Abstract
An increase in human urinary schistosome transmission in southern Lake Malaŵi has been suggested to be associated with decreased density of molluscivorous fishes due to illegal seine-net fishing from the shore. In addition, the increased density of snails (Melanoides spp.) through the invasion of an Asian morph could have changed the predators’ prey choice. At Chembe village, the intermediate host snail of urinary schistosomes, Bulinus nyassanus, constitutes <5% of the total gastropod fauna. This study was designed to compare crushing resistance of the intermediate host snails Bulinus globosus and B. nyassanus with that of Melanoides tuberculata, which dominates the gastropod fauna. A crush value index (CVI) as an indicator of potential prey value was expressed as the ratio of benefit (weight of snail tissue) to cost (crush resistance of snail shell). Bulinus globosus had the highest CVI. Using shell height as measure of snail size indicated that B. nyassanus had higher CVI than M. tuberculata within the size range of snails consumed by Trematocranus placodon, one of the molluscivore fishes. This may be one of the reasons that B. nyassanus is a preferred prey of T. placodon. In spite of this preference, the reduced population of T. placodon has not been able to control the population of B. nyassanus because of its apparent opportunistic feeding on large numbers of M. tuberculata.
Introduction
Urinary schistosomiasis, caused by Schistosoma haematobium has long been recognized as a major public health problem in lakeshore communities of Lake Malaŵi (WHO Citation1993), where transmission has occurred primarily in streams and protected areas of the lake (Madsen et al. Citation2004). An increase in transmission at Cape Maclear, Lake Malaŵi, however, has occurred in recent years (Cetron et al. Citation1996), and Stauffer et al. (Citation1997) suggested that this was due to an increase in abundance of intermediate host snails associated with declines in density of shallow-water molluscivorous cichlid fishes (e.g., Trematocranus placodon, Trematocranus microstoma, Mylochromis sphaerodon, and Mylochromis anaphyrmus). Prior to 1988, the open waters of Lake Malaŵi were believed to be free from schistosome production/transmission, but Madsen et al. (Citation2001) reported transmission along open shorelines in the southern part of the lake.
In Lake Malaŵi, there are two known intermediate hosts for S. haematobium – Bulinus globosus and Bulinus nyassanus (Madsen et al. Citation2001). Bulinus globosus is the primary host in sheltered areas of the lake as well as in pools and streams close to the lake shore and further inland (Madsen et al. Citation2004). Bulinus nyassanus is endemic to Lake Malaŵi and found along open shorelines on sediment of sand or gravel (Madsen et al. Citation2001). It has been suggested that reducing seine-net fishing in nearshore areas of the lake (Stauffer et al. Citation1997, Citation2006) would result in an increase in density (i.e., to pre-1988 levels) of molluscivorous fishes and possibly a decline in schistosome transmission. This outcome, however, requires that the molluscivores consume B. nyassanus.
Trematocranus placodon feeds on snails in the genera of both Melanoides and Bulinus (Evers et al. Citation2006). Stomach content analysis of field-collected T. placodon, however, showed that B. nyassanus was found in higher proportion in the stomachs than it was in the lake (Evers et al. Citation2006). In the environment, B. nyassanus constituted 3.5% of all snails, and Melanoides spp. comprised 94.8%. In the stomachs of T. placodon, the respective percentages were 24.6 and 72.3 (Evers et al. Citation2006). Although this would indicate a feeding preference of T. placodon, Melanoides spp. still dominate the stomach content T. placodon, probably because of this much greater environmental abundance. These results, however, may be confounded by the introduction of an exotic morph of Melanoides tuberculata (Genner et al. Citation2004). Indigenous Melanoides spp. have more sculptured shells than the introduced morph (i.e., more pronounced spiral grooves and presence of axial ribs; Genner et al. Citation2004) and this could represent an anti-predatory adaptation. The exotic morph has a rather smooth shell with only spiral lines; its shell may be less substantial than those of the indigenous Melanoides spp. Optimal foraging models indicate that prey are added to the diet in decreasing order of energetic benefit/cost ratio (Pyke et al. Citation1977; Begon et al. Citation1996). Our objective was to compare one aspect of prey suitability by comparing prey value as a crush value index (CVI) based on crushing resistance and organic dry weight (dry tissue mass) of B. globosus, B. nyassanus, and the introduced morph of M. tuberculata.
Materials and methods
Snails were collected in the field during June–August 2003, and preserved in 70% ethanol. Melanoides tuberculata (the morph form) were collected in Lake Malaŵi at Chembe village. Bulinus globosus were collected from a nearby stream. The preserved snails were rinsed in distilled water, dried at 70°C for 24 h, and placed in desiccators. For M. tuberculata, shells that were not eroded at the apex were selected so as to measure the height accurately and test the strength of complete shells. Shell height was measured to the nearest 0.05 mm using calipers. We elected to use shell height to represent snail size although shells are crushed across their width, because M. tuberculata of a width comparable to those of the two Bulinus species would be too large for the fish to consume. Individual shell widths were not measured originally but were subsequently extrapolated from shell heights on the basis of measurements made of shell height and shell width of 25 specimens from the same samples as used for the crush resistance trials and covering the same size range for each species (r 2 values were 0.7499, 0.9216, and 0.9644 for B. globosus, B. nyassanus, and M. tuberculata, respectively).
Crush resistance was determined (sensu Brodersen et al. Citation2003) by placing dried snails with the aperture facing down in a Petri dish underneath a plexiglass cylinder closed at the bottom; this procedure ensured that force was applied to snails against their minimal dimension, consistent with the way the molluscivore labrid fish manipulate snails between pharyngeal plates (Stein et al. Citation1984). The tube was gradually filled with sand until the shell was crushed. The weight of the plexi-glass tube including sand was the crush weight. The snail and shell fragments were brushed into crucibles, and the total dry weight was determined to the nearest 0.1 mg. Crucibles containing all snail parts were combusted at 500°C for 1.5 h, and subsequently reweighed to yield inorganic weight. Organic weight (ash-free dry weight) was determined by subtracting inorganic weight from the total dry weight.
The CVI was calculated as the organic content in mg divided by crush weight in kg. Differences among species were tested using analysis of covariance (p < 0.05) with shell height as covariate. Both the dependent variables and the covariate were log10 transformed.
Results and discussion
The characteristics of the three snail species are given in . The log10 transformed dry weight increased linearly with log10 transformed shell height for the three species, but B. nyassanus were heavier than either B. globosus or M. tuberculata when comparing snails of similar size.
Table 1. Characteristics of the three snail species investigated.
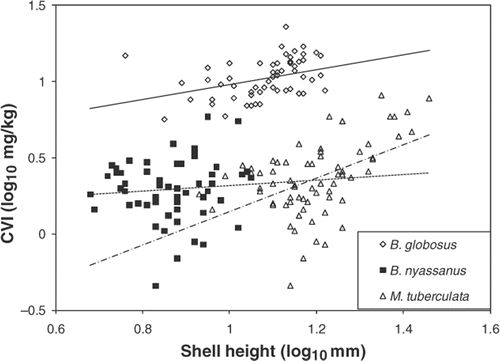
Generally, as the shell height increased, so did CVI (), although the slopes differed significantly (p < 0.001), and the slope for B. nyassanus was not significantly different from zero. Bulinus globosus had the highest CVI at a given size. Melanoides tuberculata, however, showed a much steeper increase in CVI with shell size than the two Bulinus species. Within the size range covered by B. nyassanus, it had a higher CVI than M. tuberculata and only when M. tuberculata exceeded a shell height of about 15 mm did CVI on average exceed that of the smaller sized B. nyassanus. Since fish tend to crush snails against their smaller dimension (Stein et al. Citation1984), the shell width might be a better representation of shell size than height. When CVI was plotted against shell width (data not shown), there was no indication of a difference between B. nyassanus and M. tuberculata.
Bulinus globosus has the highest CVI, which might suggest that it would be the most profitable prey for T. placodon followed by B. nyassanus and M. tuberculata. The habitat of B. globosus within the lake (sheltered areas with macrophytes or stones) only slightly overlaps with that of T. placodon and therefore, this snail species is not consumed in any appreciable numbers by T. placodon (Evers et al. Citation2006). There may be other predators of B. globosus, however, such as Metriaclima lanisticola, which is an oral sheller (Lundeba et al. Citation2007), and hence crush resistance is of little significance. The habitat of B. globosus may be in refugia provided by heavy vegetation, while B. nyassanus with its stronger shell and its behavior of descending into the top sediment is capable of choosing open sand habitats where it is more vulnerable to molluscivores. Although B. nyassanus has higher CVI than small M. tuberculata and therefore is potentially a better catch, the higher environmental abundance of M. tubeculata might make this species the most commonly consumed prey. The disproportionate relative abundance of B. nyassanus in the stomachs of T. placodon (Evers et al. Citation2006) however, would indicate preferential foraging by T. placodon.
Differences in shell morphology between Bulinus spp. and M. tuberculata are complicating comparisons between these species. If T. placodon, like some molluscivores (Stein et al. Citation1984), manipulates snails between pharyngeal plates to crush them against their minimal dimension, the shell size and crush resistance are not the only determinants of prey value. The globose shell of B. nyassanus compared to the elongated shell of M. tuberculata may make it easier to handle for a predator. Thus, shell shape and other morphological characteristics may function as anti-predator adaptations (Vermeij and Currey Citation1980; West et al. Citation1991; West and Cohen Citation1996).
Predictions about molluscivores’ prey of choice are difficult to make and are influenced by other factors than the preys’ crushing resistance. Factors such as spatial distribution, dispersion, position in sediment, vegetation attachment, shell morphology, and refugia activity also need consideration (Palmer Citation1979; Osenberg and Mittelbach Citation1989; Alexander and Covich Citation1991). The possible implications of increased density of molluscivore fishes for populations of the intermediate hosts within the lake therefore are difficult to predict. The primary snail predator along exposed shorelines with sandy sediment is T. placodon, and in this habitat B. nyassanus is the principal host for S. haematobium. It is interesting that T. placodon seems to preferably consume B. nyassanus over Melanoides spp. (Evers et al. Citation2006), possibly reflecting the larger prey value of B. nyassanus as shown in this study.
It is possible that T. placodon selects indiscriminately and thus is an opportunistic snail predator. Density of T. placodon in shallow water (to a depth of about 5 m) at Chembe was clearly lower in 2003 than it was in 1980, and B. nyassanus density peaked at water depths of 1.5–6 m (Stauffer et al. Citation2006). Preventing beach seine-net fishing would seem to be a realistic approach for snail control in Lake Malaŵi to reduce the incidence of schistosomiasis.
Acknowledgements
Funding was provided by the NSF/NIH joint program in Ecology of Infectious Diseases (DEB-0224958). The work was conducted in collaboration with Malaŵi Fisheries Department, Malaŵi Park and Wildlife Department and Bunda College of Agriculture, University of Malaŵi.
References
- Alexander , JE and Covich , AP . 1991 . Predation risk and avoidance behaviour in two freshwater snails . Biological Bulletin , 180 ( 3 ) : 387 – 393 .
- Begon , M , Harper , JL and Townsend , CR . 1996 . Ecology , 3rd , London, UK : Blackwell Science Ltd .
- Brodersen , J , Chimbari , MJ and Madsen , H . 2003 . Prosobranch mollusc species- and size- preference of Sargochromis codringtonii (Cichlidae) in Lake Kariba, Zimbabwe . African Journal of Aquatic Science , 28 ( 2 ) : 1 – 4 .
- Cetron , MS , Chitsulo , L , Sullivan , JJ , Pilcher , J , Wilson , M , Noh , J , Tsang , VC , Hightower , AW and Addiss , DG . 1996 . Schistosomiasis in Lake Malawi . The Lancet , 348 ( 9037 ) : 1274 – 1278 .
- Evers , BNH , McKaye , KM and Stauffer , JR Jr . 2006 . The schistosome intermediate host, Bulinus nyassanus, is a preferred food for the cichlid fish, Trematocranus placodon, at Cape Maclear, Lake Malaŵi . Annals of Tropical Medicine and Parasitology , 100 ( 1 ) : 75 – 85 .
- Genner , MJ , Michel , E , Erpenbeck , D , De Voogd , N , Witte , F and Pointier , JP . 2004 . Camouflaged invasion of Lake Malawi by an Oriental gastropod . Molecular Ecology , 13 ( 8 ) : 2135 – 2141 .
- Lundeba , M , Likongwe , JS , Madsen , H and Stauffer , JR Jr . 2007 . Potential of Metriaclima lanisticola (Teleostei: Cichlidae) for biological control of schistosome intermediate host snails . African Zoology , 42 ( 1 ) : 45 – 49 .
- Madsen , H , Bloch , P , Phiri , H , Kristensen , TK and Furu , P . 2001 . Bulinus nyassanus is an intermediate host for Schistosoma haematobium in Lake Malaŵi . Annals of Tropical Medicine and Parasitology , 95 ( 4 ) : 353 – 360 .
- Madsen , H , Stauffer , JR , Bloch , P , Konings , A , McKaye , KR and Lilongwe , JS . 2004 . Schistosomiasis haematobium transmission in Lake Malaŵi . African Journal of Aquatic Science , 29 ( 1 ) : 117 – 119 .
- Osenberg , CW and Mittelbach , GG . 1989 . Effects of body size on the predator-prey interaction between pumpkinseed sunfish and gastropods . Ecological Monographs , 59 ( 4 ) : 405 – 432 .
- Palmer , AR . 1979 . Fish predation and the evolution of gastropod shell sculpture: experimental and geographical evidence . Evolution , 33 ( 2 ) : 697 – 713 .
- Pyke , GH , Pullimam , HR and Charnov , EL . 1977 . Optimal foraging: a selective review of theory and tests . Quarterly Review of Biology , 52 ( 2 ) : 137 – 154 .
- Stauffer , JR , Arnegard , ME , Cetron , M , Sullivan , JJ , Chitsulo , LA , Turner , GF , Chiota , S and McKaye , KR . 1997 . Controlling vectors and hosts of parasitic diseases using fishes . BioScience , 47 ( 1 ) : 41 – 49 .
- Stauffer , JR Jr , Madsen , H , McKaye , H , Konings , A , Bloch , P , Ferreri , CP , Likongwe , J and Makaula , P . 2006 . Molluscivorous fishes – potential for biological control of schistosomiasis . EcoHealth , 3 : 22 – 27 .
- Stein , RA , Goodman , CG and Marschall , EA . 1984 . Using time and energetic measures of cost in estimating prey value for fish predators . Ecology , 65 ( 3 ) : 702 – 715 .
- Vermeij , DJ and Currey , JD . 1980 . Geographical variation in the strength of thaidid snail shells . Biological Bulletin , 158 ( 3 ) : 383 – 389 .
- West , K and Cohen , A . 1996 . Shell microstructure of gastropods from Lake Tanganyika: adaptation, convergent evolution and escalation . Evolution , 50 ( 2 ) : 672 – 681 .
- West , K , Cohen , A and Baron , M . 1991 . Morphology and behavior of crabs and gastropods from Lake Tanganyika, Africa: implications for lacustrine predator-prey coevolution . Evolution , 45 ( 3 ) : 589 – 607 .
- WHO. 1993. The control of schistosomiasis. Second report of the WHO Expert Committee. Geneva: WHO Library Cataloguing-in-Publication Data. Technical support series 830