Abstract
We examined the contribution and distribution of wild-origin chinook salmon (Oncorhynchus tshawytcha) in the Salmon River, New York, during the 2005 spawning run. To determine the origin of each fish, we used scale metrics and a recently developed discriminant function model. We estimated that approximately 32% (∼20,000 individuals) of chinook salmon were of wild-origin and that there was a greater proportion of wild fish in younger age groups than in older groups (age-1 = 48.6%, age-2 = 47.2%, and age-3 = 21.1%). We also observed spatial differences in the distribution of wild- and hatchery-derived fish, with a higher proportion of wild-origin chinook salmon in the lower section of the river (47.5%) than in the upper sections (28.2%). The lowest proportion of wild fish was found in the Salmon River hatchery (12.4%), located in the upper section of the river. These findings suggest that wild-origin fish were more evenly distributed along the river, whereas hatchery fish were more likely to reach the upper parts of the river or enter the hatchery.
Introduction
The Salmon River in northern New York is a unique river with large runs of steelhead (Oncorhynchus mykiss), brown trout (Salmo trutta), coho salmon (Oncorhynchus kisutch), and chinook salmon (Oncorhynchus tshawytcha). Chinook salmon, the largest and the most abundant of the salmonines in Lake Ontario, was first stocked during the late 1960s and has been maintained by annual stocking. Currently, about 350,000 hatchery-reared chinook salmon smolts are stocked annually into the mouth of the Salmon River from the Salmon River Fish Hatchery by the New York Department of Environmental Conservation (NYDEC). The number of returning spawners has been estimated as 48,272 and 68,878 fish in 2004 and 2005, respectively (Everitt Citation2006), with 10,149 and 8132 fish returning to the Salmon River Fish Hatchery in those years (NYDEC, unpublished data).
Natural reproduction of salmonine species has been documented in several of the tributaries of the Salmon River (Abraham Citation1979; Johnson Citation1979; Johnson and Ringler Citation1981) and in 13 other tributaries to Lake Ontario (Wildridge Citation1990). Wildridge (Citation1990) estimated that up to 40% of the naturally produced salmonines came from the Salmon River system, although the contribution of wild fish to the open-lake population was unknown. More recently, a study by Everitt (Citation2006) showed extensive reproduction along the Salmon River, with an estimate of 10.0 million (±4.8 million) chinook juveniles produced in the Salmon River in 2005.
The recently documented extensive natural reproduction of chinook salmon in the Salmon River has raised questions about the contributions of wild- and hatchery-origin chinook salmon to the overall population of Lake Ontario. Connerton et al. (Citation2009) developed a discriminant model, based on early life scale increments, to determine the origin (wild or hatchery) of adult chinook salmon in Lake Ontario. Their findings showed that on average between 1989 and 2005, 62% of age-3 chinook throughout Lake Ontario were of wild-origin. The contribution of these wild fish to the spawning runs in Lake Ontario tributaries is currently unknown and may be different from the overall lake population. We hypothesized that the proportion of wild chinook salmon spawning in the Salmon River would be higher than the proportion found in the lake population because of the salmon's natal homing instinct. Similarly, we hypothesized that the proportion of hatchery fish would be relatively higher within the population of fish entering the Salmon River Fish Hatchery, which produces all chinook salmon released by the NYDEC within New York waters.
The objectives of this study were to (1) determine the total and age-specific contribution of wild- and hatchery-derived chinook salmon in the Salmon River spawning population and (2) determine the spatial distribution of wild- and hatchery-derived spawning chinook salmon throughout the Salmon River and the Salmon River Fish Hatchery.
Materials and methods
The 71-km long Salmon River, located on the west slope of the Tug Hill Plateau, drains an area of approximately 711 km2 before entering Lake Ontario at Port Ontario. Spawning salmonids returning from Lake Ontario are restricted to the lower 28 km of the river by the Lighthouse Hill hydroelectric dam at Altmar, NY. In our study, this lower reach of the Salmon River was separated into four distinct areas to determine the spatial distribution of wild- and hatchery-origin spawning chinook salmon. These areas included: (1) the lower section of the main river from Lake Ontario to the mouth of Beaverdam Brook (River kilometer (rkm) 0–24), (2) the upper section of the main river from Beaverdam Brook to the lower Salmon River reservoir (rkm 24–28), (3) the Salmon River Fish Hatchery (located 0.5 km up Beaverdam Brook), and (4) Beaverdam Brook downstream of the hatchery discharge.
Scales were collected from 175 fresh chinook salmon carcasses found along the Salmon River during the 2005 spawning run (throughout October). Samples were also collected by the NYDEC within the hatchery during egg harvest. Scales, collected above the lateral line directly behind the dorsal fin, were used to determine the origin (wild or hatchery) and age of each fish. Only fish between the ages of 1 and 3 were used because very few older fish were found.
The hatchery versus wild status of each fish was determined by the analysis of early life scale increments following the methods of Seelbach and Whelan (Citation1988) and Connerton et al. (Citation2009). Scales were pressed onto cellulose acetate slides and then images were recorded with a digital camera. Measurements of the distance from the focus to the first circulus (C01) and the area of the focus were made using Image Pro Plus. The distance to C01 was measured three times, once along a central line through the focus and then 15° on either side of the central line. The average distance was then used in the analysis.
A discriminant model developed by Connerton et al. (Citation2009) was used to evaluate the origin of each fish. The discriminant functions used were: H = −31.75206 + 950.50454 × C01 + 485.57053 × focus area, and W = −17.57397 + 707.41244 × C01 + 360.69868 × focus area. Classification was based on the largest result from the two equations (i.e., if W > H, then that sample was classified as wild-origin and vice versa). After the origins of all fish were determined, the relative abundance (F w) and variance [VF w] of the proportion of wild-origin were calculated for each site and age class. This was done using the estimated proportion of wild fish (R w), the sample size (N), and the assignment probabilities, following Worlund and Fredin (Citation1962), Seelbach and Whelan (Citation1988), and Thompson and Ferreri (Citation2002), where
-
F w = relative abundance of wild-origin fish = (R w − P hw)/(P ww − P hw),
-
[V(F w)] = variance in relative abundance = [1/(P ww − P hw)]2 V(R w),
-
V(R w) = variance in the proportion of wild fish estimated by scale characteristics
-
= (1/N){[F w F h(P ww − P hw)2] + F w[(P ww P wh) − (P hw P hh)] + (P hw P hh)}, and
-
F h = relative abundance of hatchery-origin fish = 1 − F w.
Assignment frequencies developed by Connerton et al. (Citation2009) were also used in estimating the proportions, where
-
P ww = frequency of wild fish classified as wild = 0.878,
-
P wh = frequency of wild fish classified as hatchery = 0.122,
-
P hh = frequency of hatchery fish classified as hatchery = 0.896, and
-
P hw = frequency of hatchery fish classified as wild = 0.104.
Results and discussion
We found that across all age classes and river sections, 32% of the spawning chinook salmon in the Salmon River were of wild-origin (), which were similar to the proportion found in the open-lake during the same year. Hatchery-produced fish appear to comprise a significant fraction (about 2/3) of spawning chinook salmon in the river. However, compared to historic data (1989–2005; Connerton et al. Citation2009), this could be a single-year anomaly. Work by Connerton et al. (Citation2009) indicated that the proportion of age-3 wild fish was 24% in the 2002 year class (majority of fish spawning in 2005), which was the lowest observed in Lake Ontario between 1989 and 2002.
Figure 1. Relative abundance of wild-origin chinook salmon by age class found in the Salmon River, New York with standard error bars.
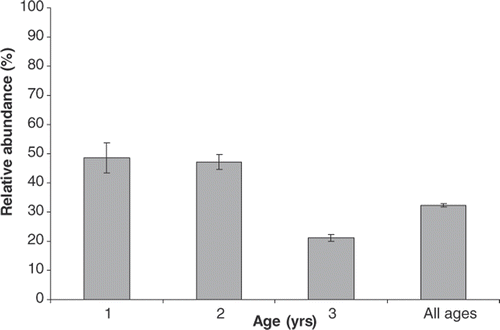
The age and origin of spawning chinook salmon collected in the Salmon River (this study) support the conclusion that 2002 produced a weak year class; 48.6% of age-1 (N = 25, 2004 year class) and 47.2% of age-2 (N = 49, 2003 year class) spawners were of wild-origin, whereas only 21.1% of age-3 (N = 101, 2002 year class) fish were wild (). In 2005, the estimated age structures of chinook salmon in the Salmon River Fish Hatchery were 2% age-1, 22% age-2, and 75% age-3 fish (Bishop and Prindle Citation2006). One of the lowest returns of age-1 chinook salmon on record occurred in 2005, although the open-lake boat census estimate was near average for 2005 (Eckert Citation2006). Using the number of returning chinook salmon in 2005 (61,878) estimated by Everitt (Citation2006), we extrapolate that approximately 20,000 wild-origin chinook salmon returned to the Salmon River to spawn in 2005.
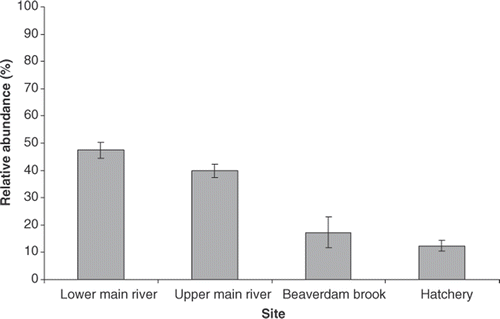
There was clear spatial segregation of wild and hatchery fish throughout the Salmon River. The proportions of wild fish in the lower (N = 50) and upper (N = 50) river were 47.5% and 39.8%, respectively, while wild-origin fish comprised only 17.3% of chinook salmon in Beaverdam Brook (N = 25) and 12.4% at the hatchery (N = 50, ). These findings showed that there was a lower percentage of wild-origin chinook salmon in the upper sections of the Salmon River system (upper main stem, Beaverdam Brook, and hatchery pooled = 28%). This suggests that almost half of the wild-origin fish either died, spawned, or were caught in the lower parts of the river system, whereas hatchery-derived fish tended to be found closer to their natal origin (i.e., the Salmon River Fish Hatchery). Observed differences in the distribution of wild- and hatchery-spawning chinook salmon suggest that hatchery-origin chinook salmon are more likely to enter Beaverdam Brook and the hatchery. This behavior of hatchery fish returning to their respective hatcheries has been seen in other populations of chinook salmon (Hard and Heard Citation1999) as well as in other salmonids (Donaldson and Allen Citation1958).
Figure 2. Relative abundances of wild-origin chinook salmon (F w) from 2005 along the Salmon River, New York with standard error bars.
The high percentage of hatchery-origin fish found in the upper main river suggests that stocking is an important component of chinook natural reproduction in the Salmon River. Our data show that natural reproduction in the Salmon River (Everitt Citation2006) and other tributaries (Wildridge Citation1990) contributes to the chinook salmon fishery along the Salmon River and in Lake Ontario (Connerton et al. Citation2009; Murry et al. Citation2010) and to the Salmon River Food web (Johnson et al. Citation2009). A similar study conducted by Peck et al. (Citation1999) on Lake Superior showed that hatchery fish contributed 70–89% of returning spawners to stocked tributaries, whereas hatchery fish represented only 6–28% of fish in unstocked tributaries. Differences in the ratio of wild versus hatchery fish between stocked and unstocked streams have also been shown in steelhead populations in Lake Michigan tributaries (Seelbach and Whalen Citation1988). This suggests that the contribution of wild-origin chinook salmon to the Lake Ontario spawning stock may be greater than what is shown in the Salmon River, because only 8 other tributaries are stocked in New York, whereas 28 tributaries are known to have runs of chinook salmon (Prindle et al. Citation2005).
Naturally produced chinook salmon are an unintended result from the introduction of this species to Lake Ontario in the late 1960s. The principal objectives of the chinook salmon stocking program when it began were to provide a biological control for an overabundant alewife population and to enhance the sport fishery of Lake Ontario. Natural reproduction is now an important component of the chinook salmon sport fisheries in Lake Ontario and its tributaries and also contributes to high predation pressure on alewife, the primary pelagic forage fish (Murry et al. Citation2010). Similarly, naturalization of chinook salmon has also been documented in the other Laurentian Great Lakes. In fact, the contribution of stocked fish in Minnesota waters was so low that stocking was discontinued after 2006 (Schreiner et al. Citation2006; Negus et al. Citation2008).
Ironically, naturally produced chinook salmon may also contribute to sustaining adequate escapement of salmon from the Salmon River fishery to the hatchery. The fishing pressure on the Salmon River is very high, with nearly 77,072 angler trips occurring from 9 September to 26 November 2005 (Prindle et al. Citation2005). At least half of the salmon run is harvested, and half of the unharvested fish die prior to spawning (Everitt Citation2006). Based on our results, stocking may still be needed to ensure maximum returns of adult chinook salmon to the hatchery and to maintain a broodstock since the majority of salmon in the hatchery were hatchery-derived fish.
References
- Abraham , WJ . 1979 . Natural reproduction of salmonids in NY tributaries of Lake Ontario , Agenda Item 5.A.2. 157 – 162 . Albany (NY) : New York State Department of Environmental Conservation .
- Bishop , DL and Prindle , SE . 2006 . Population characteristics of Pacific salmonines collected at the Salmon River Hatchery 2005 , Section 9 in 2005 Annual Report. Ann Arbor (MI) : Bureau of Fisheries Lake Ontario Unit and St. . Lawrence River Unit to the Great Lakes Fishery Commission's Lake Ontario Committee
- Connerton , MJ , Murry , BA , Ringler , NH and Stewart , DJ . 2009 . Majority of age-3 chinook salmon (Oncorhynchus tshawytscha) in Lake Ontario were wild from 1992 to 2005, based on scale pattern analysis . Journal of Great Lakes Research , 35 : 419 – 429 .
- Donaldson , LR and Allen , GH . 1958 . Return of silver salmon, Oncorhynchus kisutch (walbaum) to point of release . Transactions of the American Fisheries Society , 86 : 13 – 22 .
- Eckert , TH . 2006 . 2005 Lake Ontario fishing boat census , Section 2 in 2005 Annual Report. Ann Arbor (MI) : Bureau of Fisheries Lake Ontario Unit and St. . Lawrence River Unit to the Great Lakes Fishery Commission's Lake Ontario Committee
- Everitt DW. 2006. Natural reproduction and spawning site characteristics of chinook salmon (Oncorhynchus tshawytscha) in the Salmon River, New York [Master's thesis]. [Syracuse, NY]: State University of New York, College of Environmental Science and Forestry
- Hard , JJ and Heard , WR . 1999 . Analysis of straying variation in Alaskan hatchery chinook salmon (Oncorhynchus tshawytscha) following transplantation . Canadian Journal of Fisheries and Aquatic Science , 56 : 578 – 589 .
- Johnson JH. 1979. Natural reproduction and juvenile ecology of Pacific salmon and steelhead trout in tributaries of the Salmon River, New York [Master's thesis]. [Syracuse, NY]: State University of New York, College of Environmental Science and Forestry
- Johnson , JH , Nack , CC and Chalupnicki , MA . 2009 . Predation by fallfish (Semotilus corporalis) on Pacific salmon eggs in the Salmon River, New York . Journal of Great Lakes Research , 35 : 630 – 633 .
- Johnson , JH and Ringler , NH . 1981 . Natural reproduction of juvenile ecology of Pacific salmon and rainbow trout in tributaries of the Salmon River, New York . New York Fish and Game Journal , 28 : 49 – 60 .
- Murry , BA , Connerton , MJ , O’Gorman , R , Stewart , DS and Ringler , NH . 2010 . Lake-wide estimates of alewife biomass and chinook salmon abundance and consumption in Lake Ontario, 1989–2005: implications to prey fish sustainability . Transactions of the American Fisheries Society , 139 : 223 – 240 .
- Negus , MT , Schram , ST , Seider , MJ and Pratt , DM . 2008 . Bioenergetics evaluation of the fish community in the western arm of Lake Superior in 2004 . North American Journal of Fisheries Management , 28 : 1649 – 1667 .
- Peck , JW , Jones , TS , MacCallum , WR and Schram , ST . 1999 . Contribution of hatchery-reared fish to chinook salmon populations and sport fisheries in Lake Superior . North American Journal of Fisheries Management , 19 : 155 – 164 .
- Prindle , SE , Bishop , DL and Penney , ME . 2005 . Fall 2005 Lake Ontario tributary angler survey , Section 10 in 2005 Annual Report. Ann Arbor (MI) : Bureau of Fisheries Lake Ontario Unit and St. . Lawrence River Unit to the Great Lakes Fishery Commission's Lake Ontario Committee
- Schreiner , DR , Ostazeski , JJ , Halpern , TN and Geving , SA . 2006 . Fisheries management plan for the Minnesota waters of Lake Superior , St. Paul (MN) : Minnesota Department of Natural Resources. . Special Publication 163, Duluth
- Seelbach , PW and Whelan , GE . 1988 . Identification and contribution of wild and hatchery steelhead stocks in Lake Michigan tributaries . Transactions of the American Fisheries Society , 117 : 444 – 451 .
- Thompson , BE and Ferreri , CP . 2002 . Population biology of steelhead spawning runs in three Pennsylvania tributaries to Lake Erie . Journal of Great Lakes Research , 28 : 264 – 275 .
- Wildridge PJ. 1990. Natural reproduction of Pacific salmon and steelhead trout in Lake Ontario tributaries, New York [Master's thesis]. [Syracuse, NY]: State University of New York, College of Environmental Science and Forestry
- Worlund , DD and Fredin , RA . 1962 . “ Differentiation of stocks ” . In Symposium on pink salmon , Edited by: Wilimovsky , NJ . 143 – 153 . Vancouver : University of British Columbia .