Abstract
Cultures of Microcystis aeruginosa were inoculated into media with different nitrogen concentrations (5%, 10%, 25%, 100% N of standard BG-11 media) and cultured under two light intensities for 35 days. The populations of M. aeruginosa tended to increase with decreased nitrogen concentrations in both the high light intensity and the low light intensity. Cell densities in high light intensity were significantly higher than those in low light intensity under lower nitrogen concentrations (5%, 10%, 25% N), whereas the result was reversed under the high nitrogen concentration (100% N). A four-parameter logistic model fitted all the population dynamics well. Soluble extracellular polysaccharide content of M. aeruginosa cultured in the 100% N media was significantly higher than in M. aeruginosa cultured in the lower nitrogen media; however, the bound polysaccharide and total polysaccharide of M. aeruginosa cultured in 5% N media were significantly higher than those of M. aeruginosa cultured in all other nitrogen concentration media. Significant differences in bound polysaccharide and total polysaccharide were also detected between the high light intensity and the low light intensity. There was a statistically significant interaction between light intensity and nitrogen concentration on the soluble extracellular polysaccharide content but not on bound polysaccharide or total polysaccharide.
Introduction
Microcystis aeruginosa is one of the dominant species of cyanobacteria that form surface water blooms in eutrophic lakes and has received much attention because of water management problems associated with its blooms (Dokulil and Teubner Citation2000). The distribution and abundance of M. aeruginosa are to a large extent affected by factors such as light and nutrient availability acting synergistically with other physical and chemical factors. Nitrogen and phosphorus are generally considered as the main nutrients for algal growth in temperate and subtropical lakes. It was suggested that the lower ratio between these two nutrients may promote the development of cyanobacterial blooms (Smith Citation1983). There are some studies about the impact of nitrogen concentration on M. aeruginosa from different perspectives, such as growth (e.g., Fujimoto et al. Citation1997; Boumnich et al. Citation2001; Ou et al. Citation2004), microcystin content (e.g., Orr and Jones Citation1998; Vezie et al. Citation2002), and buoyancy (e.g., Konopka et al. Citation1987; Brookes and Ganf Citation2001; Chu et al. Citation2007). It is well known that light intensity and nutrient have important effect on growth and polysaccharide production of algae (Friedman et al. Citation1991; Moreno et al. Citation1998; Corzo et al. Citation2000; Guerrini et al. Citation2000; Staats et al. Citation2000; Granum et al. Citation2002; Otero and Vincenzini Citation2003; Magaletti et al. Citation2004). However, little information is available about the combined effects of nitrogen concentration and light intensity on population dynamics and polysaccharide content in M. aeruginosa. We conducted a laboratory experiment to evaluate the effect of various nitrogen concentrations in combination with light intensities on growth and polysaccharide content of M. aeruginosa in batch culture.
Materials and methods
Organisms and cultivation
Microcystis aeruginosa (FACHB 927) was obtained from the Institute of Hydrobiology, Chinese Academy of Sciences. The cyanobacterium was batch-cultured axenically in liquid BG-11 medium (Rippka et al. Citation1979) in 1 L flasks at 25°C and under a fluorescent light intensity of 35 µmol photons m−2 s−1 with a light–dark period of 12:12 h.
Experimental design
Experiments were carried out at four nitrogen levels (5%, 10%, 25%, and 100% of nitrogen in BG-11) in combination with two light intensities (35 and 80 µmol photons m−2 s−1). To minimize intracellular nutrient storage and nitrogen in the medium, prior to the experimental phase, M. aeruginosa cultures in exponential growth were harvested by centrifugation and then resuspended in nitrogen-free BG-11 medium for 2 days, and then this process was repeated. After 4 days, the suspensions were collected by centrifugation and the pellets were inoculated into the modified BG-11 medium with different proportions of nitrogen (5%, 10%, 25%, and 100% of N in BG-11). The measured nitrogen concentrations were 12.73, 25.08, 61.63, and 254.73 mg L−1, respectively. Inoculum of each of the four groups was grown in batch culture in 500-mL Erlenmeyer flasks containing 200 mL of above liquid medium under light intensities of 35 and 80 µmol photons m−2 s−1. The initial cell density was 2.067 × 105 cells mL−1. The experiment was cultured at 25°C and run in triplicate for 35 days. The cyanobacterium cultures within the flasks were shaken two times each day.
Growth
Samples were collected every other day and fixed in Lugol's solution (2%), and cell density was determined using a blood-cell counting chamber under a microscope.
Polysaccharide contents assay
At the end of the experiment, 10 mL samples were taken from the cultures for the extraction of the polysaccharide fractions. The total amount of polysaccharide was equated to the sum of the soluble extracellular and the bound fractions. The methods used to measure polysaccharide followed the procedure outlined by Yang et al. (Citation2010).
Statistical analyses
All data were presented as means ± 1 SE. Cell density was analyzed by three-way ANOVA, whereas polysaccharide was analyzed by two-way ANOVA (α = 0.05). To reveal the population dynamics of M. aeruginosa cultured under different conditions, the four-parameter logistic model, Y = a/(1 + (X/X 0) b ) + Y 0, was chosen to fit cell density data, where Y is the cell density, X is the time, a is the maximum increase of cell density, Y 0 is the initial cell density, a + Y 0 is the maximum cell density, X 0 is the time that cell density increases to 50% of the maximum cell density, and b is a constant. All statistical analyses were carried out with SigmaPlot 11.0.
Results and discussion
Algal cell densities increased significantly (p < 0.001) under all conditions over the course of the experiment (). The growth of M. aeruginosa tended to increase with decreased nitrogen concentrations in both the high light intensity and the low light intensity (i.e., growth was inhibited with increased nitrogen concentration; p < 0.001). This is very similar to the other studies on other algae (Hwang and Lu Citation2000; Shi et al. Citation2005; Chen et al. Citation2009). This may be due to the fact that algae cultured under high nitrate concentrations can accumulate intracellular nitrite, which is produced by nitrate reductase and can inhibit the growth of algae (Chen et al. Citation2009). These findings suggested that although nitrate is a macronutrient and can serve as a good nitrogen source for many species of phytoplankton, high nitrate concentrations do not benefit the growth of phytoplankton.
Figure 1. Population dynamics of M. aeruginosa cultured at four nitrogen concentrations (5%, 10%, 25%, and 100% of nitrogen in BG-11) in combination with two light intensities. The formula in the figure follows four-parameter logistic model. Vertical lines represent ±SE.
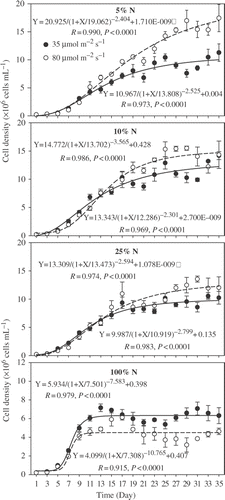
Significant differences in cell densities were also observed between high light intensity and low light intensity at all nitrogen concentrations (p < 0.001). In detail, cell densities in high light intensity were significantly higher than those in low light intensity under lower nitrogen concentration (5%, 10%, 25% N), whereas the result was reversed under high nitrogen concentration (100% N). Three-way ANOVA indicated that there was a statistically significant interaction between nitrogen concentration in media and light intensity on cell density of M. aeruginosa (p < 0.001). Also, there was a statistically significant interaction between time, nitrogen concentration in media, and light intensity on cell density (p < 0.001; ).
Table 1. Summary of three-way ANOVA on the interactions between time, light intensity, and N concentration on cell density.
The four-parameter logistic model fitted the population dynamics well (). From the parameters derived from logistic model, cell density of M. aeruginosa cultured in 100% N media increased more rapidly and reached the stationary phase much earlier than in the other treatments; however, its maximum cell density was lower at 100% N than in lower nitrogen concentrations, indicating that growth decreased with increased nitrogen concentration within the range of nitrogen concentration we used. The fact that M. aeruginosa grew well in the lowest nitrogen concentration media indicated that the nitrogen concentrations we used were all above the nitrogen-limited level; otherwise, growth would have been inhibited as reported in some studies (e.g.,, Fujimoto et al. Citation1997; Boumnich et al. Citation2001; Sabour et al. Citation2009).
Soluble extracellular polysaccharide content of M. aeruginosa cultured in the 100% N media was significantly higher than those of M. aeruginosa cultured in low nitrogen media; however, the bound polysaccharide and total polysaccharide of M. aeruginosa cultured in 5% N media were significantly higher than those of M. aeruginosa cultured in other media, suggesting that nitrogen concentration has a significant impact on different polysaccharide fractions (). Significant differences in bound polysaccharide and total polysaccharide were also detected between the high light intensity and the low light intensity (), indicating that light intensity also has an important effect on polysaccharide production of M. aeruginosa, as found in some other algae (Friedman et al. Citation1991; Moreno et al. Citation1998; Otero and Vincenzini Citation2003). Two-way ANOVA indicated that there was a statistically significant interaction between light intensity and nitrogen concentration on the soluble extracellular polysaccharide contents but not on bound polysaccharide and total polysaccharide (). Previous studies have reported on the increased rate of release of extracellular polysaccharides in many different species of algae subjected to P or N deficiency (Moreno et al. Citation1998; Corzo et al. Citation2000; Guerrini et al. Citation2000; Staats et al. Citation2000; Granum et al. Citation2002; Magaletti et al. Citation2004). The increase in extracellular polysaccharide release by algae cultured under nutrient deficiency has been considered as a metabolic response to an unbalanced nutrient ratio in the medium and to decreased growth (Myklestad and Haug Citation1972; Guerrini et al. Citation2000). In this study, based on the results of growth, nitrogen content in all media should be above the limiting level as M. aeruginosa did not experience nitrogen stress; however, M. aeruginosa cultured under lower nitrogen content media still produced much more polysaccharide compared with those cultured in higher nitrogen media, suggesting that nitrogen status profoundly affects polysaccharide production.
Figure 2. Contents of soluble extracellular polysaccharide, bound polysaccharide, and total polysaccharide content of M. aeruginosa cultured at four nitrogen concentrations (5%, 10%, 25%, and 100% of nitrogen in BG-11) in combination with two light intensities by the end of the experiments. Vertical lines represent ±SE.
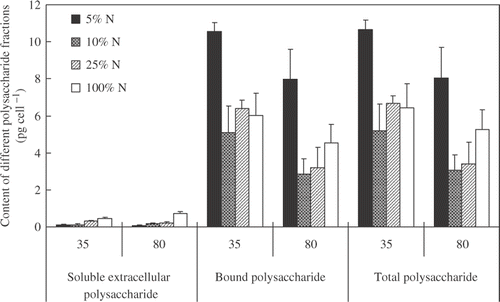
Table 2. Summary of two-way ANOVA on the interactions between light intensity and N concentration on different polysaccharide fractions.
Acknowledgements
We thank Ewan Minter for some suggestions and linguistic improvement. This study was supported by the National Natural Science Foundation of China (30670404, 30970500), the Natural Science Foundation of Jiangsu Province (BK2007743), and the Open Foundation of State Key Laboratory of Lake Science and Environment (2010SKL009).
References
- Boumnich , L , Derraz , A , Naji , B and Dauta , A . 2001 . Influence of light-temperature and nutrient conditions on the growth and intracellular storage (nitrogen and phosphorus) of Microcystis aeruginosa Kützing isolated from the El Kansera (Morocco) eutrophic impoundment . Annales de Limnologie – International Journal of Limnology , 37 : 191 – 198 .
- Brookes , J and Ganf , GG . 2001 . Variations in the buoyancy response of Microcystis aeruginosa to nitrogen, phosphorus and light . Journal of Plankton Research , 23 : 1399 – 1411 .
- Chen , W , Zhang , Q and Dai , S . 2009 . Effects of nitrate on intracellular nitrite and growth of Microcystis aeruginosa . Journal of Applied Phycology , 21 : 701 – 706 .
- Chu , ZS , Jin , XC , Yang , B and Zeng , QR . 2007 . Buoyancy regulation of Microcystis flos-aquae during phosphorus-limited and nitrogen-limited growth . Journal of Plankton Research , 29 : 739 – 745 .
- Corzo , A , Morillo , JA and Rodríguez , S . 2000 . Production of transparent exopolymeric particles (TEP) in cultures of Chaetoceros calcitrans under nitrogen limitation . Aquatic Microbial Ecology , 23 : 63 – 72 .
- Dokulil , MT and Teubner , K . 2000 . Cyanobacterial dominance in lakes . Hydrobiologia , 438 : 1 – 12 .
- Friedman , C , Dubinsky , Z and Arad , SM . 1991 . Effect of light-intensity on growth and polysaccharide production in red and blue-green Rhodophyta unicells . Bioresource Technology , 38 : 105 – 110 .
- Fujimoto , N , Sudo , R , Sugiura , N and Inamori , Y . 1997 . Nutrient-limited growth of Microcystis aeruginosa and Phomzidium tenue and competition under various N:P supply ratios and temperatures . Limnology and Oceanography , 42 : 250 – 256 .
- Granum , E , Kirkvold , S and Myklestad , SM . 2002 . Cellular and extracellular production of carbohydrates and amino acids by the marine diatom Skeletonema costatum: diel variations and effects of N depletion . Marine Ecology Progress Series , 242 : 83 – 94 .
- Guerrini , F , Cangini , M , Boni , L , Trost , P and Pistocchi , R . 2000 . Metabolic responses of the diatom Achnanthes brevipes (Bacillariophyceae) to nutrient limitation . Journal of Phycology , 36 : 882 – 890 .
- Hwang , DF and Lu , YH . 2000 . Influence of environmental and nutritional factors on growth toxicity and toxin profile of dinoflagellate Alexandrium minutum . Toxicon , 38 : 1491 – 1503 .
- Konopka , A , Kromkamp , JC and Mu , LR . 1987 . Buoyancy regulation in phosphate-limited cultures of Microcystis aeruginosa . FEMS Microbiology Ecology , 45 : 135 – 142 .
- Magaletti , E , Urbani , R , Sist , P , Ferrari , CR and Cicero , AM . 2004 . Abundance and chemical characterization of extracellular carbohydrates released by the marine diatom Cylindrotheca fusiformis under N- and P-limitation . European Journal of Phycology , 39 : 133 – 142 .
- Moreno , J , Vargas , MA , Olivares , H , Rivas , J and Guerrero , MG . 1998 . Exopolysaccharide production by the cyanobacterium Anabaena sp . ATCC 33047 in batch and continuous culture. Journal of Biotechnology , 60 : 175 – 182 .
- Myklestad , DS and Haug , A . 1972 . Production of carbohydrates by the marine diatom Chaetoceros affinis var . willei (Gran) Hustedt. I. Effect of the concentration of nutrients in the culture medium. Journal of Experimental Marine Biology and Ecology , 9 : 125 – 136 .
- Orr , PT and Jones , GJ . 1998 . Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures . Limnology and Oceanography , 43 : 1604 – 1614 .
- Otero , A and Vincenzini , M . 2003 . Extracellular polysaccharide synthesis by Nostoc strains as affected by N source and light intensity . Journal of Biotechnology , 102 : 143 – 152 .
- Ou , MM , Zhou , BX , Xie , WJ , Jiang , JH and Cai , WM . 2004 . Effects of nutrients on Microcystis growth more easily forming bloom . Journal of Environmental Science , 16 : 934 – 937 .
- Rippka , R , Deruelles , J , Waterbury , J , Herdman , M and Stanier , R . 1979 . Generic assignments, strain histories and properties of pure cultures of cyanobacteria . Journal of General Microbiology , 111 : 1 – 61 .
- Sabour , B , Loudiki , M and Vasconcelos , V . 2009 . Growth responses of Microcystis ichthyoblabe Kützing and Anabaena aphanizomenoides Forti (cyanobacteria) under different nitrogen and phosphorus conditions . Chemistry and Ecology , 25 : 337 – 344 .
- Shi , Y , Hu , H and Cong , W . 2005 . Positive effects of continuous low nitrate levels on growth and photosynthesis of Alexandrium tamarense (Gonyaulacales, Dinophyceae) . Phycological Research , 53 : 43 – 48 .
- Smith , VH . 1983 . Low nitrogen to phosphorus ratios favor dominance by blue-green algae in lake phytoplankton . Science , 221 : 669 – 671 .
- Staats , N , Stal , LJ and Mur , LR . 2000 . Exopolysaccharide production by the epipelic diatom Cylindrotheca closterium: effects of nutrient conditions . Journal of Experimental Marine Biology and Ecology , 249 : 13 – 27 .
- Vezie , C , Rapala , J , Vaitomaa , J , Seitsonen , J and Sivonen , K . 2002 . Effect of nitrogen and phosphorus on growth of toxic and nontoxic Microcystis strains and on intracellular microcystin concentrations . Microbial Ecology , 36 : 181 – 192 .
- Yang , Z , Liu , Y , Ge , J , Wang , W , Chen , YF and Montagnes , D . 2010 . Aggregate formation and polysaccharide content of Chlorella pyrenoidosa Chick (Chlorophyta) in response to simulated nutrient stress . Bioresource Technology , 101 : 8336 – 8341 .