Abstract
Flooded swamps in the Atchafalaya River basin, Louisiana, USA, are thought to be a major mechanism for removing excess riverine nitrate that may be causing hypoxia in coastal Louisiana. Two denitrification methods, the 15N2 flux and acetylene inhibition technique, were compared and evaluated. Sediment samples were collected from three baldcypress swamp sites located within the Atchafalaya River basin. Highly 15N-labeled (61.7 at.%) and unlabeled nitrate (100 mg NO3–N L−1) were applied to the floodwater of separate sets of sediment cores. Denitrification potential was measured 2, 6, 10, and 16 days after nitrate addition. Two days after nitrate addition, no significant differences in denitrification rates were observed between methods. On days 6, 10, and 16, denitrification measured by the 15N2 method was higher compared to the acetylene inhibition procedure. When significance was observed, 15N2 denitrification was approximately 5.3 times greater.
Introduction
The Mississippi–Atchafalaya River basin (MARB) drains approximately 41% of the conterminous United States (all or part of 31 states). Nitrogen fertilizer applied to MARB farmland has increased from less than one million metric tons in 1951 to about seven million metric tons in 1996 (Goolsby et al. Citation2001).
Approximately 90% of the annual discharge of nitrogen (N) to the Gulf of Mexico (GOM) is transported by the Mississippi River and Atchafalaya River (Dunn Citation1996). Average annual flux of N (1981–2005) to the GOM was 1,470,000 metric tons of which 61% was inorganic nitrate (Aulenbach et al. Citation2007). About 90% of the nitrate entering the GOM emanates from nonpoint pollution sources (mainly agriculture), with highest discharges observed in the spring and early summer.
Excess N (mainly nitrate) from the MARB may be one of the major causes of low dissolved oxygen (<2 mg L−1) in a large hypoxic area along the Louisiana–Texas coasts (Rabalais et al. Citation2002). Hypoxia can cause increased stress or death to coastal bottom-dwelling organisms that cannot flee the oxygen depleted zone. The size of the GOM hypoxic zone is highly variable and has increased over the past two decades. The zone averaged approximately 8300 km2 from 1985 to 1992, but from 1993 to 2001 averaged 16,000 km2 (Rabalais et al. Citation2002). The size of the hypoxic zone in 2007 was estimated at 20,500 km2 and may increase over the next few years due to increased ethanol production from corn in the MARB (USGS Citation2007).
The Mississippi River/Gulf of Mexico Watershed Nutrient Task Force (Citation2001) developed an action plan to reduce hypoxia and the size of the zone to less than 5000 km2 by 2015 for the northern GOM. Enhancing and/or restoring natural denitrification has been proposed to reduce nitrogen loads. Remediation actions to increase denitrification include restoring and creating riparian buffers and coastal wetlands and diverting nitrate-elevated river water into Louisiana's coastal ecosystems (USEPA Citation2001; Mississippi River/Gulf of Mexico Watershed Nutrient Task Force Citation2008).
Denitrification is the microbial reduction of nitrate () and nitrite (
) to gaseous end products nitric oxide (NO), nitrous oxide (N2O), and dinitrogen (N2) which represents a major pathway for N removal from aquatic and wetland ecosystems (Mitsch and Gosselink Citation2000). Denitrification processes are affected by soil moisture and texture, pH, microbial community structure, sediment O2,
concentration, organic matter, and temperature (Groffman Citation1994; Clement et al. Citation2002; Seitzinger et al. Citation2006; Hernandez and Mitsch Citation2007). Denitrification is fueled by
produced from mineralization of inorganic
and by water column
diffusion into reduced sediments.
Direct (measurement of N2O and/or N2) and indirect procedures have been used to estimate denitrification. Denitrification methods include N2 quantification, 15N stable isotope tracers, N2 : Ar ratios, acetylene inhibition, nitrogen mass balance, nitrate disappearance, molecular techniques, and stoichiometric approaches. All denitrification methods have advantages, disadvantages, limitations, and assumptions that must be evaluated prior to method selection (Cornwell et al. Citation1999; Steingruber et al. Citation2001; Groffman et al. Citation2006; Wallenstein et al. Citation2006).
The objective of this investigation was to compare and evaluate two denitrification methods after addition to flooded sediments. Potential denitrification rates were measured using the 15
→ 15N2 flux method and the acetylene inhibition technique.
Materials and methods
Baldcypress sediments were collected from three flooded locations within the Atchafalaya River basin. Sites were located along Bayou Cowan (BC), Jones Bayou (JB), and Bee Bayou (BB). Bulk sediment (0–15 cm depth) was manually collected, sealed in polyethylene bottles, and iced. Sediments were transported to the laboratory and stored at 1–2°C. Total sediment organic matter was measured by loss of ignition at 550°C for 2 h and total N was determined with a Leco C and N analyzer (Leco Corp., St. Joseph, Michigan, USA). Clay content was determined by the pipette method. Sediment organic matter, total N, and clay content ranged from 16.8 to 32.3, 0.4 to 0.8, and 53% to 66%, respectively. The lowest concentrations of these components were observed for BB, and highest were for BC. Substrate pH values varied from 4.4 (JB) to 5.5 (BB).
For each sediment type (site), six glass incubation jars (9 cm diameter by 16 cm height) were filled with sediment to a height of 6 cm, and the sediment was then packed to field bulk density. A 4-cm layer of nitrate-free distilled water was added, and cores were allowed to incubate (22°C) for 1 week to re–establish the thin surface oxidized layer (observed in the field) overlaying the reduced zone. Then, 180 mg of 15N labeled (61.7 at.%) KNO3 (dissolved) was added to the floodwater of two sediment cores, and 180 mg of dissolved KNO3 (unlabeled) was added to the floodwater of the remaining four cores. A high concentration of NO3–N was required to assure sediment cores were not nitrate-limited and to detect 15N2 in the headspace volume (containing 78% N2) at the end of the 6-hour incubation. Labeled nitrate was only added to two cores due to cost. Gas samples were collected at 2, 6, 10, and 16 days after nitrate addition.
Acetylene inhibition method
The acetylene (C2H2) inhibition method was used to estimate potential denitrification (Groffman Citation1994). After unlabeled nitrate (dissolved) was added to the floodwater, the incubation jars were sealed (screw caps fitted with rubber septa), and purified C2H2 was then added to the headspace volume (∼10% v/v) and floodwater. Headspace gas samples were collected 2 and 6 h after C2H2 addition with a 2-mL gas-tight syringe to estimate the increase of N2O. Two hours were allowed for C2H2 diffusion through the floodwater into the sediment before gas collection. After 6 h, screw caps were removed and cores were exposed to the atmosphere. Floodwater/sediment cores were only exposed to C2H2 for a short time to minimize effects on microorganisms (Watts and Seitzinger Citation2000). Nitrous oxide fluxes were calculated using the closed chamber equation of Rolston (1986): F = (V/A)(273/T)(ΔC/ΔT), where V is core headspace volume, A the core sediment area, T the absolute temperature of headspace gases, and ΔC/ΔT the change in N2O concentration per unit of time. The Bunsen absorption coefficient was used to estimate N2O dissolved in the floodwater. Nitrous oxide flux is reported as mg N evolved m−2 d−1 which represents total denitrification (N2O + N2).
Nitrous oxide concentrations were determined with a Shimadzu GC-14A gas chromatograph (Shimadzu Scientific Instruments, Inc., Columbia, Maryland, USA) fitted with a 1-mL sample loop, Poropak Q 1.8 m ss column, and electron capture detector (ECD) and calibrated with certified N2O reference standards (Scott Specialty Gases, Inc., Plumsteadville, Pennsylvania, USA). Nitrogen was the carrier gas and the instrument operated at 40, 100, and 290°C for the oven, injector, and ECD, respectively (Lindau et al. Citation1998).
15N2 method
Highly 15N-labeled nitrate was added to the floodwater of two sediment cores collected from each of the three sites. Incubation jars were sealed and headspace gas samples were collected at 0 (background) and 6 h with a gas-tight syringe. Collected gas samples were immediately transferred into evacuated glass Vacutainers (100 mm length by 16 mm i.d.). Prior to gas collection, Vacutainers (Becton Dickinson, Franklin Lakes, New Jersey, USA) were placed on a high-vacuum system to remove residual gases. A slight over-pressure of headspace gas was injected into each Vacutainer to prevent atmospheric gas contamination. Screw caps were removed at the end of each 6 h incubation.
Stable 15N2 isotopic distribution (28, 29, and 30 masses) was determined with a gas chromatograph coupled to a triple collector 20/20 isotope ratio mass spectrometer (GC–IRMS, Robo Prep-G Plus, Europa Scientific, SerCon Ltd., UK). Fluxes of 15N2 from the floodwater/sediment cores were calculated using the equations and 15XN and d values outlined by Hauck and Bouldin (Citation1961) and Mulvaney and Boast (Citation1986). Emission of 15N2 is reported as mg N evolved m−2 d−1.
Statistical methods
All statistical analyses were conducted using SAS 9.1.3 (SAS Institute Inc., Cary, North Carolina). Denitrification data was evaluated by the analysis of variance (ANOVA) test procedure and significant differences between treatment means were calculated by Duncan's multiple range test. An alpha level of 0.05 was used for all statistical analyses.
Results
Denitrification rates measured by the 15N2 method generally increased (after addition) from day 2 to day 10 and then decreased on day 16. Denitrification potential measured by the C2H2 technique steadily decreased over the 16 day incubation. On days 6, 10, and 16, 15N2 denitrification fluxes were higher compared to C2H2 treatment means ().
Figure 1. 15N2 and C2H2 methods potential denitrification means (mg N m−2 d−1) for Bayou Cowan (BC), Bee Bayou (BB), and Jones Bayou (JB) days after NO3–N addition to sediment cores. Error bars are one standard deviation.
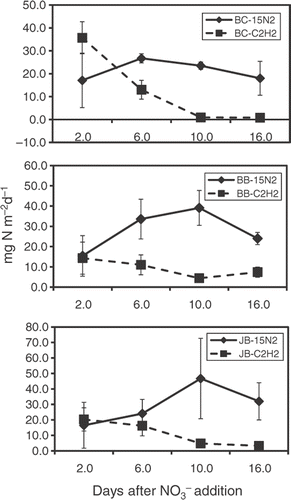
BC 15N2 denitrification ranged from 17.1 to 26.7 mg N evolved m−2 d−1 and averaged 21.3 mg N m−2 d−1 over the 16-day incubation. With the C2H2 procedure, BC denitrification ranged from 0.8 to 35.7 mg N m−2 d−1 with an average flux of 12.6 mg N m−2 d−1. Over the entire incubation, 15N2 denitrification was approximately 1.7 times greater. On day 2, denitrification potential 15N2 and C2H2 flux means were not significantly different. On days 6, 10, and 16, denitrification measured using the 15N2 method was significantly higher ().
Denitrification measured on BB sediment varied from 15.4 to 39.1 mg N m−2 d−1 and averaged 28.0 mg N m−2 d−1 (15N2 method). Potential denitrification ranged from 4.4 to 14.4 mg N m−2 d−1 with an average of 9.3 mg N evolved m−2 d−1 using the C2H2 method (). Dinitrogen fluxes from 15N cores were about 3.0 times higher compared to C2H2 method denitrification means. On days 6, 10, and 16, 15N2 denitrification means were significantly higher, but on day 2, no significant differences were observed between the methods.
Using the 15N2 method, JB denitrification rates varied from a low of 16.5 to a maximum of 46.7 mg N m−2 d−1 and averaged 29.9 mg N m−2 d−1 over the sampling period. Acetylene inhibition denitrification means were lower and ranged from 3.3 to 20.3 mg N m−2 d−1 () and averaged 11.2 mg N m−2 d−1 over the 16 days. 15N-labeled denitrification values were approximately 2.7 times greater compared to the C2H2 procedure. JB denitrification fluxes measured by the 15N2 method were significantly higher on days 10 and 16.
Discussion
Across locations, denitrification rates measured by the 15N2 flux and C2H2 methods were not significantly different 2 days after nitrate addition. For the remaining days (6, 10, and 16), 15N2 denitrification was significantly higher eight out of nine sampling times and was approximately 5.3 times greater than C2H2 method denitrification. Denitrification 15N2 fluxes evolved into the floodwater/sediment core headspace may significantly underestimate sediment 15N2 production. In a 1988 study, Lindau et al. (Citation1988) observed approximately 34% of the applied 15N was entrapped as 15N2 in flooded rice soil 33 days after 15N addition. The 15N2 flux rates are a function of production rate, solubility, mass flow, and diffusion (Well and Myrold Citation1999).
The C2H2 inhibition and 15N2 flux methods have limitations and assumptions that need to be considered for flooded sediments. Limitations of the C2H2 technique include: inhibition of nitrification by C2H2, which can lead to underestimation of total denitrification; incomplete blockage of N2O to N2 by C2H2; presence of hydrogen sulfide; slow diffusion of C2H2 through the floodwater and sediment; sediment respiration enhancement; metabolism and/or decomposition of C2H2; and contaminated C2H2 (Seitzinger et al. Citation1993; Cornwell et al. Citation1999; Groffman et al. Citation2006). Assumptions for the 15N2 method include: 14N and 15N atoms are randomly distributed between N2 molecules produced during the denitrification process; total amount of 28N2 in core headspace does not change over the incubation period; and the 15N label of the added nitrate in the sediment is uniform (Siegel et al. Citation1982; Mulvaney and Boast Citation1986).
Numerous sediment denitrification methods have been compared, including: 15N isotope pairing and C2H2 inhibition (Lohse et al. Citation1996); N2 flux and C2H2 blockage (Watts and Seitzinger Citation2000); N2 flux, 15N isotope pairing and mass balance techniques (van Luijn et al. Citation1996); membrane inlet mass spectrometry and isotope pairing mass spectrometry (Smith et al. Citation2006); and isotope pairing and N2:Ar ratios (Ferguson and Eyre Citation2007). Comparison of our denitrification methods (15N2 flux and C2H2 inhibition) to published research is difficult due to a wide variety of laboratory procedures, sediment properties, applied nitrate and ammonium concentrations, denitrification calculations, incubation methods and times, and gas collection techniques used. In a 1993 experiment, Seitzinger et al. compared three denitrification methods (N2 flux, 15N tracer, and C2H2 inhibition) using aquatic sediments. Denitrification rates due to addition of nitrate to the sediment core floodwater using the 15N tracer method were about 35% of those measured by the C2H2 inhibition method.
Over a short incubation time (2 days), our denitrification results measured with the C2H2 method were comparable to the 15N2 flux method; however, over extended incubation periods (6, 10, and 16 days), the C2H2 inhibition method significantly underestimated potential denitrification. Coupled nitrification–denitrification would not be captured due to C2H2 inhibition of nitrification in sediments. In addition, slow diffusion of C2H2 through the floodwater, incomplete blockage of N2O by C2H2, and C2H2 metabolism by microorganisms may also be responsible for underestimating denitrification. For our study, denitrification due to coupling was thought to be a minor component compared to the denitrification contribution due to the high level of NO3–N added (100 mg N L−1).
Further studies are needed to compare denitrification methods at Mississippi River water in situ NO3–N concentrations (∼1–3 mg N L−1) entering the Atchafalaya River basin and across all basin habitats. In addition, denitrification methods need to be evaluated for incubation periods less than 2 days. If the C2H2 inhibition method can be calibrated against the 15N2 flux procedure, considerable expense and time can be saved on denitrification analyses. Quantification of denitrification is needed to evaluate the contribution of denitrification to nitrogen removal within the Atchafalaya River basin.
Acknowledgements
This project was funded by the Louisiana Governor's Office of Coastal Activities and the US Army Corps of Engineers.
References
- Aulenbach , BT , Buxton , HT , Battaglin , WA and Coupe , RH . 2007. Streamflow and nutrient fluxes of the Mississippi–Atchafalaya River basin and subbasins for the period of record through 2005. United States Geological Survey. Open File Report 2007-1080
- Clement , JC , Pinay , G and Marmonier , P . 2002 . Seasonal dynamics of denitrification along topohydrosequences in three different riparian wetlands . Journal of Environmental Quality , 31 ( 3 ) : 1025 – 1037 .
- Cornwell , JC , Kemp , WM and Kana , TM . 1999 . Denitrification in coastal ecosystems: methods, environmental controls, and ecosystem level controls, a review . Aquatic Ecology , 33 ( 1 ) : 41 – 54 .
- Dunn , DD . 1996 . Trends in nutrient inflows to the Gulf of Mexico from streams draining the conterminous United States 1972–93 , Austin, Texas : USGS Report 96-4113 .
- Ferguson , AJP and Eyre , BD . 2007 . Seasonal discrepancies in denitrification measured by isotope pairing and N2:Ar techniques . Marine Ecology Progress Series , 350 ( 3707 ) : 19 – 27 .
- Goolsby , DA , Battaglin , WA , Aulenbach , BT and Hooper , RP . 2001 . Nitrogen input to the Gulf of Mexico . Journal of Environmental Quality , 30 ( 2 ) : 329 – 336 .
- Groffman , PM . 1994 . Denitrification in freshwater wetlands . Current Topics in Wetland Biogeochemistry , 1 : 15 – 36 .
- Groffman , PM , Altabet , MA , Bohlke , JK , Butterbach-Bahl , K , David , MB , Firestone , MK , Giblin , AE , Kana , TM , Nielsen , LP and Voytek , MA . 2006 . Methods for measuring denitrification: diverse approaches to a difficult problem . Ecological Applications , 16 ( 6 ) : 2091 – 2122 .
- Hauck , RD and Bouldin , DR . 1961 . Distribution of isotopic nitrogen in nitrogen gas during denitrification . Nature , 191 : 871 – 872 .
- Hernandez , ME and Mitsch , WJ . 2007 . Denitrification potential and organic matter as affected by vegetation community, wetland age, and plant introduction in created wetlands . Journal of Environmental Quality , 36 ( 1 ) : 333 – 342 .
- Lindau , CW , Delaune , RD , Collins , JW , Bollich , PK , Scott , LM and Lambremont , EN . 1998 . Methane and nitrous oxide evolution and N-15 and Ra-226 uptake as affected by application of gypsum and phosphogypsum to Louisiana rice . Agriculture, Ecosystems and Environment , 68 : 165 – 173 .
- Lindau , CW , Patrick , WH , Delaune , RD , Reddy , KR and Bollich , PK . 1988 . Entrapment of nitrogen-15 dinitrogen during soil denitrification . Soil Science Society of America Journal , 52 : 538 – 540 .
- Lohse , L , Kloosterhuis , HT , van Raaphorst , W and Helder , W . 1996 . Denitrification rates as measured by the isotope pairing method and by the acetylene inhibition technique in continental shelf sediments of the North Sea . Marine Ecology Progress Series , 132 : 169 – 179 .
- Mississippi River/Gulf of Mexico Watershed Nutrient Task Force. 2001. Action plan for reducing, mitigating, and controlling hypoxia in the northern Gulf of Mexico. Washington, DC
- Mississippi River/Gulf of Mexico Watershed Nutrient Task Force. 2008. Gulf hypoxia action plan 2008 for reducing, mitigating, and controlling hypoxia in the northern Gulf of Mexico and improving water quality in the Mississippi River basin. Washington, DC
- Mitsch , WJ and Gosselink , JG . 2000 . Wetlands , New York : Van Nostrand Reinhold .
- Mulvaney , RL and Boast , CW . 1986 . Equations for determination of nitrogen-15 labeled dinitrogen and nitrous oxide by mass spectrometry . Soil Science Society of America Journal , 50 : 360 – 363 .
- Rabalais , NN , Turner , RE and Wiseman , WJ . 2002 . Gulf of Mexico, aka “the dead zone” . Annual Review of Ecology and Systematics , 33 : 235 – 263 .
- Rolston DE. 1986. Gas flux In: Methods of soil analysis, Part 1. Klute A, editor, Physical and mineralogical methods. Madison, Wisconsin: Soil Science Society of America. p. 1103–1119
- Seitzinger , SP , Harrison , JA , Bohlke , JK , Bouwman , AF , Lawrance , R , Peterson , B , Tobias , C and van Drecht , G . 2006 . Denitrification across landscapes and waterscapes: a synthesis . Ecological Applications , 16 : 2064 – 2090 .
- Seitzinger , SP , Nielsen , LP , Caffrey , J and Christensen , PB . 1993 . Denitrification measurements in aquatic sediments: a comparison of three methods . Biogeochemistry , 23 : 147 – 167 .
- Siegel , RS , Hauck , RD and Kurtz , LT . 1982 . Determination of 30N2 and application to measurement of N2 evolution during denitrification . Soil Science Society of America Journal , 46 : 68 – 74 .
- Smith , LK , Voyek , MA , Bohlke , JK and Harvey , JW . 2006 . Denitrification in nitrate-rich streams: application of N2:Ar and 15N-tracer methods in intact cores . Ecological Applications , 16 : 2191 – 2207 .
- Steingruber , SM , Friedrich , J , Gachter , R and Wehrli , B . 2001 . Measurement of denitrification in sediments with the 15N isotope pairing technique . Applied and Environmental Microbiology , 67 : 3771 – 3778 .
- DC USEPA 2001. Action plan to reduce the size of the “dead zone” in the Gulf of Mexico. Washington . 4503F . Office of Water , EPA 841-F-01-001
- USGS 2007 . The Gulf of Mexico hypoxic zone [Internet]. [Accessed 2010 October 7]. Available from: http://toxics.usgs.gov./hypoxia/hypoxic_zone.html
- van Luijn , F , Boers , PCM and Lijklema , L . 1996 . Comparison of denitrification rates in lake sediments obtained by the N2 flux method, the 15N isotope pairing technique and the mass balance approach . Water Resources , 30 : 893 – 900 .
- Wallenstein , MD , Myrold , DD , Firestone , M and Voytek , M . 2006 . Environmental controls on denitrifying communities and denitrification rates: insights from molecular methods . Ecological Application , 16 : 2143 – 2152 .
- Watts , SH and Seitzinger , SP . 2000 . Denitrification rates in organic and mineral soils from riparian sites: a comparison of N2 flux and acetylene inhibition methods . Soil Biology and Biochemistry , 32 : 1383 – 1392 .
- Well , R and Myrold , DD . 1999 . Laboratory evaluation of a new method for in situ measurement of denitrification in water-saturated soils . Soil Biology and Biochemistry , 31 : 1109 – 1119 .