ABSTRACT
Stimulating CT-afferents by forearm caresses produces the subjective experience of pleasantness in the receiver and modulates subjective evaluations of viewed affective images. Receiving touch from another person includes the social element of another person’s presence, which has been found to influence affective image evaluations without involving touch. The current study investigated whether these modulations translate to facial muscle responses associated with positive and negative affect across touch-involving and mere presence conditions. Female participants (N = 40, M(age) = 22.4, SD = 5.3) watched affective images (neutral, positive, negative) while facial electromyography was recorded (sites: zygomaticus, corrugator). Results from ANOVAs showed that providing touch to another person or oneself modulated zygomaticus site responses when viewing positive images. Providing CT-afferent stimulating touch (i.e., forearm caresses) to another person or oneself dampened the positive affective facial muscle response to positive affective images. Providing touch to another person generally increased corrugator facial muscle activity related to negative affect. Receiving touch did not modulate affective facial muscle responses during the viewing of affective images but may have effects on later cognitive processes. Together, previously reported social and touch modulations of subjective evaluations of affective images do not translate to facial muscle responses during affective image viewing, which were differentially modulated.
KEYWORDS:
Social touch is known to play a crucial role in mature development (literature reviews by T. Field, Citation2010, Citation2019; Gallace, Citation2010) and has, thus, important long-term effects on the individual receiving the touch. However, social touch also takes immediate effect, e.g. touch can positively influence the relationship between partners during conflict (e.g. Jakubiak & Feeney, Citation2019) and receiving touch from a partner can reduce stress and pain (T. Field, Citation2019). Affiliative touch seems to cause affect-related changes in the recipient’s body. Nummenmaa et al. (Citation2016) used positron emission tomography while male participants were pleasurably (but non-sexually) touched on the body by their romantic partner. Results showed a reduction in parts of the endogenous opioid system (ACC, aINS, thalamus) involved in experiencing pain. Such findings lead to assume that social and affiliative touch can hamper negative stimuli.
Social and affiliative touch can take various forms ranging from hugs and caresses to short taps on the shoulder and depending on the location of the body the touch is applied, its effects vary. It is known that caresses on hairy skin with a velocity of 1–10 cm/sec (Löken et al., Citation2009) stimulate CT-afferents, which has further been found to produce the subjective experience of pleasantness in the person receiving the touch (T. Field, Citation2019; Löken et al., Citation2009; Morrison, Citation2016). It should be noted that most research on touch focusses on the effects of being touched. Given that people provide touch to themselves (see Boehme & Olausson, Citation2022) and that being touched also involves touching with its own sensation, the effects of self-provided touching and providing touch to another person alongside receiving touch should gain further attention. A study including these three instances found that being stroked on the forearm was rated as more pleasant than stroking the partner’s forearm or self-stroking although all three ratings were in the positive range (Triscoli et al., Citation2017). As such, CT-afferent stimulation might lead to the experience of positive affect.
Facial muscle responses in the corrugator and zygomaticus facial muscles sites have consistently been linked to negative and positive affect (e.g. Fridlund et al., Citation1984; Golland et al., Citation2018; Schwartz et al., Citation1976; Witvliet & Vrana, Citation1995), the processing of negative and positive stimuli (e.g. Dimberg, Citation1988, Citation1990; Larsen et al., Citation2003), and specifically CT-afferent stimulation (Mayo et al., Citation2018). The corrugator supercilii is generally associated with negative affect and its activation results in frowning, and the zygomaticus major is associated with positive affect as its activation results in smiling. A study looking at facial muscle responses associated with affect (i.e. zygomaticus major site activation related to positive affect) found greater zygomaticus site activation in response to CT-afferent stimulating forearm-stroking than stroking of the palm, which lacks CT-afferents (Pawling et al., Citation2017). Thus, CT-afferent stimulation and the resulting feeling of pleasantness seem to reflect in facial muscle activity associated with the experience of positive affect.
In line with physiological research, neuroscientific research has shown that the insula, a structure that is associated with emotional processing, is activated when CT-afferents are stimulated (Olausson et al., Citation2002). Neuroscientific research identified further brain regions related to social cognition and affect to be activated during CT-afferent stimulation, i.e. the amygdala, the right posterior superior temporal sulcus, the medial prefrontal cortex, and dorso anterior cingulate cortex (Gordon et al., Citation2013). As such, affiliative touch including CT-afferent stimulation from another person might be the link between the received touch and the feeling of pleasantness, affect, and potentially also respective cognitive evaluations.
A potential modulation of affective image valence evaluations due to social and affiliative touch manipulations including CT-afferent stimulation (i.e. forearm-caressing) was investigated by Wingenbach et al. (Citation2019). Similar to Triscoli et al. (Citation2017), the study included experimental conditions varying whether participants were receiving affiliative touch, were providing affiliative touch, or were self-providing the touch to shed light on the social component of touch regarding affective judgements. In addition, there was a no-touch conditions with another person present in the room and a condition where the participant was alone to shed light on the social aspect in valence judgements of affective images. Results showed that negative images were evaluated as less negative in the conditions where participants were receiving affiliative touch by another person and by themselves compared to participants providing touch to another person, having another person present, and being alone in the room. Markedly, the conditions with CT-afferent stimulation had these positive effects on negative affective image evaluation. Creating a social situation without touch also had a positive effect on negative image evaluation compared to participants being alone in the room. No effects were found on the evaluation of neutral images, but positive images were evaluated as more positive with another person present than when receiving or providing caresses. For positive effects on positive affective image evaluation, the social aspect of another’s presence seems to have played a more important role than the touch. This study showed that social aspects with and without affiliative touch differentially impact affective image evaluations. Self-reported evaluations are naturally subjective and involve a great cognitive component and may not reflect a person’s affective experience on a more objective level.
Facial EMG allows to assess facial muscle responses which may be outside of the awareness of the participant and not visible to the naked eye and can be used as an objective and sensitive measure of affect (see Wingenbach, Citation2023). Facial EMG can thus be used to investigate whether facial muscle responses related to affect during affective image viewing are modulated by social as well as affiliative touch manipulations and how these aspects interact; a question that is unanswered by the published literature to date.
The main aim of the current study was to investigate the potential of the presence of another person (social aspect), affiliative touch (social touch aspect), and CT-afferent stimulation (social vs non-social) to modulate facial muscle responses associated with positive and negative affect during affective image viewing. The touch was applied skin-to-skin through forearm caresses with a CT-optimal velocity (i.e. 7 cm/sec). Assuming the general response pattern in zygomaticus and corrugator facial muscle sites in relation to affect, it was expected to replicate published findings of higher corrugator site activity in response to negative than positive affective and neutral stimuli, and higher zygomaticus site activity in response to positive than negative affective and neutral stimuli.
When viewing negative affective images, it was hypothesised that corrugator facial muscle site activity would be:
lower in the presence of another person than when alone (social aspect).
lower when affiliative touch (by another person and to another person) is involved than self-providing touch (social touch aspect).
lower when CT-afferent stimulation is applied (self-provided and by another person) than when providing touch to another person (CT-touch aspect).
lower when CT-afferent stimulation is applied by another person than when self-applied (CT-touch social aspect).
When viewing positive affective images, it was hypothesised that zygomaticus facial muscle site activity would show the opposite effects with expected higher activity in hypotheses (a)–(d).
Method
Participants
Participants were 46 heterosexual female participants (M(age) = 22.8, SD = 5.2) who were recruited and tested at the laboratory (sample size estimation is reported in Wingenbach et al., Citation2019). Six participants did not fully comply with the instructions of the task and were excluded from statistical analyses. Thus, a total of 40 participants were included in the results reported here (M(age) = 22.4, SD = 5.3). All participants were undergraduate students at the Mackenzie Presbyterian University with the majority from law (n = 24) and psychology (n = 11). All participants reported normal or corrected-to-normal vision.
Affective stimuli and experiment
The affective stimuli used for the current study were taken from the International Affective Picture Set (Lang et al., Citation2008). Precisely, 156 images were selected; 50 images for each of the 3 valence categories: positive, neutral, and negative. An additional six images were selected to be used as practice trials. While positive images represented the categories “animals”, “food”, “people”, “landscapes”, negative images represented the categories “mutilations”, “death”, “disasters”, “war”, “disgust”, and neutral images belonged to the category “objects”. The selection of the images was based on the valence ratings from the Brazilian norm ratings (Ribeiro et al., Citation2004). The experiment consisted of 5 experimental conditions of 30 trials with each condition containing 10 images of each image valence category. Touch was always provided skin-to-skin, i.e. palm stroking forearm with a velocity of 7cm/sec, in line with effective C-afferent stimulation (e.g. Löken et al., Citation2009). The experimental conditions of the task were:
Providing: The participant was stroking the experimenter’s hairy side of the forearm with their palm in the area between wrist and elbow.
Presence: The participant sat next to the experimenter, separated by a curtain, while undergoing the condition without touch.
Self-providing: The participant was stroking the hairy side of their own forearm with their palm in the area between wrist and elbow.
Receiving: The participant had the hairy side of their forearm stroked by the experimenter with their palm in the area between wrist and elbow.
Alone: The participant underwent the condition alone in the room and without touch.
The valence ratings for each image valence category were kept constant across the experimental conditions: providing (M(negative) = 1.14, SD = 0.12; M(neutral) = 5.15, SD = 0.20; M(positive) = 8.63, SD = 0.18), receiving (M(negative) = 1.15, SD = 0.12; M(neutral) = 5.15, SD = 0.19; M(positive) = 8.63, SD = 0.17), self-providing (M(negative) = 1.15, SD = 0.12; M(neutral) = 5.15, SD = 0.20; M(positive) = 8.61, SD = 0.14), presence (M(negative) = 1.15, SD = 0.12; M(neutral) = 5.16, SD = 0.19; M(positive) = 8.62, SD = 0.14), alone (M(negative) = 1.15, SD = 0.12; M(neutral) = 5.16, SD = 0.19; M(positive) = 8.61, SD = 0.14). A table displaying the image numbers and their distribution across the experimental conditions of the task applied in the current study can be found in Wingenbach et al. (Citation2019).
The stimulus presentation software E-Prime 2.0 (Psychology Software Tools, Pittsburgh, PA) was used to programme and run the experiment. A 32” PC screen (resolution: 1280 × 720, refresh rate: 60Hz) was used to present the experiment. The images were displayed at 75% of the experiment resolution (640 × 480). The total number of experimental trials was 150 plus 6 practice trials before starting the experiment. Specific instruction was provided for each experimental condition. A trial started with a fixation cross (2 s), followed by the stimulus (4 s) after which the answer screen containing the Self-Assessment-Manikins (Bradley & Lang, Citation1994) rating scale for valence (1 = “negative” to 9 = “positive”) appeared. The numbers 1–9 on the keyboard were set as corresponding input. The time to answer was not restricted and an allowed key press initiated the next trial. Visualisation of the trial procedure can be found in in Wingenbach et al. (Citation2019).
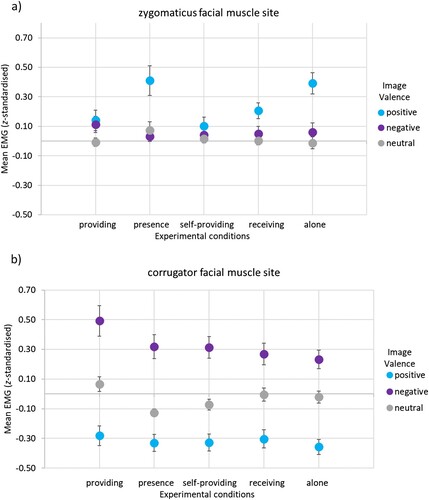
Figure 1 Facial muscle responses to affective stimuli per experimental condition. Note. The EMG signal representing facial muscle responses was z-standardised and baseline-corrected. The figures present (a) zygomaticus facial muscle responses and (b) corrugator facial muscle responses to positive, negative, and neutral affective stimuli for each of the five experimental conditions. Error bars display the SE of the M.
EMG recording
The BIOPAC MP150 System with the Acqknowledge software (Version 4, Biopac Systems, Inc., Goleta, CA) and EMG110C units for each of the two facial muscle sites (corrugator supercilii and zygomaticus major) were used for recording of the EMG data. Pairs of shielded surface silver–silver chloride (Ag-AgCl) electrodes (EL254S) filled with conductive gel (saline-based Signa Gel) and with a 4 mm diameter were used. The EMG signal was bandpass filtered online from 10 Hz to 500 Hz. Grounding was achieved with an additional electrode on the forehead. The sampling rate was 1000 Hz throughout the experiment.
Procedure
Ethical approval for the study was provided by the Mackenzie Ethics Committee in line with the Declaration of Helsinki. Every recruited participant filled out the Portuguese versions of the Beck’s Depression Inventory and the Beck’s Anxiety Inventory (Gorenstein & Andrade, Citation1996) online. Participants’ scores needed to be < 18 and < 16, respectively, to get invited to the testing session at the laboratory. This procedure was applied to exclude individuals with potential mood disorders, as these could influence their affective responses during the experiment and bias the results. Data collection was carried out by the same two female experimenters of which one provided the verbal instructions to the participant and attached the electrodes (experimenter 1) and the other provided/received the touch (experimenter 2). These roles were fixed. When participants arrived at the laboratory, they were greeted by experimenter 1, and the study procedures explained to them without revealing the hypotheses. Participants then provided written informed consent. Experimenter 2 remained unseen to the participant until the end of the testing session.
The laboratory room was set-up akin to the procedure by Schirmer et al. (Citation2011). That is, a black curtain separated two chairs with arm rests while the PC screen was shared and placed approximately 1m from participants. This set-up assured that participants would not see the experimenter 2. in Wingenbach et al. (Citation2019) displays this set-up. Since the curtain had a small hole at arm rests level, the touch was provided while the arm was resting on the arm rest. Experimenter 2 was trained by the last author prior to data collection in touch administration with a velocity of approximately 7cm/sec as necessary for effective CT-afferent stimulation. Four strokes were carried out during stimulus presentation.
After consent for participation in the study was obtained, the facial electromyography (EMG) electrodes were attached by experimenter 1. First, participants’ faces were cleaned with alcohol swabs to remove oils and dead skin cells from the skin for better attachment of the electrodes with double-sided adhesive rings. The electrodes were attached in accordance with the guidelines for facial EMG by Fridlund and Cacioppo (Citation1986). That is, the two electrode pairs were placed on the left side of the face. One pair was placed halfway on the imaginary line between the mouth corner and the zygomatic depression to assess zygomaticus major facial muscle site responses. For assessing the corrugator supercilii facial muscle site, one electrode was placed directly above the eyebrow lined up with the inner commissure of the eye fissure and the other electrode was placed lateral and slightly above the first one above the eyebrow. Each pair of electrodes was placed in proximity (1 cm between the electrode centres). A ground electrode was placed on the forehead. Participants were kept blind about the purpose of the electrodes until after completion of the study.
Every participant underwent the five experimental conditions in random order. Before each condition, experimenter 1 explained the nature of the condition to the participant, i.e. whether experimenter 2 would be present behind the curtain and what type of touch would be involved. Before starting the conditions where the participant was providing touch to herself or experimenter 2, the touch administration was demonstrated to the participant by experimenter 1 and practised with the participant. It was explained to participants that four strokes need to be carried out for the entire duration of the image presentation (i.e. 4 s). Participants were instructed to lightly caress the hairy side of their forearm in the area between wrist and elbow with slow movements and to rest the palm above the wrist in-between images. Since experimenter 2 was present in the conditions where the participant was providing touch and was able to see the movement on their arm, participants were excluded if the touch was incorrectly administered.
Participants were instructed to watch the images and evaluate their valence; the results are presented in Wingenbach et al. (Citation2019). The touch conditions included an additional rating. After concluding a touch condition, participants rated the pleasantness of the touch on a 9-point Likert-scale (1 = “unpleasant” to 9 = “pleasant”). During the whole duration of the experiment, participants were observed through a webcam mounted to the PC screen by experimenter 1. The webcam did not produce any recordings and merely served the aim to be able to take note of movement artefacts in the EMG signal. Participants were told about the webcam. The electrodes were taken off after completion of the experiment, participants debriefed, and granted course credit for participation.
Data preparation and analyses
The EMG data was prepared using Matlab 2016b. A 60 Hz notch filter was applied in addition to a high pass filter of 28 Hz. The signal was rectified and smoothed with a moving average of 50 ms. Artefacts (e.g. yawns), of which there were few, were removed based on the experimenters’ documentation; the respective time segments were set as missing values. However, no trials were excluded due to artefacts, which mainly occurred in time segments not included in the analysis. A pre-stimulus baseline of 500 ms and an event window of 4 s from stimulus onset were defined. This time period was segmented into 100 ms bins and the mean EMG activity for each of these bins extracted. Each 100 ms time bin was z-standardised; this standardisation was performed for each participant individually across experimental conditions but per muscle site. Afterwards, the pre-stimulus baseline was subtracted from each bin. A trial was excluded when the difference from one baseline-corrected 100 ms bin to the next exceeded -/+ 3 SD of the mean. For the corrugator facial muscle site, 1529 of the trials (25%) of the overall 6000 trials were omitted and 1130 trials (19%) for the zygomaticus facial muscle site. The number of trials excluded per participant, on average, for each stimulus category were in the corrugator site: positive (3), negative (2), neutral (3), and in the zygomaticus site: positive (1), negative (2), neutral (2). With the remaining trials, means were created for the EMG activity of 2 s from stimulus onset until offset. Research using affective scenes in images has shown that an orienting response in the corrugator site occurs around 500 ms after stimulus onset (Mavratzakis, Cornelia, & Peter, Citation2016). This orienting response is independent from the affective valence of the stimulus. Around 500–750 ms after scene stimulus onset, the response to the affective value of the stimulus starts to become observable in the corrugator site (Mavratzakis et al., Citation2016) and develops further until the maximum change to baseline is reached. With the aim to include the affective responses to the visual stimuli without the orienting and development to peak response, the second half of the stimulus presentation was used as the event window and entered into the analysis as averaged responses.
Data preparation and analyses were conducted in SPSS version 24 (SPSS IBM, Armonk, U.S.A.). The EMG means per facial muscle site for the three image valences within each of the 5 experimental conditions were inspected for outliers. Boxplots were used for identification of outliers per variable and all data points that were +/- 3x IQR from the median were defined as outliers. Identified outliers were winsorised instead of eliminated, as suggested by Field (Citation2009). This did not change the rank of those cases but made them less extreme to account for the sensitivity of ANOVA to extreme values.
Two repeated measures analyses of variance (ANOVAs) were conducted, one for each facial muscle site, with stimulus Valence category (3 levels [positive, negative, neutral]) and Experimental Condition (5 levels [alone, presence, self-provided, receiving, providing]) as within-subject factors. Adjustment of degrees of freedom was applied when sphericity was violated using Greenhouse-Geisser when the Greenhouse-Geisser estimate of sphericity was < .75 and Huynh-Feldt when the Greenhouse-Geisser estimate of sphericity was > .75 (Field, Citation2009). Partial η² is presented as effect size measure for the main effects and interactions. Significant interaction effects were qualified with ANOVAs and planned contrasts conducted to test the hypotheses for negative and positive images.
To test the social aspect, presence without touch condition was compared to the alone condition.
To test the social touch aspect, the receiving touch from another person and providing touch to another person conditions combined were compared to the self-providing touch.
To test the CT-afferent stimulation aspect, the conditions of caresses self-provided and by another person combined were compared to the providing touch to another person condition.
To test the social touch aspect within CT-afferent stimulation, the receiving caresses by another person condition was compared to the self-providing touch condition.
Significant effects with no prior hypothesis were followed up with post-hoc paired comparisons or paired samples t-tests and Bonferroni–Holm correction (Holm, Citation1979) was applied to the p-values to account for multiple comparisons. The presented p-values are after correction. Cohen’s d was calculated for the t-tests results as effect size measure.
Results
Zygomaticus facial muscle site responses
The 3 × 5 (Valence [positive, negative, neutral]) x (Experimental Conditions [providing, presence, self-providing, receiving, alone]) repeated measures ANOVA for the z-standardised zygomaticus facial muscle site responses showed a significant main effect of Valence, F(2, 78) = 20.21, p < .001, partial η² = .34, power = 1.00. Planned contrasts showed higher zygomaticus site activity (M = 0.25, SD = 0.05) in response to positive stimuli than to negative (M = 0.06, SD = 0.03), F(1, 39) = 19.10, p < .001, partial η² = .33, power = .99, and neutral stimuli (M = 0.01, SD = 0.02), F(1, 39) = 31.61, p < .001, partial η² = .45, power = 1.00.
The main effect of Experimental Condition was significant, F(4, 156) = 3.14, p = .016, partial η² = .08, power = .81. Post-hoc paired comparisons with Bonferroni–Holm adjustment showed, the z-standardised zygomaticus site activity was significantly higher in the presence condition (M = 0.17, SD = 0.04) than in the self-providing condition (M = 0.05, SD = 0.03), p = .020. The z-standardised zygomaticus site activity was trending towards being significantly higher in the alone condition (M = 0.15, SD = 0.04) than in the self-providing condition (M = 0.05, SD = 0.03), p = .063. No other comparisons reached statistical significance, p’s > .472.
Means and standard deviations for the z-standardised activity in the zygomaticus muscle site in response to positive, negative, and neutral valence stimuli for each of the experimental conditions are presented in .
Table 1. Means and Standard Deviations (z-standardised) across experimental conditions, stimulus valance, and facial muscle sites.
The interaction of Valence × Experimental Condition was significant, F(8, 312) = 3.05, p = .007, partial η² = .07, power = .91; see (A). A repeated measures ANOVA for the z-standardised zygomaticus site activity in response to positive stimuli with Experimental Condition as factor was conducted to qualify the interaction. Results showed a significant main effect of Experimental Condition for positive stimuli, F(4, 156) = 4.87, p = .003, partial η² = .11, power = .91. Planned contrasts showed no significant social effect (a) in z-standardised zygomaticus facial muscle site activity, F(1, 39) = 0.03, p = .866, partial η² = .00, power = .05. There was also neither a significant touch effect (b) in z-standardised zygomaticus facial muscle site activity, F(1, 39) = 1.00, p = .324, partial η² = .03, power = .16, nor a CT-afferent effect (c), F(1, 39) = 0.04, p = .852, partial η² = .00, power = .05, or CT-afferent social effect (d), F(1, 39) = 1.92, p = .174, partial η² = .05, power = .27.
To identify which experimental conditions differed from each other in z-standardised zygomaticus facial muscle site activity during positive affective image viewing, paired samples t-tests with Bonferroni–Holm correction for the eight tests were conducted. Results showed that the z-standardised zygomaticus facial muscle site activity was significantly higher in the presence condition than the self-providing condition, t(39) = 3.27, p = .014, d = .53, but not the providing condition, t(39) = 2.28, p = .120, d = .36, or receiving condition, t(39) = 1.82, p = .228, d = .28. The z-standardised zygomaticus facial muscle site activity was significantly higher in the alone condition than the providing condition, t(39) = −3.04, p = .024, d = .48, and the self-providing condition, t(39) = −4.44, p < .001, d = .70, but not significantly different from the receiving condition, t(39) = −2.35, p = .120, d = .38. The z-standardised zygomaticus facial muscle site activity was not significantly different in the providing condition compared to the self-providing condition, t(39) = 0.43, p = .840, d = .07, or the receiving condition, t(39) = 0.82, p = .840, d = .14.
Neither the main effect of Experimental Condition for the z-standardised zygomaticus site activity in response to negative stimuli was not significant, F(4, 156) = 0.53, p = .711, partial η² = .01, power = .18, nor was the main effect for neutral stimuli, F(4, 156) = 0.95, p = .421, partial η² = .02, power = .27.
Corrugator facial muscle site responses
The 3 × 5 (Valence [positive, negative, neutral] x Experimental Conditions [providing, presence, self-providing, receiving, alone]) repeated measures ANOVA for the z-standardised facial responses showed a significant main effect of Valence, F(2, 78) = 60.77, p < .001, partial η² = .61, power = 1.00. Planned contrasts showed, the z-standardised corrugator site activity in response to negative stimuli (M = 0.33, SD = 0.06) was significantly higher than to positive (M = −0.32, SD = −0.40), F(1, 39) = 76.99, p < .001, and neutral stimuli (M = −0.03, SD = −0.03), F(1, 39) = 32.71, p < .001.
The main effect of Experimental Condition was significant, F(4, 156) = 3.77, p = .006, partial η² = .09, power = .79. Post-hoc paired comparisons with Bonferroni–Holm correction for the ten comparisons showed, the z-standardised corrugator site activity was significantly higher in the providing condition (M = 0.09, SD = 0.05) than in the alone condition (M = −0.05, SD = 0.03), p = .020. There were no significant differences involving the presence (M = −0.05, SD = 0.04), self-providing (M = −0.03, SD = 0.03), and receiving (M = −0.01, SD = 0.03) conditions as no other comparisons reached statistical significance, p’s > .171.
Means and standard deviations for the z-standardised activity in the corrugator muscle site in response to positive, negative, and neutral valence stimuli for each of the experimental conditions are presented in . The interaction of Valence x Experimental Condition was not significant, F(8, 312) = 1.27, p = .28, partial η² = .03, power = .44; see (B). Thus, no further hypotheses tests were conducted.
Discussion
The current study investigated the potential modulating effects of affiliative touch and social presence on facial muscle responses while viewing affective images. The typical changes in facial muscle activity in the corrugator and zygomaticus facial muscle sites to positive, neutral, and negative valence images were replicated, in line with the expectation. The specific a-priori hypotheses on social, touch, and CT-afferent stimulation effects on zygomaticus and corrugator facial muscle responses while viewing positive and negative affective images were not supported. This hints at a complex interplay between these aspects. Nonetheless, touch modulations of zygomaticus facial muscle site responses were present during viewing of positive stimuli but not neutral or negative stimuli. Particularly, providing touch to oneself or a stranger seems to attenuate zygomaticus facial muscle site responses to positive affective images. Corrugator facial muscle site responses were amplified (across image valence categories) when providing touch to a stranger. Overall, providing touch to a stranger seems to attenuate positivity and increase negativity when viewing affective images, as reflected in facial muscle activity.
The facial muscle responses differed across image valence categories in the current study following the typical pattern. That is, the zygomaticus facial muscle site activity was greater when viewing positive than negative or neutral images, while the corrugator facial muscle site activity was greater during negative images viewing than positive or neutral images. These results are in line with the published literature on affective stimuli processing and facial muscle responses (e.g. Dimberg, Citation1988, Citation1990; Larsen et al., Citation2003). The current study shows, the valence category of viewed affective images reflects in corrugator and zygomaticus facial muscle site responses across social, touch, and CT-afferent stimulation manipulations.
Based on subjective image evaluations reported in Wingenbach et al. (Citation2019), it was expected that the presence of another person (vs alone) would increase positive affect as measured by zygomaticus facial muscle site activity when viewing positive images. However, there were no pronounced differences in zygomaticus facial muscle site activity between the social (presence) and non-social (alone) condition. This result shows that merely creating a social situation does not markedly affect facial muscle activity associated with positive affect during positive affective image viewing. This finding aligns with the wider literature on creating a social situation while viewing affective images. For example, a study reports no difference in attention-related measures assessed using electroencephalography during viewing of affective images either alone or in the presence of another person (Mairon et al., Citation2020). It should be noted that studies reporting social effects have taken their measures after stimulus offset. In addition to Wingenbach et al. (Citation2019) reporting the most positive valence ratings for positive affective images in the presence of another person, Wagner et al. (Citation2015) found increased positive affect in the condition where participants believed to be sharing an emotional experience. Thus, while the presence of another person does not seem to affect objective physiological measures during the viewing of affective images, there seem to be effects after the viewing which emerge on self-report level.
It was hypothesised that the social aspect of affiliative touch, provided by another person or to another person (vs self-provided caresses), would amplify zygomaticus facial muscle responses during positive affective image viewing. However, the results did not support this hypothesis. The results imply that the effects of touch on positive affective image processing expressed in the zygomaticus facial muscle site are stable whether receiving or providing touch, socially or non-socially (i.e. when self-providing). It is possible that social aspects of touch also do not modulate measures like implicit facial muscle activations during the viewing of affective images but explicit subjective measures afterwards.
It was further expected that receiving CT-afferent-stimulating caresses during positive affective image viewing, by another person or self-provided (vs to another person), would amplify zygomaticus facial muscle site activity, and more so when received from another person (vs self-provided). However, the zygomaticus facial muscle site activity during positive affective image viewing supported neither assumption. This result seems to contrast reports of increased visual attention to affective images and modulation of their processing based on electroencephalography from received forearm squeezes (Schirmer et al., Citation2011). However, forearm squeezes, unlike caresses, do not stimulate CT-afferents, and could have served as primes to the affective images driving the results. Importantly, the results from the current study align with reports of no reflection of perceived pleasantness from received forearm stroking at CT-afferent-stimulating velocity in the zygomaticus facial muscle site in the absence of any further stimuli (Ree et al., Citation2020). Possibly, the zygomaticus facial muscle site does not reflect increased subjective pleasantness from CT-afferent stimulation, at least when provided by a stranger who, in addition, was outside the participant’s visual field. Future research should investigate effects on the zygomaticus facial muscle site when touch is applied by a friend or partner vs strangers as well as manipulating their visibility to the participant.
Notably, in the current study, self-stimulating of CT-afferents in the forearm through caresses descriptively led to the lowest zygomaticus facial muscle site responses of all conditions during positive affective image viewing and significantly lower than the presence and alone conditions. Further, providing forearm caresses to another person showed significantly lower zygomaticus facial muscle site activity than being alone in the room during positive affective image viewing. Thus, providing touch to a stranger and self-providing touch, despite CT-afferent stimulation, appears to hamper positive images’ positivity as reflected in the zygomaticus site activity. As such, zygomaticus facial muscle site responses might reflect decreased rather than increased positive affect in relation to touch. Wondering whether liking the touch or subjective valence ratings of the images could have played a role, we carried out regression analyses including the touch liking and valence ratings (ratings presented in Wingenbach et al., Citation2019). However, neither liking nor valence ratings were entered in the model as predictors of facial muscle responses to the image valence categories. Alternatively, adding a task for participants to carry out, i.e. (self-)providing touch, in these two conditions might have distracted from the stimuli reflecting in less positive affect when viewing positive affective images.
Less strong zygomaticus facial muscle site responses when self-providing or providing touch to another person touch compared to no-touch conditions align with the wider literature, for example, reports of less positive subjective evaluations of positive images after self-providing forearm caresses compared to mere presence of another person (Wingenbach et al., Citation2019). With the current results showing higher zygomaticus activity in the no-touch conditions compared to two of the three touch conditions (self-providing and providing to another person but not receiving from another person) when viewing positive images, it is unlikely that the involvement of touch per se was underlying the results. The results align with the reports by Triscoli et al. (Citation2017) of lower, albeit positive, pleasantness ratings of forearm caresses when touch was self-provided or provided to another person than when received from another person. Thus, the question arises what makes receiving touch from another person different to (self-)providing touch. Providing touch to another person should be less pleasant than receiving touch if CT-afferent stimulation is necessary to create the effect. This is because the human palm carrying out the caresses does not have the necessary CT-afferents (McGlone et al., Citation2012). However, a lack of CT-afferent stimulation did not apply to the self-caressing condition, as this condition included CT-afferent stimulation. It should be noted that Triscoli et al. (Citation2017) found a significantly decreased heart rate only in the condition where participants were receiving touch from a partner and not when self-caressing or caressing a partner. That is, receiving touch from another person differentially affects physiological responses on cardiovascular level. It is possible that there was an experience of pleasantness when receiving touch from another person or that stimulation by a partner adds qualitative value to the subjective and physiological experience leading to non-significant differences to the no-touch conditions. In addition, it is possible that requiring the participant to carry out the touch might divert some of the participant’s attention away from the stimuli and reflect in lower zygomaticus facial muscle site activity in those conditions compared to the conditions where the participant is passive.
It should be noted that the zygomaticus facial muscle site responses in the receiving touch condition were not significantly different to the presence and the alone condition when viewing positive images. Taking the alone condition as reference, the results imply that receiving affiliative touch as well as social presence do not increase positive affect expressed in zygomaticus facial muscle activity in response to positive affective images. In addition, it was expected that negativity while viewing negative images would decrease, as measured by corrugator facial muscle site activity, when CT-afferent stimulation is taking place. However, the corrugator facial muscle site responses were not differentially affected by touch or presence across the image valence categories. The lack of receiving touch modulation aligns with a study measuring zygomaticus and corrugator facial muscle responses to facial emotional expressions including tactile primes next to control conditions without touch, which also did not find a touch modulation of facial muscle responses using facial EMG (Spape, Harjunen, & Ravaja, Citation2017). These authors suggested that receiving touch does not have immediate effects on affective processing but may affect later and more cognitive processes, such as interpretation and decision-making. It is possible that later, more explicit, cognitive processes related to affective stimulus processing are modulated by touch.
Indeed, receiving arm caresses from another person during the exposure to negative affective stimuli led to less negative subjective evaluations of affective images after stimulus offset (Wingenbach et al., Citation2019). If this finding translated to corrugator muscle responses, the activity should have been lowered in the receiving touch condition. This is particularly valid to assume, as the corrugator facial muscle site has been shown to inversely reflect perceived subjective pleasantness of received CT-afferent stimulating touch, though, in the absence of affective stimuli (see Mayo et al., Citation2018; Ree et al., Citation2020). Given the corrugator facial muscle site activity varied for the affective image categories but not within stimulus valence categories, it seems that touch and presence manipulations do not modulate corrugator facial muscle responses when viewing affective images. It can be concluded that touch differentially affects various processing stages of affective images. Future research should apply touch after affective stimuli viewing, i.e. during obtaining explicit evaluations, to shed more light on the matter.
Corrugator facial muscle site activity was, independent of image valence, enhanced when participants caressed the forearm of a stranger compared to all other conditions. As mentioned above, it is possible that the caressing action added another attention-requiring element to the experiment. As attention engages the corrugator muscle (Cohen et al., Citation1992), this explanation does align with the results. Alternatively, participants may have experienced some negative affect from touching a stranger which reflected in amplified corrugator activity. Future research should investigate effects on the corrugator facial muscle site when touch is applied to a friend or partner vs strangers. In addition, an experimental condition where only touch is applied without the presentation of stimuli could help differentiate facial muscle responses related to the stimuli from the touch itself.
It should be noted that this study was conducted on female participants with female experimenters, all with heterosexual orientation, and it is, therefore, possible that the results do not generalise to all genders, gender pairs, and sexual orientations. Likewise, the sample did not reflect the general population on characteristics, such as age and education. However, it is unlikely that these characteristics would alter the results, as CT-afferent stimulation takes effect on a basic level not involving cognitive processing that would be influenced by education, age, or gender. It is possible, though, that the results change when pairs of potential attraction are created, which should be investigated by future research. Due to the touch having been provided by humans with their palm as opposed to a machine, it cannot get excluded that some variation in the stroking occurred between participants. It may further be possible that also other types of touch would show similar effects, as we only investigated stroking at CT-afferent optimal stimulation with the palm.
In sum, the current study showed that zygomaticus facial muscle site responses can be dampened during viewing of positive affective images when affiliative touch is provided to a stranger or to oneself. Providing touch to a stranger generally seems to reflect in heightened corrugator facial muscle site activation when viewing affective images. Receiving CT-afferent stimulating forearm caresses does not seem to modulate affective responses in either facial muscle site during the viewing of affective images but seems to affect later cognitive processes.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Boehme, R., & Olausson, H. (2022). Differentiating self-touch from social touch. Current Opinion in Behavioral Sciences, 43, 27–33. https://doi.org/10.1016/j.cobeha.2021.06.012
- Bradley, M. M., & Lang, P. J. (1994). Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25(1), 49–59. https://doi.org/10.1016/0005-7916(94)90063-9
- Cohen, B. H., Davidson, R. J., Senulis, J. A., Saron, C. D., & Weisman, D. R. (1992). Muscle tension patterns during auditory attention. Biological Psychology, 33(2–3), 133–156. https://doi.org/10.1016/0301-0511(92)90028-S
- Dimberg, U. (1988). Facial electromyography and the experience of emotion. Journal of Psychophysiology, 2(4), 277–282.
- Dimberg, U. (1990). Facial electromyographic reactions and autonomic activity to auditory stimuli. Biological Psychology, 31(2), 137–147. https://doi.org/10.1016/0301-0511(90)90013-M
- Field, A. (2009). Discovering statistics using SPSS. SAGE.
- Field, T. (2010). Touch for socioemotional and physical well-being: A review. Developmental Review, 30(4), 367–383. https://doi.org/10.1016/j.dr.2011.01.001
- Field, T. (2019). Social touch, CT touch and massage therapy: A narrative review. Developmental Review, 51, 123–145. https://doi.org/10.1016/j.dr.2019.01.002
- Fridlund, A. J., & Cacioppo, J. T. (1986). Guidelines for human electromyographic research. Psychophysiology, 23(5), 567–589. https://doi.org/10.1111/j.1469-8986.1986.tb00676.x
- Fridlund, A. J., Schwartz, G. E., & Fowler, S. C. (1984). Pattern recognition of self-reported emotional state from multiple-site facial EMG activity during affective imagery. Psychophysiology, 21(6), 622–637. https://doi.org/10.1111/j.1469-8986.1984.tb00249.x
- Gallace, A. (2010). The science of interpersonal touch: An overview. Neuroscience & Biobehavioral Reviews, 34(2), 246–259. https://doi.org/10.1016/j.neubiorev.2008.10.004
- Golland, Y., Hakim, A., Aloni, T., Schaefer, S., & Levit-Binnun, N. (2018). Affect dynamics of facial EMG during continuous emotional experiences. Biological Psychology, 139, 47–58. https://doi.org/10.1016/j.biopsycho.2018.10.003
- Gordon, I., Voos, A. C., Bennett, R. H., Bolling, D. Z., Pelphrey, K. A., & Kaiser, M. D. (2013). Brain mechanisms for processing affective touch. Human Brain Mapping, 34(4), 914–922. https://doi.org/10.1002/hbm.21480.
- Gorenstein, C., & Andrade, L. (1996). Validation of a Portuguese version of the beck depression inventory and the state-trait anxiety inventory in Brazilian subjects. Brazilian Journal of Medical and Biological Research = Revista Brasileira de Pesquisas Medicas e Biologicas, 29, 453–457. http://www.ncbi.nlm.nih.gov/pubmed/8736107.
- Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 6(2), 65–70.
- Jakubiak, B. K., & Feeney, B. C. (2019). Hand-in-Hand combat: Affectionate touch promotes relational well-being and buffers stress during conflict. Personality and Social Psychology Bulletin, 45(3), 431–446. https://doi.org/10.1177/0146167218788556
- Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8, University of Florida.
- Larsen, J. T., Norris, C. J. & Cacioppo, J. T. (2003). Effects of positive and negative affect on electromyographic activity over zygomaticus major and corrugator supercilii. Psychophysiology, 40(5), 776–785. http://www.ncbi.nlm.nih.gov/pubmed/14696731.
- Löken, L. S., Wessberg, J., Morrison, I., McGlone, F., & Olausson, H. (2009). Coding of pleasant touch by unmyelinated afferents in humans. Nature Neuroscience, 12(5), 547–548. https://doi.org/10.1038/nn.2312
- Mairon, N., Nahum, M., Stolk, A., Knight, R. T., & Perry, A. (2020). Behavioral and EEG measures show no amplifying effects of shared attention on attention or memory. Scientific Reports, 10(1), 8458. https://doi.org/10.1038/s41598-020-65311-7
- Mavratzakis, A., H., Cornelia, & W., Peter. (2016). Emotional facial expressions evoke faster orienting responses, but weaker emotional responses at neural and behavioural levels compared to scenes: A simultaneous EEG and facial EMG study. NeuroImage, 124, 931–946. http://doi.org/10.1016/j.neuroimage.2015.09.065
- Mayo, L. M., Lindé, J., Olausson, H., Heilig, M., & Morrison, I. (2018). Putting a good face on touch: Facial expression reflects the affective valence of caress-like touch across modalities. Biological Psychology, 137, 83–90. https://doi.org/10.1016/j.biopsycho.2018.07.001
- McGlone, F., Olausson, H., Boyle, J. A., Jones-Gotman, M., Dancer, C., Guest, S., & Essick, G. (2012). Touching and feeling: Differences in pleasant touch processing between glabrous and hairy skin in humans. The European Journal of Neuroscience, 35(11), 1782–1788. https://doi.org/10.1111/j.1460-9568.2012.08092.x
- Morrison, I. (2016). Keep calm and cuddle on: Social touch as a stress buffer. Adaptive Human Behavior and Physiology, 2(4), 344–362. https://doi.org/10.1007/s40750-016-0052-x
- Nummenmaa, L., Tuominen, L., Dunbar, R., Hirvonen, J., Manninen, S., Arponen, E., Machin, A., Hari, R., Jääskeläinen, I. P., & Sams, M. (2016). Social touch modulates endogenous μ-opioid system activity in humans. NeuroImage, 138, 242–247. https://doi.org/10.1016/j.neuroimage.2016.05.063
- Olausson, H., Lamarre, Y., Backlund, H., Morin, C., Wallin, B. G., Starck, G., Ekholm, S., Strigo, I., Worsley, K., Vallbo, ÅB, & Bushnell, M. C. (2002). Unmyelinated tactile afferents signal touch and project to insular cortex. Nature Neuroscience, 5(9), 900–904. https://doi.org/10.1038/nn896
- Pawling, R., Cannon, P. R., McGlone, F. P., & Walker, S. C. (2017). C-tactile afferent stimulating touch carries a positive affective value. PLOS ONE, 12(3), e0173457. https://doi.org/10.1371/journal.pone.0173457
- Ree, A., Bendas, J., Pabel, L., Croy, I., & Sailer, U. (2020). Right between the eyes: Corrugator muscle activity tracks the changing pleasantness of repeated slow stroking touch. Physiology & Behavior, 222, 112903. https://doi.org/10.1016/j.physbeh.2020.112903
- Ribeiro, R. L., Pompéia, S., & Bueno, O. F. A. (2004). Normas brasileiras para o international affective picture system (IAPS): comunicação breve. Revista de Psiquiatria Do Rio Grande Do Sul, 26(2), 190–194. https://doi.org/10.1590/S0101-81082004000200008
- Schirmer, A., Teh, K. S., Wang, S., Vijayakumar, R., Ching, A., Nithianantham, D., Escoffier, N., & Cheok, A. D. (2011). Squeeze me, but don’t tease me: Human and mechanical touch enhance visual attention and emotion discrimination. Social Neuroscience, 6(3), 219–230. https://doi.org/10.1080/17470919.2010.507958
- Schwartz, G. E., Fair, P. L., Salt, P., Mandel, M. R., & Klerman, G. L. (1976). Facial expression and imagery in depression: An electromyographic study. Psychosomatic Medicine, 38(5). https://journals.lww.com/psychosomaticmedicine/Fulltext/1976/09000/Facial_Expression_and_Imagery_in_Depression__An.6.aspx.
- Spapé, M. M., Harjunen, V., & Ravaja, N. (2017). Effects of touch on emotional face processing: A study of event-related potentials, facial EMG and cardiac activity. Biological Psychology, 124, 1–10. http://doi.org/10.1016/j.biopsycho.2017.01.002
- Triscoli, C., Croy, I., Olausson, H., & Sailer, U. (2017). Touch between romantic partners: Being stroked is more pleasant than stroking and decelerates heart rate. Physiology & Behavior, 177, 169–175. https://doi.org/10.1016/j.physbeh.2017.05.006
- Wagner, U., Galli, L., Schott, B. H., Wold, A., van der Schalk, J., Manstead, A. S. R., Scherer, K., & Walter, H. (2015). Beautiful friendship: Social sharing of emotions improves subjective feelings and activates the neural reward circuitry. Social Cognitive and Affective Neuroscience, 10(6), 801–808. https://doi.org/10.1093/scan/nsu121
- Wingenbach, T. S. H. (2023). Facial EMG – investigating the interplay of facial muscles and emotions. In P. S. Boggio, T. S. H. Wingenbach, M. L. da Silveira Coêlho, W. E. Comfort, L. Murrins Marques, M. V. C. Alves (Eds.), Social and affective neuroscience of everyday human interaction (pp. 283–300). Springer. https://doi.org/10.1007/978-3-031-08651-9_17.
- Wingenbach, T. S. H., Ribeiro, B., Nakao, C., Gruber, J., & Boggio, P. S. (2019). Evaluations of affective stimuli modulated by another person’s presence and affiliative touch. Emotion, 21(2), 360–375. https://doi.org/10.1037/emo0000700.
- Witvliet, C. V., & Vrana, S. R. (1995). Psychophysiological responses as indices of affective dimensions. Psychophysiology, 32(5), 436–443. https://doi.org/10.1111/j.1469-8986.1995.tb02094.x
