 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Gaze cueing effect (GCE) refers to attention orienting towards the gazed-at location, characterised by faster responses to gazed-at than non-gazed-at stimuli. A previous study investigated the effects of affective priming on GCE and reported that threatening primes enhanced GCE. However, it remains unknown whether the threat or heightened arousal potentiated GCE. We investigated how highly arousing threatening and positive primes, compared to low arousing neutral primes modulate GCE. After a brief exposure to an affective prime (pictures of threat or erotica) or a neutral prime, participants detected an asterisk validly or invalidly cued by the gaze direction of a neutral face. The results showed that the threatening primes diminished the magnitude of GCE. The highly arousing positive primes did not have an effect on GCE. Further analyses showed that, as compared to neutral priming, the reaction times after threatening primes were shortened on invalid trials. This finding was interpreted to suggest that the threatening primes enhanced goal-directed target detection and attenuated attention orienting by irrelevant gaze cues via improving executive control. In sum, the present findings indicate that threat priming modulates GCE, not because of heightened arousal but because of the threat.
1. Introduction
Gaze cueing effect (GCE) refers to attention orienting towards the gazed-at location, characterised by faster responses to gazed-at than non-gazed at stimuli (Friesen & Kingstone, Citation1998). Despite showing some characteristics of automaticity, GCE has been proved to be modulated by several contextual variables, such as characteristics of the observers, characteristics of the cueing faces, and the relationship between the cueing faces and observers (for a review, see Dalmaso et al., Citation2020). Previous studies have showed enhanced GCE by fearful faces, suggesting that this reflects the effect of the communicative intention (a potential danger in the environment) of the person’s emotional expression on the observer’s attention orienting (Lassalle & Itier, Citation2013; Tipples, Citation2006). However, because physiological arousal can regulate attentional state (Reynolds et al., Citation2013), Ishikawa et al. (Citation2021) argued that the potentiation of GCE by threat-related faces could be mediated by the observers’ heightened arousal. To address this issue, Ishikawa et al. used an affective priming paradigm in which, on every trial, threatening or neutral non-facial stimuli (primes) were presented before the gaze cues to manipulate participants’ affective arousal. The results showed that the magnitude of GCE was greater after threatening primes than after neutral primes. Ishikawa et al. interpreted their findings to suggest that the threatening primes increased the participants’ level of arousal and prepared them for an imminent danger, and, therefore, they were highly sensitive to the gaze cues signalling the location of a potential danger.
In Ishikawa et al.’s (Citation2021) study, the primes used to evoke affective arousal were both threat-related and highly arousing. However, an important question is what was the critical property of the primes resulting in the enhancement of GCE: the threatening contents, the arousing contents, or the combination of these two? One way to investigate this question is to investigate the effects of both threatening and affectively positive primes with comparable arousal levels. Although previous research investigating the effects of emotion on attentional processes has emphasised the role of valence, arousal as an important component of affective experience also serves as information signalling importance or urgency (Storbeck & Clore, Citation2008). It has been demonstrated that manipulations of the autonomic arousal level indeed influence attention orienting in spatial cueing tasks; low arousal level (e.g. exposure to nature stimuli) decreases the cueing effect (Laumann et al., Citation2003) while high arousal level (e.g. inhalation of low concentrations of carbon dioxide) increases the cueing effect (Garner et al., Citation2012).
In the current study, we investigated the effects of highly arousing threatening, neutral (low arousal), and highly arousing positive primes on GCE. To match the high arousal level of the threatening primes (pictures of scary animals), pictures of erotica were used as positive primes. The pictures used are rated as both emotionally positive and highly arousing by men and women (Lang et al., Citation2008; Wierzba et al., Citation2015). In addition, like threatening stimuli, erotic stimuli also attract attention involuntarily due to their reproduction-relevant significance (Sennwald et al., Citation2016). On each trial, after a briefly presented prime, participants were shown a gaze cue and required to detect the appearance of an asterisk following the gaze cue. Based on the findings by Ishikawa et al. (Citation2021), it was hypothesised that threat priming would enhance GCE compared to the priming by neutral primes. If the increase of GCE by threatening versus neutral primes was due to the effect of arousal only, we expected that GCE would be enhanced in the positive priming condition, too. If, instead, the results showed greater GCE for threat-primed than positively primed gaze cues, this would indicate that the threatening contents played a role in the enhanced GCE for the threatening primes. To confirm that the primes evoked corresponding affective experiences (valence and arousal) in the participants, they were also required to explicitly evaluate the primes after the gaze cueing task.
2. Methods
2.1. Participants
Participants (n = 81) were recruited via the recruitment site of Decision-Making Laboratory (DMLab) at Tampere University or by contacting students on the campus. The sample size exceeded the sample size of 52 suggested by G*Power (Faul et al., Citation2007; See Supplemental File for the detailed procedure to determine the sample size). All the participants had normal or corrected-to-normal vision and reported no neurological or psychiatric diagnoses. They participated in the experiment voluntarily, gave written informed consent, and received either course credits or a compensation of 5 euros. Ethical statement for the experiment was obtained from the Ethics Committee of Tampere region.
2.2. Stimuli
Gaze cue stimuli were modified face pictures of four different individuals (two females) selected from the Oslo Face Database (Chelnokova et al., Citation2014) (See Supplemental File for the details of the stimuli and their presentation). The stimuli were presented on a white background in the original colour subtending 13° in width and 16° in height.
The affective priming pictures were selected from the International Affective Picture System (IAPS) (Lang et al., Citation2008), Nencki Affective Picture System (NAPS) (Wierzba et al., Citation2015), and Geneva Affective PictureE Database (GAPED) (Dan-Glauser & Scherer, Citation2011). The threatening primes (80) and positive primes (80) were pictures of threatening animals (e.g. snakes) and pictures of erotica, respectively. Positive primes for female participants were pictures of erotic couples (40) and nude males (40), and for male participants they were pictures of erotic couples (40) and nude females (40). The neutral primes (80) were pictures of everyday objects. The selection of the primes was based on the normative data reported in those picture databases. The mean valence/arousal ratings were 3.02/6.19 for the threatening primes, 4.98/2.54 for the neutral primes, and 6.65/5.81 for the positive primes (See Table S1 in Supplemental File for all the primes). In the experiment, prime pictures were presented subtending 6° × 8° or 8° × 6° (height × width). The target stimulus was an asterisk subtending 1° and it was presented 10° to the left or right of the centre of the screen.
2.3. Experimental procedure
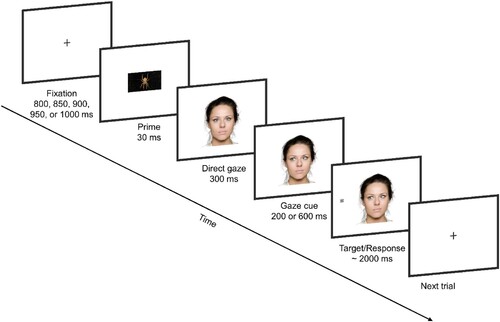
The study consisted of two tasks: a gaze cueing task and a rating task. In the gaze cueing task, the trials were presented in two blocks. In each block, there were 96 test trials and 24 catch trials. On each test trial, a fixation cross appeared first centrally on the screen with a varied duration of 800/850/900/950/1000 ms, followed by a prime for 30 ms. Then a face with direct gaze was presented for 300 ms and immediately after this, the same face with the gaze directed to the left or right was presented. This presentation gave an illusion of the eyes moving from the central position (direct gaze) to the left or right. Then, 200 or 600 ms after the presentation of the face with averted gaze (Stimulus Onset Asynchrony, SOA),Footnote1 an asterisk (the target) appeared at the gazed-at location or at the non-gazed-at location, at the same level as the eyes, while the face still stayed on the screen. The participants were required to press a response key as quickly as they could when they detected the appearance of the target. The next trial started when the participants made a response or after 2000 ms had elapsed (). It was emphasised that the gaze direction would not predict on which side of the face the target would appear. To prevent participants from responding too early, 48 catch trials (20% of the total number of trials) were inserted among the test trials. On the catch trials, no target was presented, and participants were supposed not to press the response key. Before the experimental trials, participants were allowed to practice the task for 24 trials (only neutral primes were used).
Figure 1. Sequence of events on a single trial in the gaze-cuing task. The figure illustrates a valid gaze cue trial in the threat priming condition.
In the rating task, participants were asked to rate their subjective experience of affective valence and arousal on a 1-9-point Self-Assessment Manikin scales (1 = unpleasant/calm, 9 = pleasant/arousing) (See Supplemental File for the implementation of the rating task).
2.4. Data analysis
Sexual orientation can influence whether a participant perceives nude persons of the opposite sex as positive or not (Wierzba et al., Citation2015). Thus, to exclude the potential influence of sexual orientation on the effect of positive priming, 4 females and 8 males were excluded (See Supplemental File for this exclusion criterion).
In the gaze cueing task, trials with a missing response or responses with RTs < 150 ms or > 1000 ms were excluded from the data analysis (0.2% of the trials were discarded). This was followed by another screening procedure in which responses with RTs falling outside each participant’s mean RT plus or minus 2 standard deviations were removed from participants’ data. After the exclusion, at least 13 trials remained for each participant in each condition. Then, the mean RT in each condition was calculated for each individual. For the final analysis, data from altogether 69 participants (37 females; age range = 19-50 years; M = 24.84 years, SD = 6.13) were included.
A 3 (affective priming) × 2 (SOA) × 2 (validity) repeated-measures ANOVA was performed on the RT data.Footnote2 For the rating task, one-way repeated-measures ANOVAs were conducted to test differences in the valence and arousal ratings for threatening, neutral, and positive primes. The level of significance was set at α = 0.05. Bonferroni correction was performed for all the multiple comparisons.
3. Results
3.1. Rating task
For the valence ratings of the primes, the ANOVA showed a significant effect of valence [F(2,206) = 117.62, p < 0.001, = 0.63], indicating that valence ratings of positive primes [M = 6.13, SE = 0.15] were the highest, followed by neutral primes [M = 5.32, SE = 0.12] and threatening primes [M = 3.30, SE = 0.15; ps < 0.001 for all the comparisons]. The arousal ratings also differed between the prime categories [F(2,206) = 115.21, p < 0.001,
= 0.63]. Threatening primes [M = 5.14, SE = 0.21] and positive primes [M = 4.85, SD = 0.22] were rated as more arousing than neutral primes [M = 2.47, SE = 0.13; ps < 0.001], but the arousal difference between the threatening and positive primes was not significant [p = 0.42].
3.2. Gaze cueing task
Analyses examining the effects of validity, SOA, and affective priming as well as the interaction between validity and SOA (See Supplemental Figure 1 for the interaction) are presented in the Supplemental File (also see Supplemental File for the discussion of these results).
The validity × affective priming was statistically significant [F(2,827) = 4.45, p = 0.01, = 0.06]. This interaction was broken down by analysing the effect of validity in each priming condition, separately. Pairwise comparisons indicated that the RTs on valid trials were significantly shorter than those on invalid trials in each priming condition [threatening: Mvalid = 295.28 ms vs. Minvalid = 299.92; neutral: Mvalid = 294.88 ms vs. Minvalid = 303.91 ms; positive: Mvalid = 291.65 ms vs. Minvalid = 301.90 ms; all ps < 0.01] (a). To investigate whether the validity effect (GCE) differed between the priming conditions, the magnitude of GCE (RTinvalid – RTvalid) was calculated in each priming condition. Pairwise comparisons showed that the magnitude of GCE was smaller after threatening primes [M = 4.64 ms, SE = 1.57] than after neutral [M = 9.03 ms, SE = 1.42; p = 0.04] and positive [M = 10.26 ms, SE = 1.75; p = 0.01] primes, while there was no difference in the magnitudes of GCE between neutral and positive priming conditions [p = 0.49].
Figure 2. Observed two-way interactions. The graphs show means and standard errors of the reaction times as a function of (a) Validity and Prime and (b) Prime and SOA.
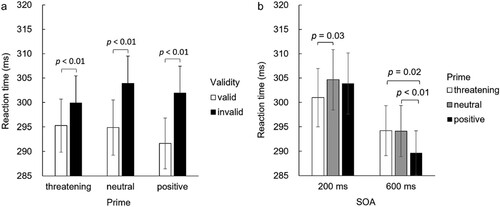
To analyse whether the smaller magnitude of GCE in the threatening vs. other priming conditions reflected the effects of priming on RTs on valid or invalid trials (or both), we further analysed the effects of primes on the RTs for valid and invalid trials, separately. On valid trials, there was no difference in RTs between the priming conditions [all ps > 0.05]. On invalid trials, the RTs were significantly shorter after the threatening than after the neutral primes [p = 0.03]. The difference between the positive and neutral priming conditions [p = 0.69] and the difference between the threatening and positive priming conditions [p = 0.93] were not significant.
Additionally, affective priming × SOA was also statistically significant [F(2,827) = 5.60, p = 0.005, = 0.08]. This interaction was broken down by analysing the effect of affective priming on RTs at the two SOAs, separately. At the SOA of 200 ms, the RTs were shortened in threat priming condition [Mthreatening = 300.98 ms, SE = 5.99] compared to neutral priming [Mneutral = 304.66 ms, SE = 6.20; p = 0.03]. The RTs in positive priming condition [Mpositive = 303.89 ms, SE = 6.30] were not significantly different from those in neutral priming [p = 1.00] and threat priming conditions [p = 0.52]. However, at the SOA of 600 ms, the RTs were shorter in the positive priming condition [M = 289.66 ms, SE = 4.54] compared to threatening [M = 294.22 ms, SE = 5.12; p = 0.02] and neutral [M = 294.13 ms, SE = 5.22; p = 0.007] priming conditions. The difference between the latter two did not reach statistical significance [threatening vs. neutral: p = 1.00] (b).
The three-way interaction between validity, affective priming, and SOA was not significant [F(2,827) = 0.12, p = 0.89].
4. Discussion
As expected, the result of the present study showed an interaction between the validity of gaze cues and affective priming. However, rather surprisingly, the magnitude of GCE was smaller after threatening than after neutral and positive primes, whereas there was no difference in the magnitudes of GCE between neutral and positive priming conditions. Thus, the results did not give any support whatsoever for the hypothesis that threat priming would enhance GCE. This is in stark contrast to the findings by Ishikawa et al. (Citation2021) showing enhancement of GCE by threat priming. Interestingly, however, there is another previous study reporting similar findings to those of the present study (Ohlsen et al., Citation2013). In that study, threatening (vs. non-threatening) pictures were presented in a one-minute-long priming block before the gaze cueing task to induce a dangerous or safe context and the results showed that threat priming by this methodology also decreased the magnitude of GCE (vs. non-threat priming).
Ishikawa et al. (Citation2021) explained their results by suggesting that observers’ sensitivity to the perceived gaze direction was enhanced when they were experiencing affective arousal via threat priming. How do we, then, explain the decreased GCE by threatening primes? A more detailed analysis of the present results indicated that the decreased GCE resulted from the effects of threatening primes on response times on invalid trials: on invalid trials, the response times were shorter in the threat priming condition compared to the neutral priming condition. We propose that the results reflect the effects of threatening primes on executive control processes. Empirical studies have shown that, in a flanker task, central presentation of threat-related stimuli preceding the target stimulus decreases the conflict evoked by incongruent flankers (e.g. ← ← → ← ←) and results in speeded responses as compared to incongruent trials preceded by a neutral stimulus (Birk et al., Citation2011; Finucane, Citation2011). The decreased congruity effect (RTincongruent – RTcongruent) has been interpreted to be the outcome of improved executive control following the perception of threatful stimuli (Birk et al., Citation2011). Based on these findings and suggestions we propose that, in the present study, the threatening primes (i) improved executive control resulting in enhanced goal-directed target detection (i.e. fast detection of the laterally presented targets) and (ii) attenuated gaze-cued attention orienting as the centrally presented, unpredictive gaze cues were irrelevant stimuli in terms of the primary task. Thus, on threat-primed invalid trials, the enhanced target detection together with the attenuated attention orienting by (invalid) gaze cues facilitated responses to the targets, compared to the invalid trials following neutral primes. On valid threat-primed trials, instead, the enhanced target detection combined with the attenuated attention orienting by gaze cues did not result in any faster responses than on neutrally primed valid trials on which attention was efficiently oriented by valid gaze cues. In order to test our proposal for the mechanism explaining the results, in future research, it would be interesting to investigate how threatening primes with different threat intensities influence GCE. A study by Zsido et al. (Citation2020) manipulated the threat intensity of threat primes and investigated their influence on performance in a visual search task. The results showed that the participants were faster in finding the targets after threat primes than after neutral primes, and more importantly, the reaction times were shorter after high-threat primes than after low-threat primes. This was interpreted to suggest that threatening primes modulated executive functions and that attentional resources to the primary task were allocated as a function of the threat intensity. Therefore, if threatening primes decreased GCE by improving executive control in the present study, it would be expected that the decrease in the magnitude of GCE would be greater for high-threat vs. low-threat primes.
Regarding the conflicting findings between the present study and the study by Ishikawa et al. (Citation2021), one possible reason is that, in the present study, a considerably high percentage of the trials were catch trials (20%) inserted randomly among the test trials. In contrast, Ishikawa et al. did not use catch trials at all in their localisation task. When the SOA was long, participants probably started to anticipate a test trial to be a catch trial, as time passed by without the appearance of a target. This could have led participants to disengage their attention from the gazed-at-location and shift it back to the face or the non-gazed-at location (to search for novelty in the environment). This might have facilitated responses to invalidly cued targets. Previous research has shown that GCE can disappear at the SOA of 600 ms and even reverse (faster responses on invalid trials than valid trials) at very long SOAs (1005 ms) because of the high probability of catch trials (33%) (Okamoto-Barth & Kawai, Citation2006). Thus, in the present study, during the anticipation of a target, the threat priming enhanced target detection, as noted above, and prompted active exploration of the possible target locations. If, on a given trial, the target did not appear at the gazed-at side shortly after the gaze cue, participants effectively disengaged their attention from the gazed-at-location and searched the target from the opposite location. This led to the shortened response times to invalidly cued targets and to the decreased GCE.
The significant interaction between affective priming and SOA revealed that at the SOA of 600 ms, the responses were overall faster after positive primes than after threatening and neutral primes. This result could be explained by proposing that positive affect elicited by positive primes broadened attentional breadth (Fredrickson & Branigan, Citation2005) and led to facilitated processing of task-relevant peripheral targets and shortened overall response times. This interpretation is consistent with the findings showing that cueing faces with a happy expression can enhance the neural processing (amplitude of the P3 response) of subsequent target stimuli appearing at both the gazed-at and non-gazed-at locations (Fichtenholtz et al., Citation2009).
The present study also aimed to investigate whether the effect of threat priming on GCE was due to arousal or threatening contents of these primes. The results from the rating task showed that participants perceived pictures of threat and erotica as significantly more negative and more positive, respectively, than the neutral primes. Moreover, the threatening and positive primes were evaluated as comparably arousing, and the arousal levels of both affective primes were higher than that of neutral primes. Thus, despite that threatening and positive primes induced comparable levels of affective arousal, the diminished GCE was observed only after threatening primes. GCE for positively primed gaze cues was roughly the same as for the neutrally primed gaze cues. Hence, our observations provided no evidence for the role of affective arousal in modulating GCE. Instead, it appeared to be the threat-related significance of the threatening primes that attenuated GCE. It is noteworthy that positive priming did not modulate GCE but facilitated the overall detection of targets at long SOA.
To conclude, the results showed that threat-related primes diminished GCE. We suggested that this could be due to improved executive control which resulted in enhanced goal-directed target detection and attenuation of the gaze-cued attention orienting. The findings provide insights into how the perception of emotional stimuli in the environment modulates gaze-mediated attention orienting.
Supplemental File_brief article.docx
Download MS Word (163.1 KB)Data availability statement
The datasets analysed during this study are available from the corresponding author, J.K.H., upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes
1 In the present study, we included two SOAs. The shifts of attention by the gaze cues are more automatic at the short than at the long SOAs as indicated, for example, by the results showing that the shifts of attention in response to counter-predictive gaze cues cannot be suppressed at short SOAs, whereas they can be suppressed at long SOAs (Driver et al., Citation1999, Experiment 3; Friesen et al., Citation2004, Experiment 1). Thus, the gaze cueing may be less susceptible to affective priming at a short than at a long SOA.
2 We also performed a four-way repeated-measures ANOVA, including the gender of the stimulus face as the fourth variable. The main effect of the gender of the stimulus face and its interactions with all the other variables were not significant (all ps > 0.05).
References
- Birk, J. L., Dennis, T. A., Shin, L. M., & Urry, H. L. (2011). Threat facilitates subsequent executive control during anxious mood. Emotion, 11(6), 1291–1304. https://doi.org/10.1037/a0026152
- Chelnokova, O., Laeng, B., Eikemo, M., Riegels, J., Løseth, G., Maurud, H., Willoch, F., & Leknes, S. (2014). Rewards of beauty: The opioid system mediates social motivation in humans. Molecular Psychiatry, 19(7), 746–747. https://doi.org/10.1038/mp.2014.1
- Dalmaso, M., Castelli, L., & Galfano, G. (2020). Social modulators of gaze-mediated orienting of attention: A review. Psychonomic Bulletin & Review, 27(5), 833–855. https://doi.org/10.3758/s13423-020-01730-x
- Dan-Glauser, E. S., & Scherer, K. R. (2011). The Geneva affective picture database (GAPED): A new 730-picture database focusing on valence and normative significance. Behavior Research Methods, 43(2), 468–477. https://doi.org/10.3758/s13428-011-0064-1
- Driver, J., Davis, G., Ricciardelli, P., Kidd, P., Maxwell, E., & Baron-Cohen, S. (1999). Gaze perception triggers reflexive visuospatial orienting. Visual Cognition, 6(5), 509–540. https://doi.org/10.1080/135062899394920
- Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. https://doi.org/10.3758/BF03193146
- Fichtenholtz, H. M., Hopfinger, J. B., Graham, R., Detwiler, J. M., & LaBar, K. S. (2009). Event-related potentials reveal temporal staging of dynamic facial expression and gaze shift effects on attentional orienting. Social Neuroscience, 4(4), 317–331. https://doi.org/10.1080/17470910902809487
- Finucane, A. M. (2011). The effect of fear and anger on selective attention. Emotion, 11(4), 970–974. https://doi.org/10.1037/a0022574
- Fredrickson, B. L., & Branigan, C. (2005). Positive emotions broaden the scope of attention and thought-action repertoires. Cognition & Emotion, 19(3), 313–332. https://doi.org/10.1080/02699930441000238
- Friesen, C. K., & Kingstone, A. (1998). The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychonomic Bulletin & Review, 5(3), 490–495. https://doi.org/10.3758/BF03208827
- Friesen, C. K., Ristic, J., & Kingstone, A. (2004). Attentional effects of counterpredictive gaze and arrow cues. Journal of Experimental Psychology: Human Perception and Performance, 30(2), 319–329. https://doi.org/10.1037/0096-1523.30.2.319
- Garner, M., Attwood, A., Baldwin, D. S., & Munafò, M. R. (2012). Inhalation of 7.5% carbon dioxide increases alerting and orienting attention network function. Psychopharmacology, 223(1), 67–73. https://doi.org/10.1007/s00213-012-2690-4
- Ishikawa, M., Haensel, J. X., Smith, T. J., Senju, A., & Itakura, S. (2021). Affective priming enhances gaze cueing effect. Journal of Experimental Psychology: Human Perception and Performance, 47(2), 189–199. https://doi.org/10.1037/xhp0000880
- Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual (Technical Report A-8). University of Florida.
- Lassalle, A., & Itier, R. J. (2013). Fearful, surprised, happy, and angry facial expressions modulate gaze-oriented attention: Behavioral and ERP evidence. Social Neuroscience, 8(6), 583–600. https://doi.org/10.1080/17470919.2013.835750
- Laumann, K., Gärling, T., & Stormark, K. M. (2003). Selective attention and heart rate responses to natural and urban environments. Journal of Environmental Psychology, 23(2), 125–134. https://doi.org/10.1016/S0272-4944(02)00110-X
- Ohlsen, G., van Zoest, W., & van Vugt, M. (2013). Gender and facial dominance in gaze cuing: Emotional context matters in the eyes that we follow. PLoS One, 8(4), e59471. https://doi.org/10.1371/journal.pone.0059471
- Okamoto-Barth, S., & Kawai, N. (2006). The role of attention in the facilitation effect and another “inhibition of return”. Cognition, 101(3), B42–B50. https://doi.org/10.1016/j.cognition.2005.11.002
- Reynolds, G. D., Courage, M. L., & Richards, J. E. (2013). The development of attention. Oxford University Press. https://doi.org/10.1093/oxfordhb/9780195376746.013.0063
- Sennwald, V., Pool, E., Brosch, T., Delplanque, S., Bianchi-Demicheli, F., & Sander, D. (2016). Emotional attention for erotic stimuli: Cognitive and brain mechanisms. Journal of Comparative Neurology, 524(8), 1668–1675. https://doi.org/10.1002/cne.23859
- Storbeck, J., & Clore, G. L. (2008). Affective arousal as information: How affective arousal influences judgments, learning, and memory. Social and Personality Psychology Compass, 2(5), 1824–1843. https://doi.org/10.1111/j.1751-9004.2008.00138.x
- Tipples, J. (2006). Fear and fearfulness potentiate automatic orienting to eye gaze. Cognition & Emotion, 20(2), 309–320. https://doi.org/10.1080/02699930500405550
- Wierzba, M., Riegel, M., Pucz, A., Leśniewska, Z., Dragan, WŁ, Gola, M., Jednoróg, K., & Marchewka, A. (2015). Erotic subset for the Nencki Affective Picture System (NAPS ERO): Cross-sexual comparison study. Frontiers in Psychology, 6(1336), 1–13. https://doi.org/10.3389/fpsyg.2015.01336
- Zsido, A. N., Matuz, A., Inhof, O., Darnai, G., Budai, T., Bandi, S., & Csatho, A. (2020). Disentangling the facilitating and hindering effects of threat-related stimuli – A visual search study. British Journal of Psychology, 111(4), 665–682. https://doi.org/10.1111/bjop.12429
