Abstract
We used the monogonont rotifer, Brachionus calyciflorus, to study the effect of ambient temperatures of 16°C, 22°C, and 29°C on longevity and life history parameters. We found that temperature had a significant relationship with longevity. At lower temperature, there was prolongation of the pre-reproductive and reproductive periods, but fecundity was reduced significantly due to suppression of the reproductive rate. When lifespan of short- and long-lived rotifers was compared, we found that the significant longevity difference in these rotifers was due to extension of reproductive and post-reproductive periods. The fecundity was significantly higher in longer lived rotifers due to the extension of the reproductive period, but the reproductive rate was significantly lower in these rotifers. A consistent negative relationship between rotifer longevity and the rate of reproduction was observed at all temperatures, and it was particularly pronounced in rotifers reproducing heavily at the end of the reproductive stage of their life cycle. The combined rate of living/oxidative damage theory may help explain the temperature effects that we observed.
Introduction
Environmental temperature has a profound effect on the life history parameters of poikilothermic organisms, and a number of studies have confirmed such effect on the lifespan and reproduction of rotifers (Meadow and Barrows Citation1971; Snell and King Citation1977; Verdone-Smith and Enesco Citation1982). Lansing (Citation1942) was among the first investigators to suggest that the prolongation of lifespan observed in temperature-based aging studies in rotifers may simply be related to the rate of metabolic activity. This view was based primarily on the rate of living theory first proposed by Pearl (Citation1928), which states that longevity is inversely proportional to metabolic rate. However, this theory has not found much support in the literature whether in dealing with rotifers (Enesco et al. Citation1990), nematodes (Houthoofd et al. Citation2002), fruit flies (Van Voorhies et al. Citation2003; Hulbert et al. Citation2004; Khazaeli et al. Citation2005), or rodents (Masoro et al. Citation1982; McCarter and Palmer Citation1992; Speakman et al. Citation2004). While the link between the metabolic rate and longevity still remains obscure and controversial, an inverse relationship between high rates of fecundity and longevity has been documented in many species (Rose and Charlesworth Citation1981; Ernsting and Isaaks Citation1991; Kaitala Citation1991; Braeckman et al. Citation2002), and it was thoroughly examined in rotifers by Snell and King (Citation1977). In their often cited work using the rotifer Asplanchna brightwelli, Snell and King (Citation1977) documented a trade-off between reproduction and future survival of rotifers maintained at three different temperatures. By examining a number of life history parameters, they noted the detrimental effect of high rates of reproduction early in life, as opposed to rotifers reproducing at low rates, while spreading their reproduction into many age classes. Other studies that examined genetic correlations between fitness components of early and late breeders also commonly found that the late breeders exhibited greater longevity (Partridge Citation1987).
Despite the rapid advances in molecular biology techniques, which resulted in discovery of a number of pathways affecting longevity in various organisms, the mechanisms responsible for the observed relationship between high reproductive effort early in adult life and accelerated senescence are not well understood. An adequate demographic description of mortality that accounts for the trade-off between reproduction and life expectancy provides a holistic approach to the study of cost of reproduction and is crucial to an understanding of the evolution of senescence (Tatar et al. Citation1993). Therefore, much like the work of Snell and King (Citation1977) on the carnivorous rotifer A. brightwelli, our study was designed to explore a number of life history parameters in relation to the cost of reproduction in the rotifer Brachionus calyciflorus at different temperatures. In addition to similar temperature-based longevity studies using B. calyciflorus in which limited numbers of life history parameters were examined (Halbach Citation1970; Xi et al. Citation2005), the detailed analysis of interrelationships between the longevity and fecundity described here could provide impetus to future studies that might explore the molecular mechanisms underlying such relationships.
Materials and methods
In this study, a Florida strain of B. calyciflorus was obtained from a pond at the University of Florida in Gainesville, Florida (Gilbert and Walsh Citation2005). Rotifers were reared on a culture of green algae Nannochloropsis sp., both obtained from Florida Aqua Farms, Dade City, Florida. Rotifer eggs were hydrated in plain medium, hatched rotifers were transferred to a flask with algal culture, and the population was allowed to increase to its exponential growth phase. For temperature experiments, life history observations were carried out on rotifers reared in the dark at 16°C, 22°C, and 29°C. Life history data were also collected on a larger sample where rotifers were maintained in continuous light at room temperature (20°C).
Culture and experimental methods
The algae and rotifers were maintained on non-sterile Guillard's Woods Hole MBL medium (Nichols Citation1973). The medium was modified for algae and rotifer cultures in accordance with Stemberger (Citation1981) by reducing K2HPO4 and NaNO3 concentrations by 50%. Boron, as H3BO3, and NH4Cl were added to yield concentrations of 1.3 × 10−4 g/L and 5.36 × 10−3 g/L, respectively. TRIS buffer was eliminated, as suggested by Guillard (Citation1975). Initially, rotifers with eggs randomly selected from a continuous culture were placed singly in the wells of 24-well tissue culture plates (LIMBRO, Flow Laboratories Inc., Virginia). Each well contained 1.5 mL of medium with 2.0 × 106 cells/mL Nannochloropsis sp. The algal density was determined using a hemacytometer and then adjusted to the desired final concentration. The rotifers in plates were observed under a dissecting microscope.
Temperature experiments
For temperature experiments, three groups of 24 rotifers were set up. Plates with experimental animals were maintained in darkness and placed in three separate incubators at temperatures of 16°C, 22°C, and 29°C. For every 3 h, plates were inspected for hatched rotifers. The times of births were recorded and the mothers were removed. Subsequently, plates were inspected every 6–11 h and scored for deaths, condition of the animals, and number of offsprings. Each time, young were counted and discarded, and the presence of eggs was noted. Every two days, the experimental rotifers were transferred to another plate with 1.5 mL of 2.0 × 106 cells/mL of fresh algae in MBL medium to ensure an ad libitum supply of fresh algae.
Two distinct subpopulations of Florida rotifers were encountered in this set of experiments. Since the first population of rotifers crashed before the completion of the experiments, a second batch of eggs was hydrated and used to complete the experiments. While identical in all life history parameters to the previous cohort at temperatures of 22°C and 29°C, the temperature of 16°C exceeded the physiological adaptation limits of rotifers from the second batch of cysts. At this temperature, a large proportion of test animals turned mictic. For this reason, one plate with the second batch of rotifers maintained at 16°C was not considered during the analysis.
Life history data on rotifers raised in controlled laboratory conditions
Observations were also made on 121 animals randomly selected from a continuous culture and maintained at room temperature (20°C) with continuous illumination. Rotifers were fed ad libitum (2.0 × 106 cells/mL) green alga Nannochloropsis sp. Experimental animals were transferred into fresh MBL medium containing fresh algae every two days. The 121 rotifer sample was a composite of three separate Florida rotifer subpopulations.
Statistical analysis
The reproductive rate (R o) was defined as the number of neonates produced per day within each reproductive period. The innate capacity for increase (r) was estimated by the iterative method, solving for r using Lotka's relationship (Lotka Citation1907),
In our temperature experiments, the collected data from three replicate runs at 29°C and 22°C were pooled. Data from only the first two replicates were pooled for the ambient temperature of 16°C, and cohort life tables were constructed for each temperature. Data were compared for length of each life cycle phase, longevity, fecundity, and R o using one way analysis of variance (ANOVA) followed by a post hoc Tukey test. In addition, Kaplan–Meier survival analysis followed by a log rank test using SPSS software was performed to confirm longevity traits at all three temperatures. Correlation analysis was used to assess the relationship among the rate of reproduction, fecundity, and various stages of the life cycle. Furthermore, R o in the first and last 20% of the reproductive period was related to other life history parameters by correlation analysis. In order to compensate for different rates of metabolism at the three different temperatures, the R o during the first and the last portion of the reproductive period was normalized against the first and the last 24-h (i.e., 20%) length of the reproductive period at a temperature of 22°C. Therefore, the reproductive effort during the first and last 20% of reproduction (41 h) was examined at 16°C, and the effort during the first and last 20% of reproduction (15 h) was considered at 29°C.
Life history data for the 121 animals maintained under continuous illumination at 20°C were collected, and cohort life tables were constructed. Data on reproductive effort during the first and last 24 h of the reproductive period were also collected. The life history parameters and the rate of reproduction of rotifers with above-average survivorship and of rotifers with below-average lifespan were compared using a t-test. Correlation analysis was used to assess the relationship between the rate of reproduction, fecundity, and various stages of life cycle.
To circumvent the drawbacks connected with the application of Bonferroni corrections (Perneger Citation1998; Nagakawa Citation2004), correlation analyses were carried out on individual replicates separately and then again on the pooled data set.
Results
As expected, there was an inverse relationship between temperature and the rotifer survivorship (). The mean lifespan of B. calyciflorus was 4.1, 6.4, and 11.3 days at 29°C, 22°C, and 16°C, respectively (p < 0.001). The difference in lifespan was predominantly due to the extension of the pre-reproductive and reproductive stages of the rotifer lifespan, while the post-reproductive period remained unchanged (F = 1.6, p = 0.198). The increase in longevity with decreasing environmental temperature was accompanied by a corresponding decrease in fecundity (F = 5.5, p = 0.005) and a somewhat higher reduction in reproductive rate (F = 109, p < 0.001). However, the mean number of offspring produced in the lifetime of a female at 22°C was not significantly different (p = 0.835) from the number of offspring produced at 16°C ().
Figure 1. Kaplan–Meier cumulative survival functions for B. calyciflorus at 16°C, 22°C, and 29°C and in continuous darkness.
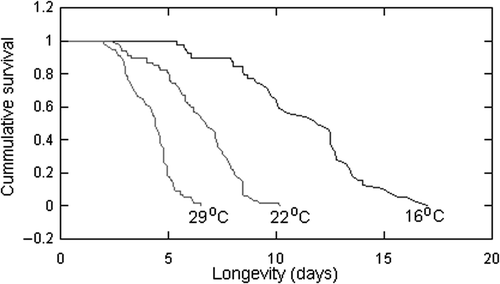
Table 1. Effect of temperature on life history parameters of B. calyciflorus at 16°C, 22°C, and 29°C in continuous darkness.
When we compared the life history parameters of long- and short-lived individuals at each of the three experimental temperatures, the trends were similar to those for rotifers maintained at room temperature (). The length of the pre-reproductive period was the same between the long- and short-lived rotifers at all three temperatures, while the lengths of the reproductive and post-reproductive periods, the mean and maximum lifespan, and the number of neonates produced were all clearly higher for the long-lived rotifers. The exception was the number of neonates produced at the temperature of 29°C. At the higher temperature, the difference in fecundity between long- and short-lived rotifers was not statistically significant (p = 0.127), possibly due to high variance in fecundity in the short-lived group. However, it was noted that the rate of reproduction was significantly lower (p = 0.017) in the long-lived group of rotifers maintained under continuous illumination at 20°C () and at 29°C in the temperature experiments. The rate of reproduction in long-lived animals as opposed to their short-lived counterparts was consistently lower at the lower temperatures as well but was statistically significant only at the 90% confidence level (data not shown). This trend was particularly pronounced when we examined the reproductive effort during the last 20% of the reproductive period. At all temperatures, rotifers reproducing heavily in their last hours of reproduction had a significantly shorter lifespan than their counterparts, which did not. The same was true for animals reproducing heavily at the beginning of reproduction but only at 29°C.
Table 2. Life history parameters of short- and long-lived B. calyciflorus maintained at room temperature with continuous light.
In all experiments R o reflected the overall increase in fecundity for the long-lived groups since R o is a function of survivorship (lx ) as well as fecundity (mx ). Our observations of innate capacity for increase (r), however, were consistent with those of Snell and King (Citation1977), where the long- and short-lived individuals achieved similar r, thus, offsetting the effect of natural selection otherwise favoring the long-lived individuals.
Discussion
The relationship between the length of the pre-reproductive period and the reproductive rate showed a clear pattern; although not significant at 29°C, it became increasingly negative at lower temperatures. This weak negative relationship at lower temperatures was accompanied by a somewhat stronger negative relationship (r = −0.578, p < 0.001) between the length of the pre-reproductive period and the total number of neonates produced at 16°C. This relationship was again confirmed when we considered the length of the pre-reproductive period and reproductive effort during the last 20% of reproduction (r = −0.423, p = 0.007). Interestingly, there was a weak positive, statistically significant relationship between the length of the pre-reproductive period and the reproductive effort at the beginning of reproduction at 29°C (r = 0.399, p = 0.002).
Correlation between any two variables does not prove causality; however, one could suggest that higher reproductive output at low temperatures would influence the length of the pre-reproductive period only in case of a pre-determined allocation of resources. If this proposition is correct, resources channeled into reproduction could be obtained at the expense of the length of the pre-reproductive period. It is interesting to note, however, that such a trade-off was pronounced only at lower temperature, while the opposite trend was observed at higher temperature.
Similarly, we observed a negative relationship between the length of post-reproductive period and the R o at low ambient temperature (r = −0.453, p = 0.004). This trend was further pronounced in the reproductive effort during the last 20% of reproductive period at 16°C (r = −0.549, p < 0.001).
We found a negative correlation between the reproductive rate and the length of the reproductive period at 29°C and 22°C, suggesting an inverse relationship between these two variables. A somewhat stronger negative relationship existed when reproductive effort during the last 20% of reproduction at both temperatures was considered. However, it was not statistically significant at 16°C.
As expected, there was a positive relationship between the number of offspring produced and both the lifespan and the length of the reproductive period. In effect, overall reproductive output is a function of the length of both, simply because the lifespan is itself a function of the length of the reproductive period. Typically, there is a strong positive relationship between the R o and the total number of offspring produced; however, here we notice a consistent weakening of this relationship as we approach lower temperatures. This can be explained by a flattening of the fecundity curve with a decrease of temperature, as observed in our study as well as that of others (Halbach Citation1970; Snell and King Citation1977; Xi et al. Citation2005).
Fecundity was lowered through the reduction of R o at lower temperatures, and this reduction of R o was coupled with a corresponding increase of lifespan. This relationship held in our temperature experiments as well as in the rotifers divided into long-, and short-lived groups. The consistent negative relationship between rotifer longevity and the R o at all temperatures observed here and as reported by Snell and King (Citation1977) in A. brightwelli indicates detrimental effects of high R o, especially when such reproductive effort was exerted during the final hours of the reproductive period.
The oxidative damage theory is based on the fact that generation of harmful free radicals is an inevitable consequence of aerobic energy metabolism (Harman Citation1956). Reactive oxygen species (ROS) thus generated cause gradual accumulation of molecular damage within cells. Such accumulated damage results in progressive decline of biological function associated with aging and ultimately cell death. Indeed, Sawada and Carlson (Citation1990) measured the level of superoxide radicals in cellular membranes of the rotifer A. brightwelli. They observed an increase of superoxide radicals in the tissues of older rotifers as compared to younger animals. They also measured the level of superoxide radicals in rotifers maintained at three different temperatures and concluded that rotifers maintained at higher temperatures retained more superoxide radicals in their tissues than individuals maintained at lower temperatures.
Due to the obvious link between oxidative metabolism and the rate of living theory (Pearl Citation1928), both hypotheses could be considered together as one rate of living/oxidative damage theory (Brys et al. Citation2007). This combined theory could explain the observed effect of reproductive effort on longevity; since fecundity can serve as an index of metabolic activity and ROS production, we could possibly observe a negative relationship between lifespan and the R o in B. calyciflorus. This notion is also in line with the observations reported by Snell and King (Citation1977) and Sawada and Carlson (Citation1990) for A. brightwelli.
The rate of ROS production and its effect on somatic tissues is mitigated by superoxide dismutase, catalase, and other somatic repair mechanisms. Obviously the efficiency of any cellular repair mechanism would be affected by the same ROS-generated damage, and therefore it is expected to decline with age. This might explain the negative effects of high R o s on lifespan in rotifers, especially when such a high R o was maintained in the final hours of reproductive period. In addition, in poikilothermic organisms, the efficiency of ROS scavenging and damage repair becomes a function of temperature. Consistent with our observations and based on the observation of Sawada and Carlson (Citation1990), it is possible to postulate that, in rotifers, as the temperature drops so does the generation of superoxide radicals and lipid peroxides and therefore the accumulation of irreversible damage. This in turn may become a contributing factor in the extension of lifespan. By the same token, however, the efficiency of the somatic maintenance and repair mechanisms may possibly be altered with temperature, depending on the thermodynamic optima of enzyme activity.
When examining the lengths of all three life history stages in B. calyciflorus, the life extension observed in our temperature experiments was effected by the prolongation of the pre-reproductive and reproductive period. When comparing long- and short-lived individuals, however, a different pattern emerged. In this case, the pre-reproductive period between the two groups was identical, and the lifespan was extended through the length of both the reproductive and post-reproductive stages of the life cycle.
The reasons and mechanisms for this intraspecific variability in life history parameters and their interrelationships are not clear. The results, however, lead us to speculate that there are at least two different aging mechanisms at work, both operating at the level of genetic control. The first of these appears to be turned on after the onset of reproduction and continues to be expressed throughout the entire reproductive and post-reproductive period. This mechanism could be directly linked to the reduction of R o observed in our long-lived rotifers. The second mechanism appears to be expressed and acts on the age and development of the animals immediately upon hatching from a parthenogenetic egg. It is temperature-dependent and operates only during the pre-reproductive and reproductive stages.
A detailed life history analysis of organisms such as rotifers, exposed to a number of life modifying treatments and as presented here, is only a first step in understanding the interactions between the organism and its environment. Further studies focusing on the accumulation of somatic damage throughout the entire lifespan, and its relation to the transcriptome and the protein expression during each developmental life stage in rotifers, may bring us closer to understanding of the molecular mechanisms of aging and the evolution of senescence.
Acknowledgements
We are indebted to anonymous reviewers for their criticism of the previous drafts of the manuscript, which resulted in substantial improvements. We thank Dominique Besso for English grammar corrections.
References
- Braeckman , BP , Houthoofd , K , De Vreese , A and Vanfleteren , JR . 2002 . Assaying metabolic activity in ageing Caenorhabditis elegans . Mechanisms of Ageing and Development , 123 ( 2–3 ) : 105 – 119 .
- Brys , K , Vanfleteren , JR and Braeckman , BP . 2007 . Testing the rate-of-living/oxidative damage theory of aging in the nematode model Caenorhabditis elegans . Experimental Gerontology , 42 ( 9 ) : 845 – 851 .
- Enesco , HE , McTavish , A and Garberi , R . 1990 . Spontaneous activity level and life span in rotifers: lack of support for the rate of living theory . Gerontology , 36 ( 5–6 ) : 256 – 261 .
- Ernsting , G and Isaaks , JA . 1991 . Accelerated aging: a cost of reproduction in the carabid beetle Notiophilus biguttatus F . Functional Ecology , 5 ( 2 ) : 299 – 303 .
- Gilbert , JJ and Walsh , EJ . 2005 . Brachionus calyciflorus is a species complex: mating behavior and genetic differentiation among four geographically isolated strains . Hydrobiologia , 546 ( 1 ) : 257 – 265 .
- Guillard , RRL . 1975 . “ Culture of phytoplankton for feeding marine invertebrates ” . In Culture of marine invertebrate animals , Edited by: Smith , WL and Chanley , MH . 29 – 60 . New York, NY : Plenum Pub. Co .
- Halbach , U . 1970 . Einfluß der Temperatur auf die Populationsdynamik des planktischen Rädertieres Brachionus calyciflorus Pallas . Oecologia , 4 : 176 – 207 .
- Harman , D . 1956 . Aging: a theory based on free radical and radiation chemistry . Journal of Gerontology , 11 ( 3 ) : 298 – 300 .
- Houthoofd , K , Braeckman , BP , Lenaerts , I , Brys , K , De Vreese , A , Van Eygen , S and Vanfleteren , JR . 2002 . No reduction of metabolic rate in food restricted Caenorhabditis elegans . Experimental Gerontology , 37 ( 12 ) : 1357 – 1367 .
- Hulbert , AJ , Clancy , DJ , Mair , W , Braeckman , BP , Gems , D and Partridge , L . 2004 . Metabolic rate is not reduced by dietary-restriction or by lowered insulin/IGF-1 signalling and is not correlated with individual lifespan in Drosophila melanogaster . Experimental Gerontology , 39 ( 8 ) : 1137 – 1143 .
- Kaitala , A . 1991 . Phenotypic plasticity in reproductive behaviour of waterstriders: trade-offs between reproduction and longevity during food stress . Functional Ecology , 5 ( 1 ) : 12 – 18 .
- Khazaeli , AA , Van Voorhies , W and Curtsinger , JW . 2005 . Longevity and metabolism in Drosophila melanogaster: genetic correlations between life span and age-specific metabolic rate in populations artificially selected for long life . Genetics , 169 ( 1 ) : 231 – 242 .
- Lansing , AI . 1942 . Some effects of hydrogen ion concentration, total salt concentration, calcium and citrate on longevity and fecundity of the rotifer . Journal of Experimental Zoology , 91 ( 2 ) : 195 – 211 .
- Lotka , AJ . 1907 . Studies on the mode of growth of material aggregates . American Journal of Science , 24 ( 4 ) : 199 – 216 .
- Masoro , EJ , Yu , BP and Bertrand , HA . 1982 . Action of food restriction in delaying the aging process . Proceedings of the National Academy of Sciences , 79 ( 13 ) : 4239 – 4241 .
- McCarter , RJ and Palmer , J . 1992 . Energy metabolism and aging: a lifelong study of Fischer 344 rats . American Journal of Physiology: Endocrinology and Metabolism , 263 ( 3 ) : E448 – E452 .
- Meadow , ND and Barrows , CH . 1971 . Studies on aging in a bdelloid rotifer. II. The effects of various environmental conditions and maternal age on longevity and fecundity . Journal of Gerontology , 26 ( 3 ) : 302 – 309 .
- Nagakawa , S . 2004 . A farewell to Bonferroni: the problems of low statistical power and publication bias . Behavioral Ecology , 15 ( 6 ) : 1044 – 1045 .
- Nichols , HW . 1973 . “ Growth media - freshwater ” . In Handbook of phycological methods , Edited by: Stein , JR . 7 – 24 . New York, NY : Cambridge University Press .
- Partidge , L . 1987 . Is accelerated senescence a cost of reproduction? . Functional Ecology , 1 ( 4 ) : 317 – 320 .
- Pearl , R . 1928 . The rate of living , London : University of London Press .
- Perneger , TV . 1998 . What's wrong with Bonferroni adjustments . British Medical Journal , 316 ( 7139 ) : 1236 – 1238 .
- Rose , MR and Charlesworth , B . 1981 . Genetics of life history in Drosophila melanogaster I. Sib analysis of adult females . Genetics , 97 ( 1 ) : 173 – 186 .
- Sawada , M and Carlson , JC . 1990 . Biochemical changes associated with the mechanism controlling superoxide radical formation in the aging rotifer . Journal of Cellular Biochemistry , 44 ( 3 ) : 153 – 165 .
- Snell , TW and King , CE . 1977 . Lifespan and fecundity patterns in rotifers: the cost of reproduction . Evolution , 31 ( 4 ) : 882 – 890 .
- Speakman , JR . 2004 . Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer . Aging Cell , 3 ( 3 ) : 87 – 95 .
- Stemberger , RS . 1981 . A general approach to the culture of planktonic rotifers . Canadian Journal of Fisheries and Aquatic Sciences , 38 : 721 – 724 .
- Tatar , M , Carey , JR and Vaupel , JW . 1993 . Long-term cost of reproduction with and without accelerated senescence in Callosobruchus maculatus: analysis of age-specific mortality . Evolution , 47 ( 5 ) : 1302 – 1312 .
- Van Voorhies , WA , Khazaeli , AA and Curtsinger , JW . 2003 . Long-lived Drosophila melanogaster lines exhibit normal metabolic rates . Journal of Applied Physiology , 95 ( 6 ) : 2605 – 2613 .
- Verdone-Smith , C and Enesco , HE . 1982 . The effect of temperature and of dietary restriction on lifespan and reproduction in the rotifer Asplanchna brightwelli . Experimental Gerontology , 17 ( 4 ) : 255 – 262 .
- Xi , Y-L , Ge , Y-L , Chen , F , Wen , X-Li and Dong , L-L . 2005 . Life history characteristics of three strains of Brachionus calyciflorus (Rotifera) at different temperatures . Journal of Freshwater Ecology , 20 ( 4 ) : 707 – 713 .