Abstract
Collection efficiencies of benthic grab samplers vary depending on the type of substrate sampled and the organism targeted. When benthic invertebrate samples obtained with two or more sampling devices are compared, it is necessary to understand the relative efficiency of each sampler and, if appropriate, apply conversion factors to the data obtained from one or more of the samplers. We used data from field trials to generate substrate- and taxon-specific conversion factors for four common benthic grabs (Shipek, petite Ponar, standard Ekman, and large Ekman). The Shipek grab collected fewer benthic invertebrates per square meters than the other samplers in silt and sand substrates. The large Ekman collected more benthic invertebrates per square meters than other samplers in silt substrate but performed poorly in sand substrate. The petite Ponar and standard Ekman showed similar performance in silt and sand substrates. The wide range of conversion factors generated from our field trials demonstrates the importance of using substrate- and taxon-specific factors when comparing samples collected with different devices.
Introduction
Until recently, studies of lake communities have focused on pelagic environments under the assumption that pelagic productivity dominates whole-lake productivity (Vadeboncoeur et al. Citation2003, Citation2008). However, benthic production has been shown to contribute substantially to whole lake and fisheries production, even in large, deep lakes (Vadeboncoeur et al. Citation2003, Citation2008; Vander Zanden et al. Citation2011). Increased recognition of the functional importance of benthic communities in large lakes, along with the general lack of understanding of these communities (Lake et al. Citation2000), has heightened interest in sampling benthic habitats. Several studies have demonstrated that tracking benthic invertebrate assemblages in large lakes over time can reveal changes to the chemical, physical, and biological characters of these lakes (Robertson and Alley Citation1966; Nalepa Citation1987, Citation1991; Stewart and Haynes Citation1994; Barton and Anholt Citation1997; Nalepa et al. Citation1998, Citation2000, Citation2003, Citation2007; Lozano et al. Citation2001). One of the problems that arise in comparisons of historical and contemporary benthic invertebrate collections, however, is a lack of comparability between samples collected with different types of samplers. Logistical and monetary constraints do not always allow for the use of similar methods over time and different samplers are appropriate for different sampling conditions (e.g., depth, substrate, and wind conditions).
The relative efficiency (success in collection of a target organism) of different benthic samplers is variable because of substantial differences in device design. The Ponar and Shipek grabs are the most effective all-purpose samplers, suitable for collection from most substrates, including harder substrates such as sand, gravel, and clay (Flannagan Citation1970; Nalepa et al. Citation1988; Mudroch and Azcue Citation1995; Clesceri et al. Citation1998). Petite and standard Ponar grabs are identical in design and weigh 11 and 23 kg, respectively. Ponar grabs have a 0.5 mm mesh screen on the top of each jaw, which allows water to flow through the sampler during decent. The Shipek grab weighs 61 kg and does not allow water to flow through the sampler. While heavier and thus more resistant to wave action than most other samplers, the Shipek is generally less efficient in benthic invertebrate collections, possibly because of the pressure wave created beneath it during decent resulting in substrate disturbance (Sly Citation1969; Flannagan Citation1970). The Ekman grab is a lightweight sampler, weighing 4–12 kg, depending on the model. Standard, tall, and large Ekman grabs are similar in design, with the tops opening during decent, nearly eliminating hydraulic disturbance prior to sample collection. The Ekman grab is the most popular freshwater benthic sampler (Downing and Rigler Citation1984), but is not as effective on hard substrates or in the presence of strong currents (Sly Citation1969; Brinkhurst Citation1975; Downing Citation1984; Murdoch and Azcue 1995).
When benthic invertebrates are collected with different samplers, it may be necessary to apply conversion factors to data before densities can be reliably compared. Several benthic researchers have outlined the advantages and disadvantages of using different types of samplers (Sly Citation1969; Flannagan Citation1970; Brinkhurst Citation1975; Clesceri et al. Citation1998) but few have provided conversion factors for sampler comparison. Moreover, the generation of conversion factors is difficult, as sampler efficiency is often substrate and taxon specific. However, when the collection efficiencies of different samplers are evaluated for specific taxa in relatively uniform substrates, conversion factors can be generated for the tested samplers. Schloesser and Nalepa (Citation2002) present conversion factors that allow four sampler types to be converted to standard Ponar equivalents for the collection of a target taxon in both soft and hard substrates. Such conversion factors can then be used to compare macroinvertebrate densities that have been obtained with different devices. For example, Reynoldson et al. (Citation1989) generated conversion factors for several different benthic grabs from reports of taxon-specific sampler efficiencies relative to diver's cores. The conversion factors were then used to track benthic invertebrate communities collected with different devices in the Great Lakes and evaluate lake condition over time. Similarly, Sly and Christie (Citation1992) obtained taxon-specific standard Ponar equivalents for several different samplers from calculations of sampler efficiency in the Great Lakes in order to examine differences in bottom-feeding amphipod densities between lakes and over time.
The relative efficiency of the standard Ponar has been extensively tested in other systems (e.g., Flannagan Citation1970; Howmiller Citation1971; Elliott and Drake Citation1981; Lewis et al. Citation1982; Nalepa et al. Citation1988; Reynoldson et al. Citation1989; Schloesser and Nalepa Citation2002); therefore, we tested the relative efficiency of four other samplers commonly used for benthic invertebrate collections: the Shipek, petite Ponar, standard Ekman, and large Ekman dredges. For photographs or illustrations of the samplers tested, see Flannagan (Citation1970) or Sly (Citation1969). Our objective was to establish conversion factors among these four samplers in two substrate types (silt and sand) for common taxa and the overall invertebrate assemblage.
Materials and methods
Field tests
We compared the efficiency in collection of benthic invertebrates of the Shipek grab (area = 0.039 m2, maximum bite depth = 10.2 cm), the petite Ponar grab (area = 0.023 m2, maximum bite depth = 10.2 cm), the standard Ekman grab (area = 0.023 m2, maximum bite depth = 15.2 cm), and the large Ekman grab (area = 0.052 m2, maximum bite depth = 22.9 cm). Samples were collected from three locations in Lake Tahoe (California/Nevada, USA) in two different substrate types. Site 1 (Tahoe City Marina) contained soft, silt-dominated substrate (diameter <0.06 mm), while site 2 (Round Hill Pines Marina), and site 3 (Dollar Point) were dominated by sand substrate (diameter 0.125–2.0 mm). Each site was chosen because of its uniformity of depth and substrate. Samples were obtained with all four samplers at site 1. Only three samplers (petite Ponar, standard Ekman, and large Ekman) were tested at site 2. Weather prevented collections from the enclosed area at site 2 with the Shipek grab, the only sampler that required a boat for deployment. In order to include the Shipek in the sampler efficiency comparisons in sand substrate, we compared the Shipek grab to the standard Ekman grab at site 3. Because it is often necessary to add weight to lightweight Ekman samplers during deepwater sampling, we added two 1.4 kg weights to the standard Ekman and four 1.4 kg weights to the large Ekman. The added weight created more practical samplers for deepwater sampling and did not allow the samplers to overturn, a common problem caused by waves during the deployment of Ekman grabs. At each site 9–11 field replicates were collected with each sampler and washed through a 0.5 mm mesh bucket sieve. Samples from each site were collected on the same day and all samplers were dropped at the same time to eliminate invertebrate disturbance from adjacent sampling. Water depth during collection was 2 m at sites 1 and 2 and 20 m at site 3. In the laboratory, samples were elutriated (if necessary) and picked using a sugar floatation and visual inspection method (Anderson Citation1959). Dominant taxa (taxa that were found in >50% of samples) were identified to class, order or family, depending on the taxon, enumerated and preserved in 70% ethanol.
Data analysis
Relative collection efficiencies of samplers tested at sites 1 and 2 were evaluated by determining differences in densities of common invertebrate taxa and the overall invertebrate assemblage collected with each sampler using one-way ANOVA and post hoc Tukey's HSD tests. Relative collection efficiencies of the two samplers tested at site 3 were determined from differences in densities of common taxa and total invertebrate density collected with each sampler using a t-test. Where significant (p ≤ 0.05) differences occurred, conversion factors were calculated from mean (± standard error; SE) densities obtained with each sampler. All density data were square-root (x + 0.5) transformed to meet assumptions of normality and homogeneity of variances (Zar Citation1999). All statistical tests were performed with JMP Version 4.0.4 (SAS Institute Inc., North Carolina, USA). Results from our field trials were compared to published results from field tests of the same samplers in other systems to determine the consistency of our findings with those of others.
Results
Field tests
The dominant taxa collected in sampler efficiency tests in silt substrate (site 1) were Oligochaeta and Chironomidae. No other taxa were found in samples collected from site 1. There were significant differences among samplers in the densities of oligochaetes (one-way ANOVA, F 3,36 = 15.41, p < 0.0001), chironomids (one-way ANOVA, F 3,36 = 13.22, p < 0.0001), and all invertebrates combined (one-way ANOVA, F 3,36 = 17.11, p < 0.0001) collected in silt substrate. The Shipek grab was significantly less efficient in the collection of oligochaetes, chironomids, and all invertebrates combined than any of the other samplers tested (; Tukey HSD, p < 0.05). The large Ekman was significantly more efficient in the collection of oligochaetes and all invertebrates combined than any of the other samplers tested and was significantly more efficient at collecting chironomids than the petite Ponar and the Shipek (; Tukey HSD, p < 0.05). The petite Ponar and standard Ekman were not significantly different in sampling efficiency of common taxa or the overall invertebrate assemblage (; Tukey HSD, p < 0.05). Mean (±SE) densities collected with each sampler in silt substrate at site 1 are presented in .
Figure 1. Box plots of dominant macroinvertebrate and total macroinvertebrate densities (No./m2) from collections with each of the tested samplers in silt substrate at site 1. The top, middle, and bottom lines of each box represent the 75th percentile, median, and 25th percentile, respectively. The top and bottom bars represent the 90th and 10th percentiles, respectively, and the points represent outliers.
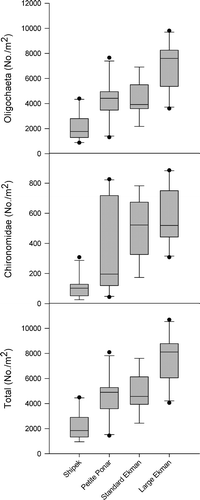
Table 1. Total benthos and dominant taxon mean (±SE) densities (No./m2) collected with different samplers at three sites during sampler efficiency testing. Logistical constraints prevented the collection of benthos with all samplers at all sites.
Conversion factors determined from mean (±SE) densities where significant differences in collection efficiencies occurred in silt substrate are given in . Benthic invertebrate densities obtained with a Shipek in silt substrate would need to be multiplied by factors ranging from 1.91 to 5.17, depending on the target taxon, in order to obtain petite Ponar, standard Ekman, or large Ekman equivalents. Benthic invertebrate densities obtained with a standard Ekman or petite Ponar would need to be multiplied by a smaller factor (1.56–1.65) to obtain large Ekman equivalents for most of the evaluated taxa, with the exception of Chironomidae, in which the collection efficiencies of standard and large Ekmans did not differ. No conversion would be necessary for standard Ekman and petite Ponar comparisons in silt substrate.
Table 2. Conversion factors determined from mean (±SE) densities collected with each sampler in silt substrate for all invertebrates combined and dominant taxa. A conversion factor was assumed to be 1.0 when there was no statistical difference (p ≤ 0.05) in mean density (No./m2) between samplers.
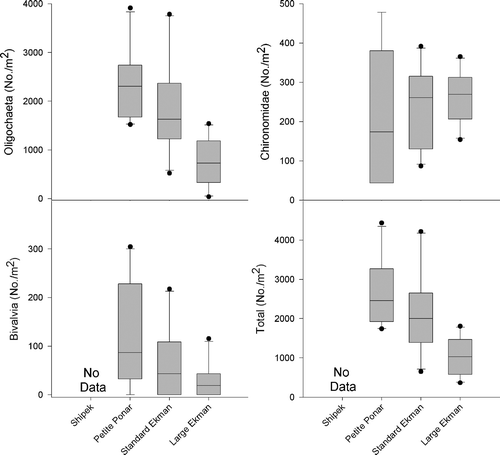
Samples collected from sites 2 and 3 were not directly comparable; therefore, efficiency comparisons between the Shipek grab and samplers other than the standard Ekman were not possible in sand substrate. The dominant taxa collected during sampler efficiency testing at site 2 were Oligochaeta, Chironomidae, and Bivalvia (Corbicula fluminea and Pisidium spp.). Ostracoda and Trombidiformes were also collected, but were not sufficient in number to use in sampler efficiency analyses. There were significant differences among samplers in the collection efficiency of oligochaetes (one-way ANOVA, F 2,27 = 12.37, p = 0.0002) and all invertebrates combined (one-way ANOVA, F 2,27 = 11.22, p = 0.0003) at site 2. The large Ekman collected significantly fewer oligochaetes and total invertebrates than the petite Ponar and standard Ekman in sand substrate (; Tukey HSD, p < 0.05). There were no significant differences among the samplers in the collection of chironomids (; one-way ANOVA, F 2,27 = 0.86, p = 0.433) or bivalves (; one-way ANOVA, F 2,27 = 2.26, p = 0.123) in sand substrate. Mean (±SE) densities collected with each sampler in sand substrate at site 2 are presented in .
Figure 2. Box plots of dominant macroinvertebrate and total macroinvertebrate densities (No./m2) from collections with each of the tested samplers in sand substrate at site 2. The top, middle, and bottom lines of each box represent the 75th percentile, median, and 25th percentile, respectively. The top and bottom bars represent the 90th and 10th percentiles, respectively, and the points represent outliers.
The dominant invertebrates collected at site 3 were oligochaetes and chironomids. Amphipoda, Gastropoda, Ostracoda, and Trombidiformes were also collected but were not dominant and were not used in sampler efficiency analyses. The Shipek was significantly less efficient than the standard Ekman in the collection of oligochaetes (; t-test, F 1,18 = 11.69, p = 0.003) and all invertebrates combined (; t-test, F 1,18 = 8.38, p = 0.01). There were no significant differences between the Shipek and standard Ekman in densities of chironomids collected (; t-test, F 2,27 = 1.04, p = 0.32). Mean (±SE) densities collected with each sampler in sand substrate at site 3 are presented in .
Figure 3. Box plots of dominant macroinvertebrate and total macroinvertebrate densities (No./m2) from collections with each of the tested samplers in sand substrate at site 3. The top, middle, and bottom lines of each box represent the 75th percentile, median, and 25th percentile, respectively. The top and bottom bars represent the 90th and 10th percentiles, respectively, and the points represent outliers.
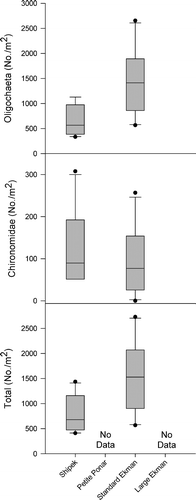
Conversion factors calculated from mean (±SE) densities that were significantly different between samplers tested at sites 2 and 3 are given in . Total benthic invertebrate and oligochaete densities obtained with a Shipek sampler would need to be multiplied by 1.93 and 2.14, respectively, to obtain a standard Ekman equivalent in sand substrate. There were no differences in collection efficiency of the standard Total invertebrate and oligochaete densities determined from petite Ponar collections would need to be multiplied by 0.40 and 0.34, respectively, to obtain a large Ekman equivalent. Total invertebrate and oligochaete densities obtained with a standard Ekman would need to be multiplied by 0.50 and 0.42, respectively, to obtain a large Ekman equivalent. No conversion would be necessary when comparing samples collected with the petite Ponar and standard Ekman in sand substrate.
Table 3. Conversion factors determined from mean (±SE) densities collected with each sampler in sand substrate for all invertebrates combined and dominant taxa. A conversion factor was assumed to be 1.0 when there was no statistical difference (p ≤ 0.05) in mean density (No./m2) between samplers. Conversion factors could not be calculated for all sampler comparisons because benthos were not collected with all samplers at all sites (see ).
We found only three other published accounts of sampler efficiency testing that compared the samplers of interest in this study (). In these accounts, there was some deviation from the sediment types and target taxa used in our study. For example, Schloesser and Nalepa (Citation2002) targeted a mayfly taxon and Shostell and Williams (Citation2005) sampled a benthic environment composed of clay, gravel, and mud in one of their field trials. Nonetheless, conversion factors produced by these studies were comparable to those produced from our sampler efficiency trials.
Table 4. Studies that have tested the efficiency of benthic samplers of interest in this study. Although other samplers may have been tested in the listed studies, only the Shipek, petite Ponar, standard Ekman, and large Ekman are included in the summary. Conversion factors were calculated from significantly different (p ≤ 0.05) sample means when not provided directly by the author(s).
Discussion
Conversion factors generated from relative sampler efficiencies varied substantially and appeared to be highly dependent on the type of substrate from which the sample was collected. For example, the large Ekman was 1.5–5.2 times more efficient than other samplers in silt substrate, but was one-third to one-half as efficient as the other samplers in sand substrate. Discrepancies between our data and findings of others also appear to be related to differences in substrate type. Our study suggests that, in both sand and silt substrate, the petite Ponar and the standard Ekman have similar collection efficiencies for all taxa. Shostell and Williams (Citation2005) found that the petite Ponar was significantly more efficient in the collection of the overall benthic invertebrate assemblage at a site composed of clay, gravel, and mud. The conflicting nature of these results was likely due to the different types of substrate tested in each study. Therefore, caution should be exercised in applying the conversion factors given here. If clay or gravel substrates are present, it is likely that heavier samplers designed to sample such environments (e.g., Ponar and Shipek grabs) will perform better than lighter samplers such as the Ekman.
Conversion factors generated from our data also varied substantially depending on the taxon targeted. For example, in sand substrate, the Shipek was less efficient than the standard Ekman in the collection of oligochaetes and the overall invertebrate assemblage, but the samplers did not differ in collection efficiencies of chironomids. In all of the field trials in sand substrate, no significant differences in sampler efficiency were found among any of the samplers in the collection of chironomids or bivalves. The differences in collection efficiency of different taxa are likely related to their position within the substrate. It is possible that chironomids and bivalves buried in sand substrate (as compared to oligochaetes partially exposed at the surface) are resistant to hydraulic disturbance caused by samplers during decent, especially in relatively solid sand substrates. When comparing our results to findings by Schloesser and Nalepa (Citation2002) in Lake Erie and Lake St. Clair, we found further evidence of differences between samplers in taxon-specific collection efficiencies. Schloesser and Nalepa (Citation2002) showed a large Ekman and petite Ponar to have similar efficiencies in the collection of mayfly nymphs in soft substrate, which contrasts with the results of our study in which the large Ekman was 1.6 times more efficient than the petite Ponar in the collection of oligochaetes and chironomids. The discrepancy between these results, therefore, may be due to differences inherent to the target organisms of each respective study.
Our results were consistent with results of others when sampler efficiency tests were conducted on similar substrate types and target organisms. In our field trials, the standard Ekman and petite Ponar showed similar performance in soft substrate in the collection of all taxa. Shostell and Williams (Citation2005) also found that densities of benthic invertebrates collected with a standard Ekman and petite Ponar did not differ significantly in soft-bottomed reservoirs in Arkansas. Results from field trials by Flannagan (Citation1970) suggested that a double Shipek was one-third as efficient in the collection of benthic invertebrates as a standard Ekman in soft substrate. We found a single Shipek to be about half as efficient in collecting benthic invertebrates as a standard Ekman in soft substrate. The discrepancy between these results could be due to the formation of a greater pressure wave below a double Shipek during its decent as compared to a single Shipek, given the substantial hydraulic pressure buildup below such heavy samplers (Flannagan Citation1970). Indeed, during Flannagan's trials with a single Shipek, the relative efficiency of the Shipek was similar to that found in our study, but replication was not sufficient to detect a significant difference between samplers.
Comparisons of sampler efficiency are difficult because the inherent spatial heterogeneity of benthic invertebrate assemblages often results in large deviations from sample means. We attempted to reduce the amount of variation in benthic invertebrate densities by sampling relatively homogeneous benthic environments (two marinas and a flat, sand-dominated shelf). Although the marinas do not represent natural areas of a lake, we felt that homogeneity was more important than the representation of a natural, heterogeneous environment in sampler efficiency testing. Our results were consistent with those of sampler efficiency trials in other systems, indicating that the conversion factors provided in this article can be applied to collections from other freshwater systems. The conversion factors generated in this study ranged from 0.3 to 5.2. Thus, quantitative comparisons of samples collected with different devices without accounting for differential sampler efficiency may be inaccurate and result in spurious conclusions. Of the samplers tested in this study, only the standard Ekman and petite Ponar could be reliably compared without the use of conversion factors. We could not determine relative sampling efficiencies of the Shipek for some of the taxon and substrate specific comparisons because of logistical constraints during field trials. Further sampler efficiency testing is needed to generate conversion factors that are not provided here. However, we hope that this article demonstrates that, where comparisons are lacking, it is relatively simple to test samplers and produce conversion factors if substrate and target taxon efficiency differences are considered.
Valuable information can be gleaned from spatial and temporal comparisons of benthic invertebrate communities in lakes. However, if collections are made with different benthic grabs, data cannot be reliably compared without the employment of conversion factors. Ideally, benthic researchers interested in monitoring an aquatic system over time and space should select a sampler suitable for the water bodies of interest and conduct all sampling with the same sampler. In situations where this is not possible, we hope that conversion factors provided in this article prove useful for sampler comparison.
Acknowledgments
The authors thank the managers of Round Hill Pines Marina and Tahoe City Marina for access to the marinas, and Brant Allen (UC Davis Tahoe Environmental Research Center) for boat and logistics assistance. The project was funded in part by funds to Drs Sudeep Chandra and Geoff Schladow from the California Tahoe Conservancy and Army Corps of Engineers.
References
- Anderson , RO . 1959 . A modified flotation technique for sorting bottom fauna samples . Limnology and Oceanography , 4 : 223 – 225 .
- Barton , DR and Anholt , B . 1997 . The macrobenthos of Lake Ontario during 1964 to 1966, and subsequent changes in the deepwater community . Aquatic Sciences , 59 : 158 – 175 .
- Brinkhurst , RO . 1975 . The benthos of lakes , New York , NY : St. Martin's Press .
- Clesceri , LS , Greenberg , AE and Eaton , AD eds . 1998 . Standard methods for the examination of water and wastewater, 20th ed , Washington , DC : American Public Health Association .
- Downing , JA and Rigler , FH . 1984 . A manual on methods for the assessment of secondary production in freshwaters , Oxford (England) : Blackwell Scientific. . Chapter 4, Sampling the benthos of standing waters; p. 87–130
- Elliott , JM and Drake , CM . 1981 . A comparative study of seven grabs used for sampling benthic macroinvertebrates in rivers . Freshwater Biology , 11 : 99 – 120 .
- Flannagan , JF . 1970 . Efficiencies of various grabs and corers in sampling freshwater benthos . Journal of the Fisheries Research Board of Canada , 27 : 1691 – 1700 .
- Howmiller , RP . 1971 . A comparison of the effectiveness of Ekman and Ponar grabs . Transactions of the American Fisheries Society , 3 : 560 – 564 .
- Lake , PS , Palmer , MA , Biro , P , Cole , J , Covich , AP , Dahm , C , Gibert , J , Goedkoop , W , Martens , K and Verhoeven , J . 2000 . Global change and the biodiversity of freshwater ecosystems: impacts on linkages between above-sediment and sediment biota . BioScience , 50 : 1099 – 1107 .
- Lewis , PA , Mason , WT Jr and Weber , CI . 1982 . Evaluation of three bottom grab samplers for collecting river benthos . Ohio Journal of Science , 82 : 107 – 113 .
- Lozano , SJ , Scharold , JV and Nalepa , TF . 2001 . Recent declines in benthic macroinvertebrate densities in Lake Ontario . Canadian Journal of Fisheries and Aquatic Sciences , 58 : 518 – 529 .
- Mudroch , A and Azcue , JM . 1995 . Aquatic sediment sampling , Boca Raton , FL : CRC Press .
- Nalepa , TF . 1987 . Long-term changes in the macrobenthos of southern Lake Michigan . Canadian Journal of Fisheries and Aquatic Sciences , 44 : 515 – 524 .
- Nalepa , TF . 1991 . Status and trends of the Lake Ontario macrobenthos . Canadian Journal of Fisheries and Aquatic Sciences , 48 : 1558 – 1567 .
- Nalepa , TF , Fanslow , DL , Lansing , MB and Lang , GA . 2003 . Trends in the benthic macroinvertebrate community of Saginaw Bay, Lake Huron, 1987 to 1996: responses to phosphorus abatement and the zebra mussel, Dreissena polymorpha . Journal of Great Lakes Research , 29 : 14 – 33 .
- Nalepa , TF , Fanslow , DL , Pothoven , SA , Foley , AJ III and Lang , GA . 2007 . Long-term trends in benthic macroinvertebrate populations in Lake Huron over the past four decades . Journal of Great Lakes Research , 33 : 421 – 436 .
- Nalepa , TF , Hartson , DJ , Fanslow , DL , Lang , GA and Lozano , SJ . 1998 . Declines in benthic macroinvertebrate populations in southern Lake Michigan, 1980–1993 . Canadian Journal of Fisheries and Aquatic Sciences , 55 : 2402 – 2413 .
- Nalepa , TF , Lang , GA and Fanslow , DL . 2000 . Trends in benthic macroinvertebrate populations in southern Lake Michigan . Verhandlungen des Internationalen Verein Limnologie. , 27 : 2540 – 2545 .
- Nalepa , TF , Quigley , MA and Ziegler , RW . 1988 . Sampling efficiency of the Ponar grab in two different benthic environments . Journal of Great Lakes Research , 14 : 89 – 93 .
- Reynoldson , TB , Schloesser , DW and Manny , BA . 1989 . Development of a benthic invertebrate objective for mesotrophic Great Lakes waters . Journal of Great Lakes Research , 15 : 669 – 686 .
- Robertson , A and Alley , WP . 1966 . A comparative study of Lake Michigan macrobenthos . Limnology and Oceanography , 11 : 576 – 583 .
- Schloesser , DW and Nalepa , TF . 2002 . Comparison of 5 benthic samplers to collect burrowing mayfly nymphs (Hexagenia spp.:Ephemeroptera:Ephemeridae) in sediments of the Laurentian Great Lakes . Journal of the North American Benthological Society , 21 : 487 – 501 .
- Shostell , JM and Williams , BS . 2005 . A new benthic sampling device for soft sediments in shallow habitats . Journal of Freshwater Ecology , 20 : 595 – 602 .
- Sly , PG . 1969 . Bottom sediment sampling , In : Proceedings of the 12th Conference on Great Lakes Research, May 5–7, Ann Arbor (MI). . Ann Arbor (MI): International Association for Great Lakes Research. p. 883–898
- Sly , PG and Christie , WJ . 1992 . Factors influencing densities and distributions of Pontoporeia hoyi in Lake Ontario . Hydrobiologia , 235/236 : 321 – 352 .
- Stewart , TW and Haynes , JM . 1994 . Benthic macroinvertebrate communities of southwestern Lake Ontario following invasion of Dreissena . Journal of Great Lakes Research , 20 : 479 – 493 .
- Vadeboncoeur , Y , Jeppesen , E , Vander Zanden , MJ , Schierup , H , Christoffersen , K and Lodge , DM . 2003 . From Greenland to green lakes: cultural eutrophication and the loss of benthic pathways in lakes . Limnology and Oceanography , 48 : 1408 – 1418 .
- Vadeboncoeur , Y , Peterson , G , Vander Zanden , MJ and Kalff , J . 2008 . Benthic algal production across lake size gradients: interactions among morphometry, nutrients, and light . Ecology , 89 : 2542 – 2552 .
- Vander Zanden MJ, Vadeboncoeur Y, Chandra S. 2011. Fish reliance on littoral-benthic resources and the distribution of primary production in lakes. Ecosystems [Internet]. [cited 2011 August 1]; Available from: http://www.springerlink.com/content/n1uv600108254771/
- Zar , JH . 1999 . Biostatistical analysis, , 4th , Upper Saddle River , NJ : Prentice-Hall .