Citation2021), who showed participants a selection of six landmarks from a set of 12 presented in the maze. Data pertaining to these responses were missing for nine participants due to technical difficulties.
Finally, participants rated their felt nostalgia (i.e. a manipulation check) on three items (e.g. “Right now, I am feeling quite nostalgic”; 1 = strongly disagree, 6 = strongly agree; Wildschut et al., Citation2006). We created an index of felt nostalgia by averaging participants’ responses to these three items (M = 3.26, SD = 1.70, α = .97).Footnote2
Results and discussion
Manipulation check
As intended, felt nostalgia was significantly higher in the nostalgia (M = 3.92, SD = 1.59) than control (M = 2.63, SD = 0.71) condition, F(1, 58) = 9.88, p = .003, η2 = .145, 90% CI = [.032, .281].Footnote3 The nostalgic (compared to control) landmarks successfully induced felt nostalgia.
Control variables
Participants in the nostalgia (M = 5.28, SD = 1.69) and control (M = 5.84, SD = 1.55) conditions did not differ significantly on prior experience with computer games, F(1, 58) = 1.81, p = .184, η2 = .030, 90% CI = [0, .130]. The nostalgia (M = 3.45, SD = 1.86) and control (M = 3.65, SD = 1.70) conditions did not differ on prior experience with virtual-reality environments either, F(1, 58) = 0.18, p = .671, η2 = .003, 90% CI = [0, .063].
Route-learning performance
We display in mean latency to complete the baseline and experimental mazes as a function of condition (shorter latency indicates faster completion of the maze). We conducted a 2 (condition: nostalgia, control) × 2 (maze: baseline, experimental) mixed Analysis of Variance (ANOVA) with time to complete the test trials (s) as dependent variable. Results revealed a significant effect of maze, F(1, 58) = 139.51, p < .001, η2 = .706, 90% CI = [.592, .771]. Test-trial latency was shorter in the baseline maze than in the longer experimental maze. The effect of condition was not significant, F(1, 58) = 2.80, p = .10, η2 = .046, 90% CI = [0, .156]. However, the crucial Condition × Maze interaction was significant, F(1, 58) = 5.98, p = .018, η2 = .093, 90% CI = [.009, .221]. To probe this interaction, we conducted a simple effects analysis. The effect of condition was not significant in the baseline maze, F(1, 58) = 0.12, p = .729, η2 = .002, 90% CI = [0, .056], but was so in the experimental maze, F(1, 58) = 5.28, p = .025, η2 = .083, 90% CI = [.006, .208]. Next, we repeated the analysis of test-trial latency in the experimental maze, controlling for test-trial latency in the baseline maze (by entering it as a covariate). The difference between the nostalgia and control conditions remained significant, F(1, 57) = 5.92, p = .018, = .094, 90% CI = [.009, .220]. As hypothesised, in the experimental maze, participants in the nostalgia condition completed the test trial faster than those in the control condition.
Recalled direction at landmarks
We showed participants six pictures, five of which had been included in the experimental maze, and asked them which direction they took at these landmarks. Supporting the postulated semantic salience of nostalgic (compared to control) landmarks, the number of directions that were correctly associated with the included landmarks was significantly higher in the nostalgia (M = 4.48, SD = 0.71) than control (M = 3.69, SD = 1.32) condition, F(1, 49) = 6.95, p = .011, η2 = .124, 90% CI = [.016, .268].
Experiment 2
Experiment 1 findings offered initial support for the hypothesis that nostalgic (compared to control) landmarks enhance route learning in a virtual environment. The objectives of Experiment 2 were to replicate conceptually this effect, to test its potential boundary conditions, and to examine its downstream consequences in terms of goal setting.
We tested the effect of nostalgic (compared to control) landmarks on route learning in two mazes. These mazes were designed to examine two potential boundary conditions for the effect of nostalgic landmarks on performance. In Experiment 1, the nostalgic/control landmarks were always located at decision points (e.g. intersections) within the maze. Kessels et al. (Citation2011) found that, after seeing a film of a virtual maze, participants’ recognition accuracy for landmarks that were placed at non-decision points (i.e. simple forced turns) was lower than for landmarks that were placed at decision points, indicating that they had attended less to the former than the latter. This finding suggests the first potential boundary condition: do nostalgic (compared to control) landmarks still facilitate route learning when the landmarks are only placed at non-decision points within the maze? We addressed this question in Maze 1 by placing the nostalgic/control landmarks at non-decision points and the neutral landmarks at decision points.
Notwithstanding Kessels et al.’s (Citation2011) finding, if the action of the nostalgic cues is governed by associative principles, we expected to replicate Experiment 1 results in Maze 1. This expectation is based on the principle of potentiation, which entails that a more salient cue facilitates the association between a simultaneously-presented less salient cue and the outcome or response (Bouton et al., Citation1987). In the current paradigm, we would expect semantically salient nostalgic landmarks (compared to control) to potentiate the neutral landmarks at the decision points, thereby allowing the neutral landmarks to more readily form an association with the correct response, and thus facilitate route learning.
There have been several demonstrations of this potentiation principle in the spatial domain (Cole et al., Citation2011; Graham et al., Citation2006; Pearce et al., Citation2006). Horne and Pearce (Citation2011), for example, required rats to find one of two submerged platforms that were situated in diagonally opposite corners of a rectangular swimming pool. In the experimental condition, during acquisition, wall-mounted black or white landmarks were attached to the walls forming the corners where the platforms were located (in a control condition the landmarks were in the correct corners on only half of the acquisition trials and thus uncorrelated with platform position). The landmarks and platforms were removed on the test trial. When trained with black landmarks, rats in the experimental condition spent more time searching near the geometrically correct corners of the pool (i.e. those associated with the platforms, where the short wall was to the left of the long wall) even though the landmarks were not present. The black landmarks had potentiated the geometric cues; associations formed between landmark and geometric cues during acquisition meant that seeing the geometric cues on test evoked a memory of the landmark that was strongly associated with the platform (Pearce, Citation2009). Rats trained with less salient white landmarks did not evince this potentiation effect. In the current experiment, we expected that the semantically salient nostalgic (compared to control) landmarks would more strongly potentiate the neutral landmarks at decision points and thus facilitate route-learning.
We now turn to the second boundary condition. In Maze 1, the nostalgic/control landmarks were located at non-decision points but were still present on the test trial. Horne and Pearce’s (Citation2011) findings suggest that, as long as the nostalgic/control landmarks are present on the acquisition trials, they need not be present on the test trial to facilitate performance. We implemented this second boundary condition in Maze 2. In this maze, the nostalgic/control landmarks were located at decision points during acquisition but removed altogether during the test trial, leaving only neutral distal landmarks beyond the maze boundaries. Again, we expected stronger potentiation of the neutral distal landmarks, and thus enhanced route-learning, in the nostalgia condition than in the control condition.
A second objective of Experiment 2 was to test the effect of nostalgic (compared to control) landmarks on future goal setting. We hypothesised that, if nostalgia enhances route-learning, this should have positive downstream consequences for goal setting in the spatial domain. According to the expectancy-value perspective on goal setting (Levy & Baumgardner, Citation1991), goal choice is a function of estimated ability to achieve the goal and the value assigned to the goal. If nostalgia enhances route-learning, it should increase perceived ability to complete a challenging future route-learning task and thus result in higher goal setting. We tested this hypothesis after participants completed Maze 1 by offering them a choice between navigating an additional maze that was either easy or difficult (all participants completed the same Maze 2).
Our third objective was to address the potential role of positive affect (PA) and negative affect (NA). Previous studies have used pictures from the International Affective Picture System (IAPS; Lang et al., Citation2008) to examine the effect of positive and negative affective landmarks on route learning and topographical memory in virtual environments, with mixed results. Whereas some findings indicated that negative affective landmarks facilitate performance more than do positive ones (Balaban et al., Citation2017), other evidence pointed to the opposite conclusion (Ruotolo et al., Citation2019), or indicated that positive and negative affective landmarks have equivalent facilitative effects (Palmiero & Piccardi, Citation2017). In contrast to our present focus on the discrete emotion of nostalgia, these previous studies focused on generalised PA and NA. For example, whereas some used pictures of “beach”, “skier”, and “sailing” as positive affective landmarks (Palmiero & Piccardi, Citation2017), others used pictures of “smiling babies”, “puppies”, and “kiss scene” (Ruotolo et al., Citation2019). Despite this crucial difference with our present approach, it is prudent to assess whether the effect of nostalgic (compared to control) landmarks is due to PA/NA.
Method
Participants
We aimed to recruit at least the same number of participants as in Experiment 1, and exceeded this target. Sixty-two high-school students (48 women, 14 men; M = 16.95, SD = 0.46) attended a series of open days at the School of Psychology, University of Southampton. On a voluntary basis, participants completed a 30-minute study as part of a research demonstration activity. We randomly assigned them to the nostalgia (n = 31; 23 women, 8 men) or control (n = 31; 25 women, 6 men) conditions.Footnote4
Materials and procedure
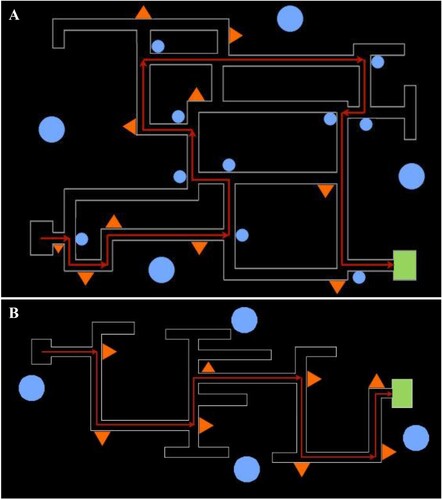
We ran the experiment in a computer classroom containing 35 computers. As in Experiment 1, the mazes offered a first-person perspective and the nostalgic/control landmarks in the maze were similar to those used in Experiment 1. The mazes included neutral distal landmarks that could be seen beyond the walls and from most points within the maze. The experimenter instructed participants to navigate through the mazes using directional arrows and to pay attention to the local and distal landmarks to help learn the route. Maze 1 (, upper panel) involved three acquisition trials and one test trial (Mtest trial 1 = 57.47 s, SDtest trial 1 = 25.95 s). Following completion of Maze 1, we assessed goal setting by asking participants if they would like to complete either a difficult or easy maze next; 52 out of 62 participants indicated that they wanted to complete a difficult maze (84%). In fact, all participants completed the same maze next. Maze 2 (, lower panel) again involved three acquisition trials and one test trial (Mtest trial 2 = 53.22 s, SDtest trial 2 = 24.17 s). In Maze 1, the nostalgic/control landmarks were present only at non-decision points in the acquisition trials as well as the test trial. In Maze 2, the nostalgic/control landmarks were present in the acquisition trials, but we removed them on the test trial so only neutral distal landmarks remained.
Figure 5. Route layout for Maze 1 and Maze 2 in Experiment 2.
Note. Schematic of Maze 1 (A) and Maze 2 (B) layout, route, and landmark positions. The red arrows illustrate the specified route and the green box indicates the end destination. The large blue circles mark the position of neutral distal landmarks. The small blue circles indicate the position of neutral local landmarks. The orange triangles indicate the position of nostalgic/control local landmarks.
Following this, participants completed the same 3-item measure of felt nostalgia (i.e. manipulation check) as in Experiment 1 (e.g. “Right now, I am feeling quite nostalgic”; 1 = strongly disagree, 6 = strongly agree; M = 2.83, SD = 1.29, α = .97). In addition, they completed 2-item measures of PA (e.g. “During the navigation task, I felt happy”; 1 = strongly disagree, 6 = strongly agree; M = 4.61, SD = 0.94, α = .91) and NA (e.g. “During the navigation task, I felt sad”; 1 = strongly disagree, 6 = strongly agree; M = 1.56, SD = 0.76, α = .83).
Next, in a free recall task, participants wrote down as many of the landmarks they had seen in the mazes as they could recall. We included this free recall task to ascertain that the popular music artists and TV characters presented in the nostalgia and control conditions were equally familiar and recognisable to participants; that is, to rule out the possibility that nostalgic (compared to control) landmarks facilitated performance because they were more familiar or recognisable. Finally, as a control variable, we assessed how experienced participants were in playing computer games (1 = I play every day, 4 = I never play; M = 3.13, SD = 0.88).Footnote5
Results and discussion
Manipulation check
As intended, participants in the nostalgia condition (M = 3.22, SD = 1.25) felt more nostalgic than those in the control condition (M = 2.44, SD = 1.23), F(1, 60) = 6.02, p = .017, η2 = .091, 90% CI = [.009, .216]. The manipulation was effective.
Control variables
Participants in the nostalgia (M = 3.00, SD = 1.03) and control (M = 3.26, SD = 0.68) conditions did not differ significantly on computer game experience, F(1, 60) = 1.35, p = .250, η2 = .022, 90% CI = [0, .113]. The nostalgia (M = 7.16, SD = 1.97) and control (M = 7.39, SD = 2.32) conditions did not differ significantly on the number of landmarks recalled either, F(1, 60) = 0.17, p = .681, η2 = .003, 90% CI = [0, .060]. Thus, there was no evidence to suggest that the nostalgic landmarks were more familiar or recognisable than the control landmarks (the numerical pattern was in the opposite direction, with more landmarks recalled in the control than nostalgia condition).
Route-learning performance
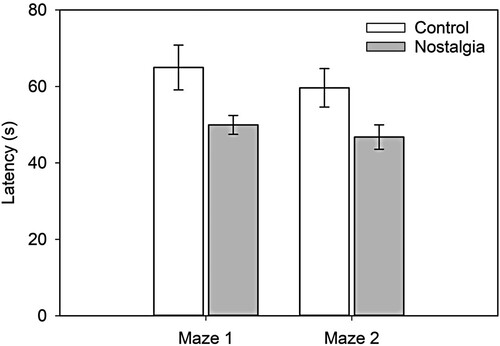
We display in the test-trial latencies in Mazes 1 and 2 as a function of condition. A 2 (condition: nostalgia, control) × 2 (maze: Maze 1, Maze 2) mixed ANOVA on time to complete the test trials (s) revealed that the maze main effect was not significant, F(1, 60) = 1.24, p = .269, = .020, 90% CI = [0, .110]. A significant effect of condition indicated that, as hypothesised, latencies were significantly shorter in the nostalgia than control condition, F(1, 60) = 8.28, p = .005,
= .121, 90% CI = [.021, .252]. The Condition × Maze interaction was not significant, F(1, 60) = 0.08, p = .779,
= .001, 90% CI = [0, .047]. Latencies to complete the test trial were shorter in the nostalgia than control condition and this effect did not vary as a function of maze. These results conceptually replicate and extend Experiment 1 findings.
Goal setting
We obtained a significant effect of condition on goal setting, χ2(1, N = 62) = 4.29, p = .038, φ = .263, Odds Ratio = 5.04. The percentage of participants who, after completing Maze 1, preferred to complete a difficult (than easy) maze next was higher in the nostalgia condition (29/31 = 94%) than in the control condition (23/31 = 74%). Nostalgia (compared to control) conduced to more ambitious goal setting. We tested whether this effect of nostalgia on goal setting was mediated by (i.e. flowed from) nostalgia’s positive effect on route-learning performance. That is, better performance on the prior route-learning task (i.e. shorter latencies) may increase confidence in the spatial domain, resulting in more ambitious goal setting. To test the indirect effect, we implemented the bootstrapping method (MacKinnon et al., Citation2004) with the PROCESS macro (Hayes, Citation2022; 5,000 bootstrap samples).Footnote6 Indeed, the indirect effect (ab) of nostalgia (compared to control) on goal setting via route-learning performance (indexed as Maze 1 latency) was significant, ab = 0.76, 95% CI = [0.076, 2.260]. The residual direct effect of nostalgia (compared to control) on goal setting was not significant, b = 0.68, 95% CI = [−0.949, 2.686], Z = 0.94, p = .349.
Positive affect and negative affect
Participants in the nostalgia (M = 4.69, SD = 0.93) and control (M = 4.53, SD = 0.96) conditions did not differ significantly on PA, F(1, 60) = 0.45, p = .503, η2 = .008, 90% CI = [0, .079]. The nostalgia (M = 1.52, SD = 0.80) and control (M = 1.60, SD = 0.74) conditions did not differ on NA either, F(1, 60) = 0.17, p = .681, η2 = .003, 90% CI = [0, .060]. Participants reported significantly more positive than negative affect (i.e. a positivity offset) in both the nostalgia (F[1, 30] = 143.84, p < .001, η2 = .827, 90% CI = [.713, .875]) and control (F[1, 30] = 124.50, p < .001, η2 = .806, 90% CI = [.679, .859]) condition. The facilitative effect of nostalgic (compared to control) landmarks on route learning and ensuing goal setting was not due to differences in generalised PA or NA.
Summary
We had three objectives in Experiment 2. Relevant to the first, key objective, we replicated and extended Experiment 1 findings. Consistent with the principle of potentiation (Cole et al., Citation2011; Graham et al., Citation2006; Horne & Pearce, Citation2011; Pearce, Citation2009; Pearce et al., Citation2006), nostalgic (compared to control) landmarks facilitated route learning when nostalgic/control landmarks were placed at non-decision points (Maze 1) and when nostalgic/control landmarks were placed at decision points during acquisition but removed on the test trial (Maze 2). These results underscore that route learning involves more than the formation of strong associations between salient landmarks and directions. Via potentiation, salient landmarks can also facilitate associations between less salient neighbouring landmarks and directions. Turning to our second objective, results supported the hypothesis that, by virtue of their enhanced route-learning performance, participants in the nostalgia (vs. control) condition evinced more ambitious goal setting. That is, compared to controls, those in the nostalgia condition were more likely to select a difficult (than easy) future navigation task and this effect was mediated by prior route-learning performance (i.e. Maze 1 latency). As to our third objective, we ruled out a potential role for generalised PA or NA in accounting for these nostalgia effects.
General discussion
Summary of findings
We demonstrated that, compared to more recent but equally recognisable landmarks, nostalgic landmarks enhanced spatial performance on a route learning task. In Experiment 1, participants in the nostalgia (vs. control) condition identified more correct directions at landmarks shown in isolation from the maze, supporting the postulated semantic salience of the nostalgic (vs. control) landmarks. In Experiment 2, nostalgic landmarks enhanced spatial performance even when they were not placed at decision points (Maze 1) and when they were removed on the test trial (Maze 2), attesting to the robustness of their facilitative effect. These findings suggest that the salient nostalgic landmarks potentiated neutral landmarks within and outside the maze. Consistent with the expectancy-value perspective on goal setting (Levy & Baumgardner, Citation1991), Experiment 2 further revealed that participants in the nostalgia (vs. control) condition evinced more ambitious goal setting and that this effect was mediated by better route learning performance in the preceding route learning task. Commitment to specific, challenging goals is a robust predictor of future performance (Locke et al., Citation1981), pointing to potential further beneficial downstream consequences of nostalgia.
Implications for mechanisms governing spatial navigation
Our theoretical point of departure was that (a) a salient landmark that identifies a correct navigational response becomes associated with that response more readily than a less salient one (Redhead et al., Citation2013), and (b) nostalgic landmarks possess a high level of semantic salience owing to their connection to unique and meaningful personal memories (Sedikides et al., Citation2015). Our findings are consistent with these tenets and corroborate previous research showing that semantically salient cues enhance route learning performance (Ruotolo et al., Citation2019). Importantly, Experiment 2 participants recalled an equivalent number of nostalgic and control cues, suggesting that facilitation was not due to the nostalgic cues being simply more familiar.
Previous comparisons of cues within a spatial paradigm have either demonstrated overshadowing (Redhead & Hamilton, Citation2007), whereby the salient cues stop participants learning about the other cues, or potentiation of less salient cues (Cole et al., Citation2011), which is what we found here. According to Urcelay (Citation2017), the determining factor as to which outcome would arise is contiguity between the competing cues, with increased spatial contiguity leading to overshadowing. Here, we manipulated between experiments the contiguity of the nostalgic cues and the cues at the decisions point. In Experiment 1 the nostalgic cues were at decision points, whereas in Maze 1 of Experiment 2 they were at a distance from the decision point. In both cases, we observed potentiation. Such a finding would be more compelling if cue contiguity was manipulated within an experiment.
Pearce (Citation2009) suggested that potentiation was due to the associations formed between salient landmarks and the other less salient structural cues of the environment during training. The novel finding in Experiment 1 – that participants more readily identified the correct direction when the nostalgic (compared to control) cues were presented in isolation – points to the association between the nostalgic cue and the response performed at the cue rather than to the associations between cues. Previous demonstrations of associations between spatial cues have been with watermaze paradigms that rely more on classical conditioning of associations between cues (i.e. position of platform relative to the position of the landmarks). The current route learning paradigm capitalises on the associations between the response and outcomes of operant conditioning. It would therefore be useful to examine the effects of nostalgic cues within a paradigm that promotes associations between cues, such as the watermaze.
Broader implications
Landmarks are visual anchors that help navigators develop effective route dialogues (Allen, Citation2000; Denis, Citation1997; Fontaine & Denis, Citation1999), thus using distinctive landmarks is a key wayfinding tool (Deakin, Citation1996; Denis et al., Citation1999; Michon & Denis, Citation2001). Identifying emotions that heighten landmark saliency could assist navigators in achieving wayfinding success. Our findings indicate that nostalgic design elements strengthen navigation ability. Navigating unfamiliar spaces can be challenging (Baskaya et al., Citation2004; Gärling et al., Citation1983; Haq & Zimring, Citation2003; Hölscher et al., Citation2006), particularly within large-scale, multilevel indoor environments including libraries, museums, shopping centres, and hospitals (Dogu & Erkip, Citation2000; Eaton, Citation1991; Li & Klippel, Citation2012; Mandel, Citation2017). More often than not, these environments present long corridors and repetitive designs, making them confusing and disorientating to navigate. Infusing landmarks with nostalgia could help address these design pitfalls by providing a meaningful visual aid.
The ability to find one’s way in the world is essential for independent functioning and social interaction, and the loss of this ability can have serious consequences. This is particularly the case for people with neurological conditions, such as Alzheimer’s disease, epilepsy, stroke, and topographical disorientation disorders (Barrett & Muzaffar, Citation2014; Cimadevilla et al., Citation2014; Iaria & Barton, Citation2010; Monacelli et al., Citation2003) – all of which result in devastating changes to everyday life. For instance, persons with dementia are more likely to become lost within their regular day-to-day environment, which can heighten spatial anxiety (Chiu et al., Citation2004; Kirasic, Citation2000; Tu & Pai, Citation2006) and reduce confidence in exploring environments (Lawton, Citation1994; Lawton & Kallai, Citation2002). Therefore, it is important to consider ways to provide environmental support for those with wayfinding difficulties. Environmental design guidelines highlight the need for landmarks (O’Malley et al., Citation2017) and call for artwork or items that evoke positive emotions to support orientation (Department of Health, Citation2015). As nostalgia enhances semantic salience, such guidelines should consider integrating more specific, scientifically tested guidance regarding landmark characteristics.
A burgeoning literature has established the psychological utility of nostalgia. For example, the emotion increases sociality (i.e. sense of acceptance and belongingness; Sedikides & Wildschut, Citation2019), meaning in life (Sedikides & Wildschut, Citation2018), and self-continuity (i.e. a sense of connection between one’s past and present; Sedikides et al., Citation2016), while strengthening motivation to pursue one’s goals (Sedikides & Wildschut, Citation2020). Although these benefits have been documented primarily in young-adult samples, the same benefits also accrue to older adults living with dementia (Ismail et al., Citation2018, Citation2022). Our research showcased an additional function, namely, that nostalgic landmarks aid in spatial navigation. Future studies could examine the utility of salient nostalgic landmarks for aiding spatial navigation among people living with dementia and other neurological conditions affecting wayfinding ability (Davis et al., Citation2017).
Limitations and future directions
We attributed the beneficial effects of nostalgic (compared to control) landmarks to their higher semantic salience, owing to nostalgia’s connection with fond and personally meaningful memories of childhood and/or close relationships (Hepper et al., Citation2012, Citation2014). We aimed to rule out potential alternative explanations in terms of visual and structural salience by carefully matching the nostalgic images with parallel control images. We controlled for visual salience by always using same-sized colour images of the same individuals. We controlled for structural salience by placing these matched images in the same point within the maze. Crucially, free-recall data in Experiment 2 demonstrated that the nostalgic landmarks were not more recognisable than the control landmarks (the numerical pattern was even in the opposite direction, with more landmarks recalled in the control than nostalgia condition). Yet, despite our best efforts, we cannot definitively rule out alternative explanations. For example, nostalgic and control landmarks differed (by design) in terms of their recency. Future research could eliminate this potential confound by selecting nostalgic and control images stemming from the same time period. This would also provide an opportunity to further refine and improve our novel pictorial-nostalgia induction – for example, by carefully pilot testing a larger set of nostalgic and control images in order to ascertain that they differ on nostalgia but not on other dimensions, such as familiarity, liking, or vividness.
Although the stimuli used were successful in evoking nostalgia (as demonstrated by the manipulation checks in both experiments), they focused on a particular theme (i.e. popular TV shows, popular music artists) that we expected to elicit nostalgia in our high-school-aged samples; that is, we adopted a nomothetic approach to manipulating nostalgia (Dimitriadou et al., Citation2019). Future studies would do well to incorporate additional themes (e.g. special places, childhood toys, transport). Further, nostalgia is a personally meaningful and self-relevant emotion (Sedikides & Wildschut, Citation2018). To harness this personal element, it would be valuable to tailor nostalgic landmarks to individual participants’ past life experiences; that is, to adopt an idiographic approach.
Finally, previous studies have shown that virtual environments like the ones we used are interactive and life-like (Hegarty et al., Citation2006; Richardson et al., Citation1999; Ruddle et al., Citation1997), boosting ecological validity. Virtual platforms are easily controlled and allow for the creation of routes and novel landmarks, which could otherwise be more challenging to implement in the real-world. We acknowledge that everyday navigation involves kinesthetic input, which virtual environments lack. Future studies would do well to apply the present findings to naturalistic virtual surroundings (e.g. indoor residences, outdoor urban landscapes; Davis et al., Citation2017) and actual real-world environments (Nolan et al., Citation2002).
Concluding remarks
Nostalgia is generally beneficial for psychological functioning. In this article, we documented a novel benefit of the emotion: Nostalgic landmarks improved spatial navigation. Our findings contribute to basic research and have application potential.
Data availability statement
The data that support the findings of this study are available from the corresponding author, ESR, upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes
1 We used the difference between the positive-landmark (M = 7.32, SE = 0.44) and neutral-landmark (M = 5.64, SE = 0.44) condition on reproduction of the eight-square sequence on the outline of the Walking Corsi Test (Palmiero & Piccardi, Citation2017, p. 5).
2 For exploratory purposes, we administered several additional scales that were not pertinent to our research objectives, including measures of trait-level (i.e. dispositional) spatial anxiety and trait-level nostalgia.
3 We report 90% confidence intervals for eta squared, because the F distribution is one sided (Steiger, Citation2004). This ensures that inferences based on p-values will agree with the lower confidence limit.
4 Four teachers who accompanied the students on their visit also completed the experiment. We excluded them from the final data analysis, because the nostalgic and control landmarks were tailored to high-school-aged individuals. Inclusion of the teachers’ responses did not alter the pattern of significant and non-significant findings.
5 For exploratory purposes, we administered several additional scales that were unrelated to our research objectives, including measures of trait-level (i.e. dispositional) spatial anxiety, psychological benefits of nostalgia, and task enjoyment.
6 Bootstrapping is a non-parametric resampling procedure that approximates the sampling distribution of the indirect effect (i.e. the ab product) by repeatedly sampling the dataset.
References
- Allen, G. L. (2000). Principles and practices for communicating route knowledge. Applied Cognitive Psychology, 14(4), 333–359. doi:10.1002/1099-0720(200007/08)14:4<333::AID-ACP655>3.0.CO;2-C
- Balaban, C. Z., Karimpur, H., Röser, F., & Hamburger, K. (2017). Turn left where you felt unhappy: How affect influences landmark-based wayfinding. Cognitive Processing, 18(2), 135–144. https://doi.org/10.1007/s10339-017-0790-0
- Barrett, A. M., & Muzaffar, T. (2014). Spatial cognitive rehabilitation and motor recovery after stroke. Current Opinion in Neurology, 27(6), 653–658. https://doi.org/10.1097/WCO.0000000000000148
- Baskaya, A., Wilson, C., & Özcan, Y. Z. (2004). Wayfinding in an unfamiliar environment different spatial settings of two polyclinics. Environment and Behavior, 36(6), 839–867. doi:10.1177/0013916504265445
- Bouton, M. E., Dunlap, C. M., & Swartzentruber, D. (1987). Potentiation of taste by another taste during compound aversion learning. Animal Learning & Behavior, 15(4), 433–438. doi:10.3758/BF03205053
- Caduff, D., & Timpf, S. (2008). On the assessment of landmark salience for human navigation. Cognitive Processing, 9(4), 249–267. https://doi.org/10.1007/s10339-007-0199-2
- Chamizo, V. D., Rodrigo, T., Peris, J. M., & Grau, M. (2006). The influence of landmark salience in a navigation task: An additive effect between its components. Journal of Experimental Psychology: Animal Behavior Processes, 32(3), 339–344. doi:10.1037/0097-7403.32.3.339
- Chiu, Y.-C., Algase, D., Whall, A., Liang, J., Liu, H.-C., Lin, K.-N., & Wang, P.-N. (2004). Getting lost: Directed attention and executive functions in early Alzheimer’s disease patients. Dementia and Geriatric Cognitive Disorders, 17(3), 174–180. https://doi.org/10.1159/000076353
- Cimadevilla, J. M., Lizana, J. R., Roldán, M. D., Cánovas, R., & Rodríguez, E. (2014). Spatial memory alterations in children with epilepsy of genetic origin or unknown cause. Epileptic Disorders, 16(2), 203–207. https://doi.org/10.1684/epd.2014.0661
- Cole, M. R., Gibson, L., Pollack, A., & Yates, L. (2011). Potentiation and overshadowing of shape by wall color in a kite-shaped maze using rats in a foraging task. Learning and Motivation, 42(2), 99–112. https://doi.org/10.1016/j.lmot.2010.11.001
- Davis, R., Ohman, J. M., & Weisbeck, C. (2017). Salient cues and wayfinding in Alzheimer’s disease within a virtual senior residence. Environment and Behavior, 49(9), 1038–1065. https://doi.org/10.1177/0013916516677341
- Davis, R. L., & Therrien, B. A. (2012). Cue color and familiarity in place learning for older adults. Research in Gerontological Nursing, 5(2), 138–148. https://doi.org/10.3928/19404921-20111004-01
- Deakin, A. K. (1996). Landmarks as navigational aids on street maps. Cartography and Geographic Information Systems, 23(1), 21–36. doi:10.1559/152304096782512159
- Denis, M. (1997). The description of routes: A cognitive approach to the production of spatial discourse. Current Psychology of Cognition, 16(4), 409–458.
- Denis, M., Pazzaglia, F., Cornoldi, C., & Bertolo, L. (1999). Spatial discourse and navigation: An analysis of route directions in the city of venice. Applied Cognitive Psychology: The Official Journal of the Society for Applied Research in Memory and Cognition, 13(2), 145–174. https://doi.org/10.1002/(SICI)1099-0720(199904)13:2<145::AID-ACP550>3.0.CO;2-4
- Department of Health. (2015). Dementia-friendly design guidance for Health and Social Care Environments (Health Building Note, 08-02). Department of Health and Social Care. https://www.england.nhs.uk/wp-content/uploads/2021/05/HBN_08-02-1.pdf.
- Dimitriadou, M., Maciejovsky, B., Wildschut, T., & Sedikides, C. (2019). Collective nostalgia and domestic country bias. Journal of Experimental Psychology: Applied, 25(3), 445–457. https://doi.org/10.1037/xap0000209
- Dogu, U., & Erkip, F. (2000). Spatial factors affecting wayfinding and orientation: A case study in a shopping mall. Environment and Behavior, 32(6), 731–755. https://doi.org/10.1177/00139160021972775
- Eaton, G. (1991). Wayfinding in the library: Book searches and route uncertainty. Reference Quarterly, 30, 519–527. https://doi.org/10.2307/25828878
- Faul, F., Erdfelder, E., Lang, A., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. https://doi.org/10.3758/BF03193146
- Fontaine, S., & Denis, M. (1999). The production of route instructions in underground and urban environments. In C. Freksa, & D. M. Mark (Eds.), Spatial information theory: Cognitive and computational foundations of geographic information science (pp. 83–94). Springer.
- Frankenbach, J., Wildschut, T., Juhl, J., & Sedikides, C. (2021). Does neuroticism disrupt the psychological benefits of nostalgia? A meta-analytic test. European Journal of Personality, 35(2), 249–266. doi:10.1002/per.2276
- Gärling, T., Lindberg, E., & Mäntylä, T. (1983). Orientation in buildings: Effects of familiarity, visual access, and orientation aids. Journal of Applied Psychology, 68(1), 177–186. https://doi.org/10.1037/0021-9010.68.1.177
- Graham, M., Good, M. A., McGregor, A., & Pearce, J. P. (2006). Spatial learning based on the shape of the environment is influenced by properties of the objects forming the shape. Journal of Experimental Psychology: Animal Behavior Processes, 32(1), 44–59. https://doi.org/10.1037/0097-7403.32.1.44
- Grzeschik, R., Hilton, C., Dalton, R. C., Konovalova, I., Cotterill, E., Innes, A., & Wiener, J. M. (2021). From repeating routes to planning novel routes: The impact of landmarks and ageing on route integration and cognitive mapping. Psychological Research, 85(6), 2164–2176. https://doi.org/10.1007/s00426-020-01401-5
- Haq, S., & Zimring, C. (2003). Just down the road a piece: The development of topological knowledge of building layouts. Environment and Behavior, 35(1), 132–160. https://doi.org/10.1177/0013916502238868
- Hayes, A. F. (2022). Introduction to mediation, moderation, and conditional process analysis (3rd ed.). Guilford.
- Hegarty, M., Montello, D. R., Richardson, A. E., Ishikawa, T., & Lovelace, K. (2006). Spatial abilities at different scales: Individual differences in aptitude-test performance and spatial-layout learning. Intelligence, 34(2), 151–176. https://doi.org/10.1016/j.intell.2005.09.005
- Henderson, J. M., Hayes, T. R., Peacock, C. E., & Rehrig, G. (2019). Meaning and attentional guidance in scenes: A review of the meaning map approach. Vision, 3(19), 1–10. https://doi.org/10.3390/vision3020019
- Hepper, E. G., Ritchie, T. D., Sedikides, C., & Wildschut, T. (2012). Odyssey’s end: Lay conceptions of nostalgia reflect its original Homeric meaning. Emotion, 12(1), 102–119. https://doi.org/10.1037/a0025167
- Hepper, E. G., Wildschut, T., Sedikides, C., Ritchie, T. D., Yung, Y.-F., Hansen, N., Abakoumkin, G., Arikan, G., Cisek, S. Z., Demassosso, D. B., Gebauer, J. E., Gerber, J. P., González, R., Kusumi, T., Misra, G., Rusu, M., Ryan, O., Stephan, E., Vingerhoets, A. J. J., & Zhou, X. (2014). Pancultural nostalgia: Prototypical conceptions across cultures. Emotion, 14(4), 733–747. doi:10.1037/a0036790
- Hepper, E. G., Wildschut, T., Sedikides, C., Robertson, S., & Routledge, C. D. (2021). Time capsule: Nostalgia shields psychological wellbeing from limited time horizons. Emotion, 21(3), 644–664. https://doi.org/10.1037/emo0000728
- Hölscher, C., Meilinger, T., Vrachliotis, G., Brösamle, M., & Knauff, M. (2006). Up the down staircase: Wayfinding strategies in multi-level buildings. Journal of Environmental Psychology, 26(4), 284–299. https://doi.org/10.1016/j.jenvp.2006.09.002
- Horne, M. R., & Pearce, J. M. (2011). Potentiation and overshadowing between landmarks and environmental geometric cues. Learning & Behavior, 39(4), 371–382. https://doi.org/10.3758/s13420-011-0032-8
- Iaria, G., & Barton, J. J. (2010). Developmental topographical disorientation: A newly discovered cognitive disorder. Experimental Brain Research, 206(2), 189–196. https://doi.org/10.1007/s00221-010-2256-9
- Ismail, S., Christopher, G., Dodd, E., Wildschut, T., Sedikides, C., Ingram, T. A., Jones, R. W., Nooman, K. A., Tingley, D., & Cheston, R. (2018). Psychological and mnemonic benefits of nostalgia for people with dementia. Journal of Alzheimer’s Disease, 65(4), 1327–1344. https://doi.org/10.3233/JAD-180075
- Ismail, S., Dodd, E., Christopher, G., Wildschut, T., Sedikides, C., & Cheston, R. (2022). The content and function of nostalgic memories of people living with dementia. The International Journal of Aging and Human Development, 94(4), 436–458. https://doi.org/10.1177/00914150211024185
- Kessels, R. P. C., van Doormaal, A., & Janzen, G. (2011). Landmark recognition in Alzheimer’s dementia: Spared implicit memory for objects relevant for navigation. PLoS ONE, 6(4), e18611. https://doi.org/10.1371/journal.pone.0018611
- Kirasic, K. C. (2000). Age differences in adults’ spatial abilities, learning environmental layout, and wayfinding behavior. Spatial Cognition and Computation, 2(2), 117–134. https://doi.org/10.1023/A:1011445624332
- Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (2008). International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida.
- Lawton, C. A. (1994). Gender differences in way-finding strategies: Relationship to spatial ability and spatial anxiety. Sex Roles, 30(11–12), 765–779. https://doi.org/10.1007/BF01544230
- Lawton, C. A., & Kallai, J. (2002). Gender differences in wayfinding strategies and anxiety about wayfinding: A cross-cultural comparison. Sex Roles, 47(9-10), 389–401. https://doi.org/10.1023/A:1021668724970
- Leunissen, J., Wildschut, T., Sedikides, C., & Routledge, C. (2021). The hedonic character of nostalgia: An integrative data analysis. Emotion Review, 13(2), 139–156. https://doi.org/10.1177/1754073920950455
- Levy, P. E., & Baumgardner, A. H. (1991). Effects of self-esteem and gender on goal choice. Journal of Organizational Behavior, 12(6), 529–541. https://doi.org/10.1002/job.4030120606
- Li, R., & Klippel, A. (2012). Wayfinding in libraries: Can problems be predicted? Journal of Map & Geography Libraries, 8(1), 21–38. https://doi.org/10.1080/15420353.2011.622456
- Locke, E. A., Shaw, K. N., Saari, L. M., & Latham, G. P. (1981). Goal setting and task performance: 1969–1980. Psychological Bulletin, 90(1), 125–152. https://doi.org/10.1037/0033-2909.90.1.125
- MacKinnon, D. P., Lockwood, C. M., & Williams, J. (2004). Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research, 39(1), 99–128. https://doi.org/10.1207/s15327906mbr3901_4
- Mackintosh, N. J. (1976). Overshadowing and stimulus intensity. Animal Learning & Behavior, 4(2), 186–192. https://doi.org/10.3758/BF03214033
- Mandel, L. H. (2017). Wayfinding research in library and information studies: State of the field. Evidence Based Library and Information Practice, 12(2), 133–148. https://doi.org/10.18438/B8395P
- Michon, P.-E., & Denis, M. (2001). When and why referring to visual landmarks in direction giving? In C. Freksa, & D. M. Mark (Eds.), Spatial information theory: Cognitive and computational foundations of geographic information science (pp. 292–305). Springer.
- Monacelli, A. M., Cushman, L. A., Kavcic, V., & Duffy, C. J. (2003). Spatial disorientation in Alzheimer’s disease: The remembrance of things passed. Neurology, 61(11), 1491–1497. doi:10.1212/WNL.61.11.1491
- Nolan, B., Mathews, R., Truesdale-Todd, G., & VanDorp, A. (2002). Evaluation of the effect of orientation cues on wayfinding in persons with dementia. Alzheimer’s Care Today, 3(1), 46–49.
- O’Malley, M., Innes, A., & Wiener, J. M. (2017). Decreasing spatial disorientation in care-home settings: How psychology can guide the development of dementia friendly design guidelines. Dementia (basel, Switzerland), 16(3), 315–328. https://doi.org/10.1177/1471301215591334
- O’Malley, M., Innes, A., & Wiener, J. M. (2018). How do we get there? Effects of cognitive aging on route memory. Memory and Cognition, 46(2), 274–284. https://doi.org/10.3758/s13421-017-0763-7
- Palmiero, M., & Piccardi, L. (2017). The role of emotional landmarks on topographical memory. Frontiers in Psychology, 8(763), 1–8. https://doi.org/10.3389/fpsyg.2017.00763
- Pearce, J. M. (2009). The 36th Sir Frederick Bartlett lecture: An associative analysis of spatial learning. Quarterly Journal of Experimental Psychology, 62(9), 1665–1684. https://doi.org/10.1080/17470210902805589
- Pearce, J. M., Graham, M., Good, M. A., Jones, P. M., & McGregor, A. (2006). Potentiation, overshadowing and blocking of spatial learning based on the shape of the environment. Journal of Experimental Psychology: Animal Behavior Processes, 32(3), 201–214. https://doi.org/10.1037/0097-7403.32.3.201
- Redhead, E. S. (2007). Multi-modal discrimination learning in humans: Evidence for configural theory. Quarterly Journal of Experimental Psychology, 60(11), 1477–1495. https://doi.org/10.1080/17470210601154560
- Redhead, E. S., & Hamilton, D. (2007). Interaction between locale and taxon strategies in human spatial learning. Learning and Motivation, 38(3), 262–283. https://doi.org/10.1016/j.lmot.2006.11.003
- Redhead, E. S., Hamilton, D. A., Parker, M. O., Chan, W., & Allison, C. (2013). Overshadowing of geometric cues by a beacon in a spatial navigation task. Learning & Behavior, 41(2), 179–191. https://doi.org/10.3758/s13420-012-0096-0
- Redhead, E. S., & Pearce, J. M. (1995). Stimulus salience and negative patterning. Quarterly Journal of Experimental Psychology, 48(1), 67–83.
- Richardson, A. E., Montello, D. R., & Hegarty, M. (1999). Spatial knowledge acquisition from maps and from navigation in real and virtual environments. Memory & Cognition, 27(4), 741–750. https://doi.org/10.3758/BF03211566
- Ruddle, R. A., Payne, S. J., & Jones, D. M. (1997). Navigating buildings in “desk-top” virtual environments: Experimental investigations using extended navigational experience. Journal of Experimental Psychology: Applied, 3(2), 143–159. https://doi.org/10.1037/1076-898X.3.2.143
- Ruotolo, F., Claessen, M. H. G., & van der Ham, I. J. M. (2019). Putting emotions in routes: The influence of emotionally laden landmarks on spatial memory. Psychological Research, 83(5), 1083–1095. https://doi.org/10.1007/s00426-018-1015-6
- Sedikides, C., & Wildschut, T. (2016). Nostalgia: A bittersweet emotion that confers psychological health benefits. In A. M. Wood, & J. Johnson (Eds.), Wiley handbook of positive clinical psychology (pp. 25–36). Wiley Blackwell. https://doi.org/10.1002/9781118468197.ch9.
- Sedikides, C., & Wildschut, T. (2018). Finding meaning in nostalgia. Review of General Psychology, 22(1), 48–61. https://doi.org/10.1037/gpr0000109
- Sedikides, C., & Wildschut, T. (2019). The sociality of personal and collective nostalgia. European Review of Social Psychology, 30(1), 123–173. https://doi.org/10.1080/10463283.2019.1630098
- Sedikides, C., & Wildschut, T. (2020). The motivational potency of nostalgia: The future is called yesterday. Advances in Motivation Science, 7, 75–111. https://doi.org/10.1016/bs.adms.2019.05.001
- Sedikides, C., Wildschut, T., Cheung, W.-Y., Routledge, C., Hepper, E. G., Arndt, J., Vail, K., Zhou, X., Brackstone, K., & Vingerhoets, A. J. J. M. (2016). Nostalgia fosters self-continuity: Uncovering the mechanism (social connectedness) and the consequence (eudaimonic well-being). Emotion, 16(4), 524–539. https://doi.org/10.1037/emo0000136
- Sedikides, C., Wildschut, T., Routledge, C., Arndt, J., Hepper, E. G., & Zhou, X. (2015). To nostalgize: Mixing memory with affect and desire. Advances in Experimental Social Psychology, 51, 189–273. doi:10.1016/bs.aesp.2014.10.001
- Seetharaman, K., Shepley, M. M., & Cheairs, C. (2021). The saliency of geographical landmarks for community navigation: A photovoice study with persons living with dementia. Dementia (basel, Switzerland), 20(4), 1191–1212. https://doi.org/10.1177/1471301220927236
- Steiger, J. H. (2004). Beyond the F test: Effect size confidence intervals and tests of close fit in the analysis of variance and contrast analysis. Psychological Methods, 9(2), 164–182. https://doi.org/10.1037/1082-989X.9.2.164
- The New Oxford Dictionary of English. (1998). Oxford University Press.
- Tu, M. C., & Pai, M. C. (2006). Getting lost for the first time in patients with Alzheimer’s disease. International Psychogeriatrics, 18(3), 567–570. https://doi.org/10.1017/S1041610206224025
- Urcelay, G. P. (2017). Competition and facilitation in compound conditioning. Journal of Experimental Psychology: Animal Learning and Cognition, 43(4), 303–314. https://doi.org/10.1037/xan0000149
- Van Tilburg, W. A. P., Bruder, M., Wildschut, T., Sedikides, C., & Göritz, A. S. (2019). An appraisal profile of nostalgia. Emotion, 19(1), 21–36. https://doi.org/10.1037/emo0000417
- Van Tilburg, W. A. P., Wildschut, T., & Sedikides, C. (2018). Nostalgia’s place among self-conscious emotions. Cognition and Emotion, 32(4), 742–759. https://doi.org/10.1080/02699931.2017.1351331
- Wildschut, T., & Sedikides, C. (2020). The psychology of nostalgia: Delineating the emotion’s nature and functions. In M. H. Jacobsen (Ed.), Nostalgia now: Cross-disciplinary perspectives on the past in the present (pp. 47–65). Routledge Press.
- Wildschut, T., Sedikides, C., Arndt, J., & Routledge, C. (2006). Nostalgia: Content, triggers, functions. Journal of Personality and Social Psychology, 91(5), 975–993. https://doi.org/10.1037/0022-3514.91.5.975