Abstract
The macroinvertebrate communities and relationships with environmental variables were characterized in the Erhai basin by sampling at 44 sites. Among the 76 taxa observed, Baetis sp., Tubificidae, Simulium sp., Eukiefferiella sp., Gammarus sp. and Parakiefferiella sp. were the dominant taxa. Canonical correspondence analysis indicated that seven environmental variables, namely altitude, Ca2+, chemical oxygen demand, NO3–N, total phosphorus, streambed width, and a qualitative habitat evaluation index (QHEI), were significantly related to the distribution of benthic macroinvertebrates. Two-way indicator species analysis divided total sampling sites into four groups. Weighted-averaging regression and cross-calibration produced strong models for predicting NO3–N, altitude, and QHEI, which enabled the selection of the following benthic taxa as potentially sensitive indicators of certain levels of NO3–N, altitude, and QHEI: Aracina, Atrichops morimotoi, Ceratopsyche sp., Guttilelopia sp., and Nemoura sp. for NO3–N; Heptagenia sp. and Parakiefferiella sp. for altitude; and Aracina for QHEI.
Introduction
Biological indicators have widespread appeal to scientists, environmental managers, and the general public and are mainly used to assess the condition of the environment as early warning signals of ecological problems, and as barometers for trends in ecological resources (Niemi and McDonald Citation2004). Biological indicators may reflect the intensity of anthropogenic stress and have been used as tools to assess the impact of human-induced changes in freshwater ecosystem (Kamden-Toham and Teugels Citation1999). Benthic macroinvertebrates have long been used as indicators of stream ecosystem health, and they are the most commonly used biological indicators in running water (Rosenberg and Resh Citation1993).
Benthic macroinvertebrates are relatively easy to collect and identify, most of them are sensitive with short life histories and can respond rapidly to change, and they have a diversity of functional traits that provide a variety of responses to changing environmental conditions (Collier Citation2008). Numerous studies on the relationships between benthic macroinvertebrates and environment (e.g., Sandin Citation2003; Soldner et al. Citation2004; Mykrä et al. Citation2007) have aimed to find indicator species, community structure traits or multimetric indices to assess water quality and river ecosystem health (Li et al. Citation2010).
In China, there have been few studies on the character of benthic macroinvertebrate distribution or the selection of indicator taxa at the basin scale. Therefore, the objectives of our study were to characterize benthic macroinvertebrate community composition in the Erhai basin and identify taxa that could potentially be used as indicators of water physicochemical conditions in the basin.
Methods and materials
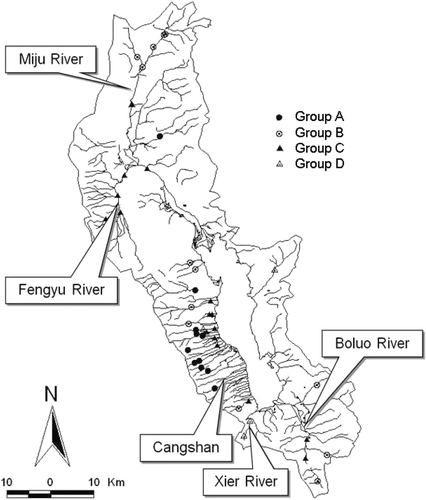
The Erhai Basin is located at 25°25′–26°10′N and 99°32′–100°27′E in Yunnan Province, China, and includes the 250-km2 Lake Erhai (). The region has a typical warm monsoon climate, with wet and dry seasons each year.
Figure 1. Location of sampling sites in the Erhai basin and identification of the four final groups denoted by TWINSPAN analysis.
During February and March of 2009, we sampled 44 sites on streams within the basin. At each site, when possible, we collected five randomly located benthic samples with a 30 × 30 cm2 Surber sampler (420 -µm mesh). Specimens were preserved in 10% formalin and were identified according to Morse et al. (Citation1994) and Merritt and Cummins (Citation1996).
A Hydrolab Minisonde (Hach Environmental, Loveland, CO) was used to measure surface water pH, conductivity, salinity, total dissolved solid (TDS), dissolve oxygen (DO), and water temperature. Surface water was also collected for analysis of the following: total nitrogen (TN), ammonium (NH4–N), nitrate (NO3–N), total phosphorus (TP), orthophosphate (PO4–P), calcium (Ca2+), magnesium (Mg2+), chlorine (Cl−), hardness, alkalinity, chemical oxygen demand (COD), and total organic carbon (TOC). All the analyses were carried out according to the standard methods (Cai Citation2007). Altitude and streambed width were also recorded for each site.
The steam habitat and geomorphology were characterized by a qualitative habitat evaluation index (QHEI; Rankin Citation1989). The QHEI assessed the substrate, riparian zone, and bank erosion and computed a summary score. QHEI scores >60 suggest high habitat quality, whereas scores <45 suggest poor habitat quality (Moerke and Lamberti Citation2004).
The relationships between environmental variables and benthic macroinvertebrate assemblages were explored using canonical correspondence analysis (CCA). All the biotic data were converted into relative abundance and log-transformed before analysis, and rare species were down-weighted in CCA. Forward selection and Monte Carlo permutations were used to select a minimum set of variables that had significant and independent relationships with the benthic macroinvertebrate distribution.
Two-way indicator species analysis (TWINSPAN) was used to classify the samples into groups based on macroinvertebrate data and identify the indicator taxa of the clusters (Dufrêne and Legendre Citation1997).
The Shannon–Wiener diversity (Shannon and Weaver Citation1949), the Pielou (Citation1966) evenness, and taxa richness were calculated. The Kruskal–Wallis test was used to compare their differences among different groups as well as differences in environmental variables.
We used weighted-averaging regression analysis to establish indicators–inference models (Birks et al. Citation1990) using C2 software (Juggins Citation2007). Taxa optima and tolerances (i.e., the mean and standard deviation of the environmental variables over all sites where a taxon occurred) were calculated. The jackknife cross-validation procedure was used to access the predicative capability of the resulting models. The coefficient of determination (R 2) between species-inferred and observed environmental variable concentrations and the root-mean-squared error (RMSE) of prediction also were measured to access the models. Based on Kilroy et al. (Citation2006) and Wu et al. (Citation2009), our criteria were (1) occurrence in at least 20 of the 44 sites and (2) tolerance to the variable of interest (i.e., <0.75 × the mean tolerance for all the taxa). Untransformed taxa and environmental data were used for the weighted-averaging regressions.
In our study, CCA was carried out by CANOCO (version 4.5); TWINSPAN was used by PC-ORD (version 4.0); Kruskal–Wallis text was conducted by SPSS 16.0 and the weighted-averaging regression by C2 (version 1.5).
Results and discussion
Macroinvertebrate sampling yielded 76 taxa in 68 genera. We defined dominant taxa as those with relative abundances >5% (Bunn et al. Citation1986). These included Baetis sp., Tubificidae, Simulium sp., Eukiefferiella sp., Gammarus sp., and Parakiefferiella sp., and their respective relative abundances were 17.34%, 13.84%, 12.17%, 9.74%, 9.44%, 7.09%, and 6.44%. Tubificidae, Eukiefferiella sp., and Parakiefferiella sp. are tolerant taxa; their presence suggests that environmental stress in this basin is considerable. The average macroinvertebrate density was 1991 ind m−2; the maximum and minimum densities were, respectively, 9355 and 303 ind m−2.
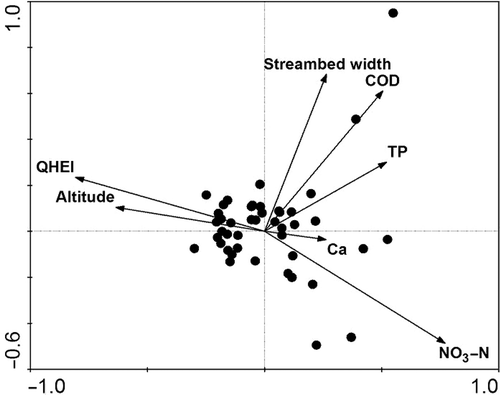
Twenty-one environmental variables, including COD, alkalinity, Ca2+, Mg2+, hardness, Cl−, TN, NO3–N, NH4–N, TP, PO4–P, TOC, conductivity, salinity, TDS, temperature, DO, pH, streambed width, altitude, and QHEI, were employed in the CCA. The result showed that the distribution of benthic macroinvertebrate was mainly related to NO3–N, QHEI, altitude, streambed width, COD, TP, and Ca2+ (Monte Carlo test p < 0.05; ).
TWINSPAN separated the 44 sites into four groups with similar characteristics (). Group A was primarily the Cangshan Mountain sites, and Group B consisted of sites located in the Miju and Bolou Rivers. The indicator taxon of Group B was Ceratopsyche sp. Group C comprised moderately disturbed sites, and Group D was seriously disturbed sites. The indicator taxon of Group C was Ceratopsyche sp., whereas the Group D indicator taxon was Tubificidae.
The Shannon–Wiener diversity, Pielou evenness and taxa richness showed significantly differences among the four groups (Kruskal–Wallis test, p < 0.05) (). Similarly, five environmental variables selected by CCA (Ca2+, NO3–N, TP, altitude, and QHEI) showed significant differences among the four groups (Kruskal–Wallis test, p < 0.05).
Table 1. Community trait and environmental variables (means) of the Erhai basin streams and results of the K–W test among the four groups.
Because the above analyses confirmed strong environmental relationships with the macroinvertebrate community, we used Ca2+, NO3–N, TP, altitude, and QHEI in weighted-averaging regression analysis. The regression and calibration produced a strong model for predicting NO3–N, altitude, and QHEI. NO3–N, altitude, and QHEI weight-averaging taxa optima were calculated using the full dataset (n = 44), and the results are presented for the taxa with effective numbers of occurrences (i.e. >20; ) Weight-averaging values of NO3–N, altitude and QHEI optima were 0.14–0.83 mgL−1, 1961–2457 m, and 29–50, respectively.
Table 2. Optimum and tolerance values generated by the weighted-averaging regression analyses of benthic macroinvertebrate for NO3–N, altitude, and QHEI in the Erhai basin.
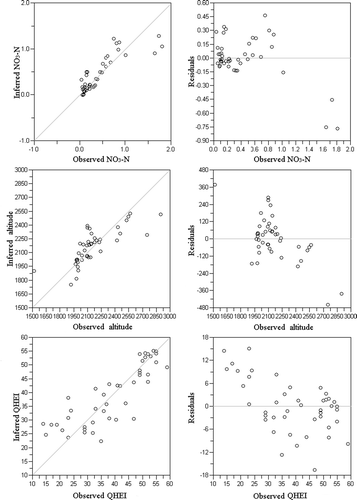
The best results used simple weight-averaging regression (tolerance down-weighting) with inverse deshrinking (NO3–N: R 2 = 0.72, apparent RMSE = 0.23; altitude: R 2 = 0.59, apparent RMSE = 152; QHEI: R 2 = 0.71, and apparent RMSE = 7). Jackknife-derived predicted NO3–N, altitude, and QHEI values matched the measured values well (), and the residuals plotted against predicted values (Racca and Prairie Citation2004) indicated no bias in the models. The models for Ca2+ and TP performed poorly (not shown).
Figure 3. Observed NO3–N, altitude, and QHEI values at the 44 sites plotted against predicted values calculated from a weighted-averaging regression model.
Macroinvertebrate taxa that met the criteria specified for potential use as indicator taxa were: Aracina (0.15 mgL−1), Atrichops morimotoi (0.18 mgL−1), Ceratopsyche sp. (0.14 mgL−1), Guttilelopia sp. (0.15 mgL−1), and Nemoura sp. (0.14 mgL−1) for NO3–N; Heptagenia sp. (2158 m) and Parakiefferiella sp. (2,061 m) for altitude; and Aracina for QHEI (49.8) ().
Ordination techniques have been used successfully to interpret macroinvertebrate community structure. In this study, macroinvertebrate distributions were mainly related to NO3–N, QHEI, altitude, streambed width, COD, TP, and Ca2+. In numerous studies (e.g., Miserendino Citation2001; Heino et al. Citation2003; Soldner et al. Citation2004), altitude and water chemistry always related to the macroinvertebrate assemblages. Altitude directly determines some environmental factors such as temperature and illumination in a region. Some studies showed that macroinvertebrate richness decreased with increased altitude (e.g., Suren Citation1994). Main nutrients (e.g., N and P) can affect benthic algal composition (Leland and Porter Citation2000), and further affect the macroinvertebrate assemblages by bottom-up control.
Our results suggest that benthic macroinvertebrate distributions can be related to NO3–N, altitude, and QHEI. The weighted-averaging models showed significant correlation and low apparent RMSE, which indicated that the models were reliable. Numerous studies have reported the altitude (e.g., Suren Citation1994), NO3–N (e.g., Richards et al. Citation1992), and QHEI (e.g., Beyene et al. Citation2009) were related to the benthic macroinvertebrate community. Environmental stress by human activity could be assessed by NO3–N and QHEI in this basin; according to our models, this stress could also be evaluated by the presence of Aracina, A. morimotoi, Ceratopsyche sp., Guttilelopia sp., and Nemoura sp.
Acknowledgments
We thank Wen Xiao, Yanpeng Li, and Jinfa Li of Dali University for their assistance with the field sampling. This study was supported financially by the Major Science and Technology Program for Water Pollution Control and Treatment (2008ZX07526-002-07) and the National Natural Science Foundation of China (30330140).
References
- Beyene , A , Addis , T , Kifle , D , Legesse , D , Kloos , H and Triest , L . 2009 . Comparative study of diatoms and macroinvertebrate as indicators of severe water pollution: case study of the Kebena and Akaki rivers in Addis Ababa, Ethiopia . Ecological Indicators , 9 : 381 – 392 .
- Birks , HJB , Line , JM , Juggins , S , Stevenson , AC and ter Braak , CJF . 1990 . Diatoms and pH reconstruction . Philosophical Transactions of the Royal Society , 327 : 263 – 278 .
- Bunn , SE , Edward , DH and Loneragan , NR . 1986 . Spatial and temporal variation in the macroinvertebrate fauna of streams of northern jarrah forest, Western Australia: community structure . Freshwater Biology , 16 : 67 – 92 .
- Cai , QH . 2007 . Protocols for standard observation and measurement in aquatic ecosystems , Beijing : China Environmental Science Press (in Chinese) .
- Collier , KJ . 2008 . Temporal patterns in the stability, persistence and condition of stream macroinvertebrate communities: relationships with catchment land-use and regional climate . Freshwater Biology , 53 : 603 – 616 .
- Dufrêne , M and Legendre , P . 1997 . Species assemblages and indicator species: the need for a flexible asymmetrical approach . Ecological Monographs , 67 : 345 – 366 .
- Heino , J , Muotka , T , Mykrä , H , Paavola , R , Hämäläinen , H and Koskenniemi , E . 2003 . Defining macroinvertebrate assemblage type of headwater streams: implications for bioassessment and conservation . Ecological Applications , 13 : 842 – 852 .
- Juggins , S . 2007 . C2 Version 1.5 User guide. Software for ecological and palaeoecological data analysis and visualisation , 73 Newcastle upon Tyne , UK : Newcastle University .
- Kamden-Toham , A and Teugels , GG . 1999 . First data on an index of biotic integrity (IBI) based on fish assemblages for the assessment of the impact of deforestation in a tropical West African river system . Hydrobiologia , 397 : 29 – 38 .
- Kilroy , C , Biggs , BJF , Vyverman , W and Broady , PA . 2006 . Benthic diatom communities in subalpine pools in New Zealand: relationships to environmental variables . Hydrobiologia , 561 : 95 – 110 .
- Leland , HV and Porter , SD . 2000 . Distribution of benthic algae in the upper Illinois River basin in relation to geology and land use . Freshwater Biology , 44 : 279 – 301 .
- Li , FQ , Cai , QH and Ye , L . 2010 . Developing a benthic index of biological integrity and some relationship to environmental factors in the subtropical Xiangxi River, China . International Review Hydrobiology , 95 : 171 – 189 .
- Merritt , RW and Cummins , KW . 1996 . An introduction to the aquatic insects of North America, , 3rd , Dubuque : Kendal/Hunt .
- Miserendino , ML . 2001 . Macroinvertebrate assemblages in Andean Patagonian rivers and streams: environmental relationships . Hydrobiologia , 444 : 147 – 158 .
- Moerke , AH and Lamberti , GA . 2004 . Restoring stream ecosystems: lessons from a midwestern State . Restoration Ecology , 12 : 327 – 334 .
- Morse , JC , Yang , L and Tian , L . 1994 . Aquatic insects of China useful for monitoring water quality , Nanjing : Hohai University Press .
- Mykrä , H , Heino , J and Muotka , T . 2007 . Scale-related patterns in the spatial and environmental components of stream macroinvertebrate assemblage variation . Global Ecology Biogeography , 16 : 149 – 159 .
- Niemi , GJ and McDonald , ME . 2004 . Application of ecological indicators . Annual Review of Ecological Evolution and Systematics , 35 : 89 – 111 .
- Pielou , EC . 1966 . The measurement of diversity in different types of biological collections . Journal of Theoretical Biology , 13 : 131 – 144 .
- Racca J, Prairie Y. 2004. Apparent and real bias in numerical transfer functions in palaeolimnology. Journal of Paleolimnology. 31:117–124
- Rankin , ET . 1989. The qualitative habitat evaluation index (QHEI): rationale, methods, and application. Ohio EPA, Division of Water Quality Planning and Assessment, Ecological Analysis Section, Columbus, Ohio. Available from: http://www.epa.state.oh.us/dsw/bioassess/BioCriteriaProtAqLife. html#QHEI
- Richards , C , Host , GE and Arthur , JW . 1992 . Identification of predominant environmental factors structuring stream macroinvertebrate communities within a large agricultural catchment . Freshwater Biology , 29 ( 2 ) : 285 – 294 .
- Rosenberg , DM and Resh , VH . 1993 . Freshwater biomonitoring and benthic macroinvertebrates , New York : Chapman and Hall .
- Sandin , L . 2003 . Benthic macroinvertebrates in Swedish streams: community structure, taxon richness, and environmental relations . Ecography , 26 : 269 – 282 .
- Shannon , CE and Weaver , W . 1949 . The mathematical theory of communication , Urbana , IL : University of Illinois Press .
- Soldner , M , Stephen , I , Ramos , L , Angus , R , Wells , NC , Grosso , A and Crane , M . 2004 . Relationship between macroinvertebrate fauna and environmental variables in small streams of the Dominican Republic . Water Research , 38 : 863 – 874 .
- Suren , AM . 1994 . Macroinvertebrate communities of streams in western Nepal: effects of altitude and land use . Freshwater Biology , 32 : 323 – 336 .
- Wu , NC , Tang , T and Qu , XD . 2009 . Spatial distribution of benthic algae in the Gangqu River, Shangrila, China . Aquatic Ecology , 43 : 37 – 49 .