Abstract
Predator–prey interactions between round goby (Neogobius melanostomus) and Dreissena are an important component of the food web in their invaded range in North America and Europe. We conducted two experiments to test the effect of round goby predation on Dreissena in coastal lakes connected to eastern Lake Michigan, USA. First, we conducted a density gradient experiment using 1-m2 cages stocked with 0 to 15 large round gobies (individuals > 7.5 cm total length (TL)). Presence of large round gobies significantly reduced Dreissena densities in all cages. Second, we conducted an exclosure experiment (treatments: none, 6-mm, and 24-mm mesh) in four coastal lakes in littoral habitats. Excluding large round gobies did not significantly affect Dreissena densities in any of the lakes, although we did find a significant effect of excluding large-bodied (i.e., animals larger than adult round goby that cannot pass through 24-mm mesh) predators in one lake. In combination, our two experiments showed that the strength of predator–prey interactions between round goby and Dreissena can vary with location and/or conditions. We hypothesize that the strength of predator–prey interactions between round goby and Dreissena vary spatially across the aquatic landscape depending on habitat (e.g., rocky vs. sandy substrates), which is critical for understanding food-web dynamics, especially in areas where the round goby and Dreissena are invasive.
Introduction
Human activities associated with the introduction of non-indigenous species are a significant component of global environmental change (Vitousek et al. Citation1997a, Citation1997b; Rahel Citation2000). In the Laurentian Great Lakes, the introduction of the zebra mussel (Dreissena polymorpha), quagga mussel (Dreissena bugensis), and round goby (Neogobius melanostomus) during 1986–1991 via ballast water from transoceanic ships (Jude et al. Citation1992; May and Marsden Citation1992; Carlton Citation2008) has resulted in significant ecological change (e.g., Vanderploeg et al. Citation2002; Corkum et al. Citation2004; Strayer Citation2009). Introduction of zebra and quagga mussels (hereafter Dreissena) to the Great Lakes may have facilitated the introduction and spread of round goby because these species have similar native ranges in the Ponto-Caspian region of Eurasia (Ricciardi Citation2001; Vanderploeg et al. Citation2002). Thus, the round goby can exploit Dreissena as prey more efficiently than native Great Lakes fishes (Ricciardi Citation2001; Vanderploeg et al. Citation2002). Moreover, the ecological effects of these invasive species are not limited to the Great Lakes basin because they have and continue to spread across North America and Europe (Corkum et al. Citation2004; Strayer Citation2009).
Large round gobies (individuals > 7.5 cm TL) are known to feed extensively on Dreissena (e.g., Ghedotti et al. Citation1995; Ray and Corkum Citation1997; French and Jude Citation2001; Campbell et al. Citation2009). Dreissena tend to be a less important component of the diet of round gobies less than 7.5 cm TL compared to larger individuals (Jude et al. Citation1995; French and Jude Citation2001). There is mounting evidence that round goby predation can affect the size structure and densities of Dreissena populations (Kuhns and Berg Citation1999; Djuricich and Janssen Citation2001; Barton et al. Citation2005; Lederer et al. Citation2006, Citation2008). Nevertheless, the importance of Dreissena in the diets of round gobies can vary among habitats (e.g., shallower vs. deeper offshore areas) as well as over time (Schaeffer et al. Citation2005) and the round goby has invaded areas that lack Dreissena (Carman et al. Citation2006). Thus, the ecological importance of predator–prey interactions between round goby and Dreissena may vary spatially across the aquatic landscape.
The goal of our study was to examine the predatory role of round goby on Dreissena densities in coastal areas of eastern Lake Michigan where predator–prey interactions between these organisms have not been previously studied. In situ enclosure and exclosure experiments are a practical way to identify strong interspecific interactions (Power Citation1992; Ruetz et al. Citation2002; Bartsch et al. Citation2005). First, we used in situ enclosures to examine the direct predatory role of large round gobies on Dreissena densities. We predicted a functional response of reduced Dreissena densities with increasing round goby densities. Second, we used in situ exclosures to examine the effect of round goby predation relative to larger predators (under natural densities) on Dreissena densities. The exclosure experiment was conducted at four study sites to determine whether the strength of the predator–prey interaction between round goby and Dreissena varied among study sites. We hypothesized that Dreissena densities would be reduced in treatments exposed to predation primarily by the round goby given their abundance in our study lakes (Cooper et al. Citation2007).
Materials and methods
Study sites
Field experiments were conducted in four coastal lakes along the eastern shore of Lake Michigan (). These lakes are all connected directly to Lake Michigan, and the round goby is present in each lake (Cooper et al. Citation2007, Citation2009).
Table 1. Site locations, lake surface area, water temperature, turbidity, Dreissena density and size, and round goby catch for the exclosure experiment. Temperature and turbidity (mean ± 1 SE) were measured during April (once), June (once in Kalamazoo and twice in other lakes), and September (once) 2005. Dreissena density (mean ± 1 SE, n = 3 bricks per lake) and size (mean and range) are based on bricks sampled at the beginning of the experiment. Round goby catch was in three fyke nets fished at each site during June 2005 (Cooper et al. Citation2007).
An enclosure experiment was conducted in Muskegon Lake at a site (N 43.244°, W 86.308°) with primarily sand substrate where submersed aquatic vegetation (SAV) was generally absent. Water depth at this site was about 60 cm, and mean daily water temperature (measured by a continuous recorder) during the experiment was 25.4°C (range = 22.2–28.2°C).
An exclosure experiment was conducted in four coastal lakes (). We selected a shallow (<1 m) site in each coastal lake that corresponded to the site Cooper et al. (Citation2007) used as a SAV habitat. Previous work in these lakes showed round goby used areas with SAV preferentially over areas with bare sediment or emergent vegetation (Cooper et al. Citation2007). Among the four study sites where we conducted the exclosure experiment, round goby densities (measured as catch in fyke nets) were the highest in Muskegon and Pentwater lakes (). Daytime water temperatures were similar among sites, although turbidity was higher in Kalamazoo Lake. Additionally, surface area varied among lakes with Muskegon Lake, the largest, and Kalamazoo Lake, the smallest.
Enclosure experiment
We tested the effect of round goby predation on Dreissena abundance by conducting a density gradient experiment in Muskegon Lake. Twelve cages (1 × 1 × 0.5 m3) with 6-mm mesh on all sides were used to manipulate round goby density. The mesh size was sufficient for the movement of invertebrate prey in and out of cages (e.g., Ruetz et al. Citation2002), while acting as a barrier to round gobies ≥ 3.8 cm TL. Cages were secured to the lake bottom about 65 m from shore in a grid formation of three rows (four cages/row) with 0.5 m between cages. Each cage was stocked with three, randomly assigned cement bricks (19.5 × 9.3 × 5.8 cm3; surface area that could be colonized = 515.4 cm2) that were colonized by Dreissena. Bricks had been incubated in Muskegon Lake for 70 days prior to the start of the experiment. The initial density of Dreissena was 16.3 individuals/brick (SE = 2.9, n = 3 bricks), and the mean size of individuals was 13.0 mm (range = 6.9–18.6 mm). The experiment began on 28 June 2005 and was concluded after 71 days. Veliger densities of Dreissena often peak in July (Fraleigh et al. Citation1993; Garton and Haag Citation1993). Thus, the response of Dreissena densities on bricks reflects the effect of predation on adult Dreissena already on bricks as well as veliger colonization throughout the experiment.
A density (0, 5, 10, or 15 fish/m2) of round gobies was randomly assigned to each cage in a row (n = 3 replicate cages). We stocked cages with round gobies > 7.5 cm TL that were collected by angling from the channel connecting Muskegon Lake to Lake Michigan. Fish of this size were selected because they often feed extensively on Dreissena (e.g., Ray and Corkum Citation1997; French and Jude Citation2001; Janssen and Jude Citation2001). Each round goby stocked in a cage was marked with a unique fin clip. We recorded TL (cm) and wet mass (g) of each fish at the time of stocking and conclusion of the experiment. Cages were monitored daily for mortality at the start of the study. Once mortalities ceased for 1 week, cages were checked weekly. Round gobies that died were replaced, and the date of stocking was recorded. At the conclusion of the experiment, Dreissena were removed from bricks in the field and transported to the laboratory for analysis.
Exclosure experiment
We tested the effect of round goby predation relative to other predators on Dreissena in four coastal lakes (). In each lake, a cement brick colonized by Dreissena (incubated for 48–55 days) was randomly assigned to a 6-mm-mesh exclosure, 24-mm-mesh exclosure, or no-mesh control (six replicates/lake = 18 bricks/lake). Exclosures had mesh on all sides (29.5 × 18.0 × 9.5 cm3), whereas there was no mesh associated with controls. Exclosures and controls were interspersed about 20 cm apart in a grid arrangement on the substrate. Mesh was cleaned every 3–5 weeks on exclosures to remove silt, algae, and macrophytes. The study was initiated on 6–13 June 2005 and concluded after 72–86 days. Mean initial densities of Dreissena in lakes ranged from 0 to 13.3 individuals/brick, and mean size of individuals in lakes ranged from 6.9 to 10.1 mm (). As noted for the enclosure experiment, the response of Dreissena densities on bricks reflects the effect of predation on adult Dreissena already on bricks as well as veliger colonization during the experiment.
We assumed that the 6-mm mesh excluded all predators of Dreissena because round gobies small enough to pass through the 6-mm exclosures (i.e., individuals < 3.8 cm TL) were likely too small to feed extensively on Dreissena (Ray and Corkum Citation1997). The 24-mm exclosures allowed round gobies (≤15.0 cm TL) access to bricks while excluding larger predators (i.e., fish, mammals, birds, and turtles that were unable to pass through the 24-mm mesh). Round gobies too large to physically pass through the 24-mm mesh are seldom encountered in and around our study sites (Clapp et al. Citation2001; Ruetz et al. Citation2007; Cooper et al. Citation2007, Citation2009). We assumed that any predation on Dreissena in the 24-mm-mesh exclosures was primarily due to the round goby because other small-bodied fishes, such as johnny darter (Etheostoma nigrum) or logperch (Percina caprodes), were unlikely to consume Dreissena (French and Jude Citation2001) and we encountered very few crayfish during our field work (CRR, personal observation). Based on those assumptions regarding our predator exclusions, we tested five ecological effects (; see ‘Statistical Analysis’ section for description of ecological effects).
Table 2. Ecological interpretations of differences in Dreissena densities in the exclosure experiment. Treatment levels were no-mesh controls (N), 24-mm exclosures (L), and 6-mm exclosures (S). We assumed that S excluded all predators of Dreissena and L excluded all predators of Dreissena except the round goby (see Materials and methods section for further discussion).
We tested the assumption that round gobies could move through the 24-mm mesh by placing a round goby (range = 11.2–14.8 cm TL) in a 37.9-L aerated tank and visually observing the tank periodically to determine whether the fish had entered a 24-mm exclosure containing a brick (cage and brick were same as used in field experiment). Trials (n = 20) were run for a maximum of 21 h with a new fish each time and were concluded the first time a round goby was observed inside the 24-mm exclosure (i.e., fish had passed through mesh to enter exclosure).
Sample collection and processing
In the field, Dreissena were scraped from each brick onto a 500-µm sieve and preserved in 70% ethanol. In the laboratory, Dreissena were enumerated, measured, and weighed. A large proportion of Dreissena was small and could not be identified to species. For samples with >100 Dreissena, we sub-sampled to estimate density (number/brick) and TL. Sub-samples contained at least 30 Dreissena, which were used to estimate their TL in millimeter and ash-free dry mass (AFDM) in grams. We measured TL of Dreissena shells with dial calipers from the umbone to the posterior margin along the ventral hinge. To estimate AFDM, Dreissena were oven dried at 55°C for at least 24 h until a constant mass was reached and ashed in a muffle furnace at 550°C for about 20 h. All samples were cooled in desiccators prior to weighing after drying and ashing.
Statistical analysis
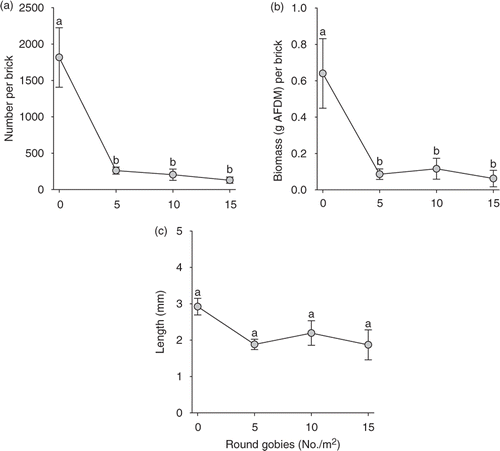
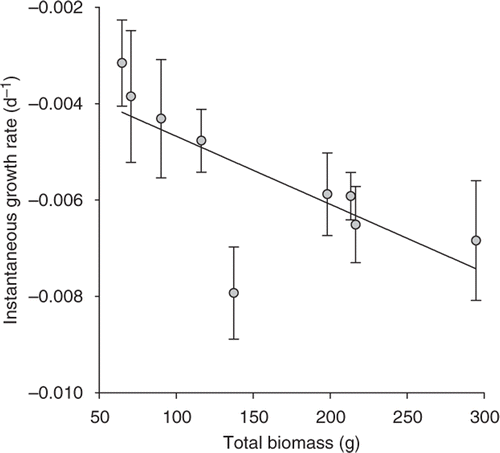
We used one-way analysis of variance (ANOVA) to assess the effect of round goby density (0, 5, 10, 15 fish/m2) on Dreissena abundance for the enclosure experiment. The response of Dreissena was measured as mean density (expressed as numbers and biomass) and length on three bricks in a cage (i.e., cage was the experimental unit). When a significant difference was detected, we made planned comparisons using orthogonal contrasts (Montgomery Citation1991) to test for an overall round goby effect (0 vs. 5 + 10 + 15 fish/cage) and to test whether the effect on Dreissena differed between the low and medium–high densities of round gobies (5 vs. 10 + 15 fish/cage) and between the medium and high densities of round gobies (10 vs. 15 fish/cage). Additionally, we examined whether the instantaneous growth rates (see Ruetz et al. Citation2006) of round gobies were a function of the initial round goby biomass stocked in a cage using linear regression. We expected that growth rates of round gobies would decrease with increasing fish biomass in a cage.
To examine the effects of excluding predators from Dreissena (), we used a nested, two-factor ANOVA to test whether Dreissena densities differed among lakes (Muskegon, Pentwater, White, and Kalamazoo) and predator exclusions (no-mesh control, 6-mm exclosure, and 24-mm exclosure) nested within lakes. Based on the findings of the enclosure experiment (i.e., statistical analyses based on Dreissena numbers and biomass did not change general findings, and length of Dreissena did not respond to round goby densities), we only examined the response of Dreissena numbers for this experiment. When a significant predator-exclusion effect was detected, we made planned comparisons (no-mesh controls vs. 6-mm exclosures, no-mesh controls vs. 24-mm exclosures, and 6-mm vs. 24-mm exclosures) within each lake using contrasts to test for differences in Dreissena density. We used these planned comparisons to test for five ecological effects (): no predation (predators did not affect Dreissena densities), cage (mesh size affected Dreissena colonization in the absence of a predator effect), round goby (predation by round gobies reduced Dreissena densities), large predator and round goby (predation by both large predators and round gobies reduced Dreissena densities), and large predator (predation by large predators – but not round gobies – reduced Dreissena densities). When using ANOVA in both experiments, we used log10 transformations on Dreissena density to correct for heteroscedasticity.
Results
Enclosure experiment
The presence of round goby significantly reduced the total number (F 3,8 = 15.84, p = 0.001; ) and biomass (F 3,8 = 4.71, p = 0.035; ) of Dreissena. Cages without round goby had significantly higher numbers and biomass of Dreissena than cages with round goby (contrasts, p < 0.008), whereas total numbers and biomass of Dreissena did not significantly differ among enclosures with 5, 10, and 15 round gobies (contrasts, p > 0.150). The mean size of Dreissena did not differ significantly within the gradient of round goby densities (F 3,8 = 2.75, p = 0.113; ). Finally, instantaneous growth rates of round gobies were negative and declined with the initial total biomass of round gobies in cages (slope: p = 0.022, R 2 = 0.549; ), suggesting that resources (e.g., food or shelters) were limited and intraspecific competition increased at higher round goby densities. In total, 40 round gobies died during the experiment (and were replaced), with 85% of the mortalities occurring during the first 7 days of the experiment.
Exclosure experiment
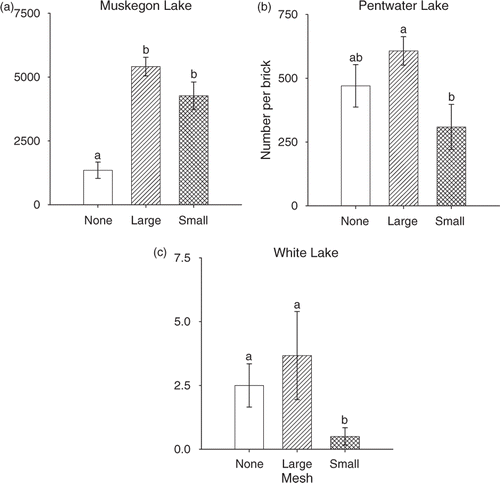
Excluding round goby from bricks did not reduce the numbers of Dreissena in any of the study lakes. Total Dreissena densities were greatest in Muskegon Lake and lowest in White Lake (). Bricks incubated in Kalamazoo Lake were not colonized by Dreissena, which could be related to differences in water quality among lakes (), and were excluded from this analysis. Dreissena densities differed significantly among lakes (F 2,45 = 735.02, p < 0.001) and across exclosure treatments nested within lakes (F 6,45 = 7.56, p < 0.001). Muskegon Lake was the only lake where we observed evidence of a predator effect (); large predators appeared to decrease Dreissena densities (). Dreissena densities in no-mesh controls were significantly lower than in 24-mm (contrast, F 1,45 = 24.95, p < 0.001) and 6-mm exclosures (contrast, F 1,45 = 17.58, p < 0.001) and Dreissena densities did not differ significantly between the 24-mm and 6-mm exclosures (contrast, F 1,45 = 0.64, p = 0.427). In Pentwater Lake, Dreissena densities in no-mesh controls did not differ significantly from 24-mm (contrast, F 1,45 = 0.97, p = 0.330) or 6-mm exclosures (contrast, F 1,45 = 2.36, p = 0.131), although Dreissena densities were significantly lower in 6-mm compared with 24-mm exclosures (contrast, F 1,45 = 6.36, p = 0.015; ). However, the direction of this effect was not clearly associated with any of our ecological hypotheses (). In White Lake, Dreissena densities in no-mesh controls did not differ significantly from 24-mm exclosures (contrast, F 1,45 = 0.40, p = 0.531) but were significantly higher than 6-mm exclosures (contrast, F 1,45 = 5.70, p = 0.021; ). Similarly, Dreissena densities in 24-mm exclosures were significantly higher than 6-mm exclosures (contrast, F 1,45 = 9.12, p = 0.004; ). The direction of this effect was not clearly associated with any of our ecological hypotheses () but could be due to a weak cage effect in which 6-mm exclosures reduced the colonization of Dreissena.
Figure 3. Mean density (±1 SE) of Dreissena in (a) Muskegon, (b) Pentwater, and (c) White lakes. All predators had access to Dreissena in no-mesh (none) controls, whereas all predators were excluded from the 6-mm (small) exclosures. Only large predators were excluded from 24-mm (large) exclosures (i.e., round goby had access to Dreissena). Means with different letters indicate significant differences (p < 0.05). Bricks in Kalamazoo Lake (not shown) were not colonized by Dreissena.
We found that round gobies ≤ 14.3 cm TL were able to pass through the 24-mm mesh in laboratory trials, demonstrating that round goby behavior should not prevent them from entering the 24-mm exclosures used in field experiments. Only three round gobies (among 20 individuals) did not pass through the mesh during a trial, and they were ≥ 14.6 cm TL. For round gobies that passed through the mesh, the fish was observed inside the 24-mm exclosure 2.01 h (SE = 1.13) after the trial began on average.
Discussion
Our results in conjunction with evidence on round goby diets (Cooper et al. Citation2009; Ruetz et al. Citation2009) suggest that round goby predation effects on Dreissena density are not uniform across coastal areas of eastern Lake Michigan. In our enclosure experiment, the presence of large round gobies (i.e., 5, 10, and 15 individuals/m2) significantly decreased Dreissena abundance in Muskegon Lake. However, based on an exclosure experiment, round goby predation did not significantly affect Dreissena density at any of the three coastal lakes we investigated, although we did find evidence that large predators reduced Dreissena density in Muskegon Lake. There was no evidence of a large predator effect in either Pentwater or White lakes.
Our enclosure experiment clearly demonstrated that large round gobies are capable of reducing Dreissena densities, which is consistent with previous investigations in southern Lake Michigan (Kuhns and Berg Citation1999; Djuricich and Janssen Citation2001), western Lake Michigan (Lederer et al. Citation2006, Citation2008), and eastern Lake Erie (Barton et al. Citation2005). Similarly, previous investigations on fish predation in areas without the round goby showed negative effects on Dreissena density (Thorp et al. Citation1998; Bartsch et al. Citation2005). In our experiment, round goby growth rates were negatively associated with initial stocking biomass in enclosures (), suggesting that food was a limiting resource in cages. This, in turn, may indicate why we did not detect differences in the strength of predation at low (5 individuals/m2) and high (15 individuals/m2) densities of round gobies (). We used round goby densities of 5–15 individuals/m2 in enclosures, which are not uncommon in the Great Lakes (Ray and Corkum Citation2001; Barton et al. Citation2005; Johnson et al. Citation2005) with densities as high as 133 individuals/m2 reported in areas of Lake Michigan (Chotkowski and Marsden Citation1999). However, the size and density of round gobies in cages were likely greater than round gobies inhabiting littoral habitats in Muskegon Lake given the size and number of round gobies collected in fyke nets (Cooper et al. Citation2007, Citation2009; Ruetz et al. Citation2007).
In the exclosure experiment, there was not a significant round goby effect on Dreissena density in any of the coastal lakes we studied. The results from our exclosure experiment contrast with other studies, including our enclosure experiment, which showed a strong effect of round goby predation on Dreissena (Kuhns and Berg Citation1999; Barton et al. Citation2005; Lederer et al. Citation2006, Citation2008). There are several possible factors that may contribute to this lack of effect, including statistical power, round goby size, habitat type, as well as predator and prey densities. We discount the likelihood that the lack of a predation effect by round goby on Dreissena was caused by low statistical power because the trends in Dreissena density are not in the direction expected (i.e., higher Dreissena densities in small-mesh relative to large-mesh exclosures; ) if the round goby had a strong effect on Dreissena density.
The size structure of round goby populations in littoral areas where we conducted exclosure experiments may be an important mechanism underlying the lack of a strong predation effect of round goby on Dreissena. The round goby is an aggressive, territorial fish (Jude and DeBoe Citation1996; Johnson et al. Citation2005; Stammler and Corkum Citation2005). Large adult round gobies prefer an environment with cover; therefore, areas with rocks and riprap are ideal habitat (Jude and DeBoe Citation1996; Ray and Corkum Citation2001). Younger, smaller round gobies often are forced out of these ideal habitats to live in less complex habitats (Ray and Corkum Citation2001; Johnson et al. Citation2005; Stammler and Corkum Citation2005). Diets of small round gobies are comprised of mostly non-mussel invertebrates, whereas diets of larger (>7.5 cm) round gobies often contain a large proportion of Dreissena (Ray and Corkum Citation1997; Skora and Rzeznik Citation2001; Barton et al. Citation2005; Campbell et al. Citation2009). This shift in diet with size is important because round gobies found near our exclosure sites had mean standard lengths of 5–6 cm (Cooper et al. Citation2007, Citation2009), whereas round gobies used in the enclosure experiment were >7.5 cm TL. Therefore, many of the round gobies living in the littoral habitats associated with the exclosure experiment may have not yet shifted their diets from non-mussel invertebrates to Dreissena, which could explain the lack of a strong round goby predation effect on Dreissena density in our exclosure experiment.
We suspect that the littoral habitats where we conducted exclosure experiments and feeding preferences of round goby may be additional factors explaining our finding that excluding the round goby did not affect Dreissena densities. The site in each coastal lake where we conducted the exclosure experiment had SAV and was found to have high numbers of round gobies compared with other types of littoral habitats with bare substrate or mono-dominant stands of Nuphar (Cooper et al. Citation2007, Citation2009). However, the site in each coastal lake where we conducted the exclosure experiment lacked preferred rocky substrate where both round goby and Dreissena are likely more abundant (Jude and DeBoe Citation1996). Although round goby diets were dominated by non-mussel invertebrates (mainly dipteran larvae and zooplankton) in coastal lakes connecting to Lake Michigan (Cooper et al. Citation2009), Dreissena are likely an important component of the diets of round gobies inhabiting areas with hard substrates in coastal lakes (e.g., riprap in channels connecting coastal lakes to Lake Michigan; Ruetz et al. 2009). Round goby often prefer non-mussel invertebrates over Dreissena in laboratory experiments (Diggins et al. Citation2002; Bauer et al. Citation2007; Polačik et al. 2009; Coulter et al. Citation2011), suggesting that densities of non-mussel invertebrates in the littoral habitats also could be an important predictor of the strength of predation by the round goby on Dreissena.
In Muskegon Lake, there was a large predator effect on Dreissena density. There are several fishes in Muskegon Lake (Ruetz et al. Citation2007; Bhagat and Ruetz Citation2011) that have been known to eat Dreissena such as the freshwater drum (Aplodinotus grunniens), pumpkinseed (Lepomis gibbosus), redhorse suckers (Moxostoma spp.), and common carp (Cyprinus carpio) (Tucker et al. Citation1996; Thorp et al. Citation1998; Magoulick and Lewis Citation2002; Andraso Citation2005; Bartsch et al. Citation2005). Large-bodied molluscivorous fishes were implicated in limiting Dreissena densities in a Lake Erie costal wetland (Bowers and de Szalay Citation2007) and in the Upper Mississippi River (Bartsch et al. Citation2005), although there was spatial variation in the magnitude of the effect in the Upper Mississippi River. In addition, there are several non-fish predators of Dreissena in Muskegon Lake, including the map turtle (Graptemys geographica; Lindeman Citation2006; Bulté and Blouin-Demers Citation2008), muskrat (Ondatra zibenthicus; Sietman et al. Citation2003), crayfish (Orconectes spp.; Martin and Corkum Citation1994; Perry et al. Citation1997), and possibly waterfowl (Hamilton et al. Citation1994; Badzinski and Petrie Citation2006). Although all these large-bodied molluscivores had access to the no-mesh exposed brick in Muskegon Lake and could have consumed Dreissena, our personal observations suggest that predation effects were caused by large-bodied fishes.
We conclude that the predation effect by the round goby on Dreissena density is spatially heterogeneous across the aquatic landscape. We hypothesize that habitat type is critical for predicting the effect of round goby predation on Dreissena density. For example, in areas of SAV in coastal lakes of eastern Lake Michigan, round goby are not likely to strongly reduce Dreissena densities because of the size structure of round gobies (i.e., more small individuals) and round gobies are likely consuming energy rich non-mussel invertebrates rather than Dreissena as their dominant food source (Cooper et al. Citation2007, Citation2009; Ruetz et al. Citation2007, Citation2009). Our hypothesis should apply beyond eastern Lake Michigan and should be tested in other areas in North America and Europe where the round goby is invading.
Acknowledgments
The authors thank Matt Cooper, Matt Breen, Aaron Parker, and Dana Strouse for assistance with fieldwork. Kristin Thomas shared ideas that helped shape the discussion. Matt Altenritter and Yakuta Bhagat provided helpful suggestions on an earlier version of this manuscript. MRR was partially supported by the Herbert L. VanderMey Internship. This research was funded by the generous support of Mr. Allen D. Hunting.
References
- Andraso , GM . 2005 . Summer food habits of pumpkinseed (Lepomis gibbosus) and bluegill (Lepomis macrochirus) in Presque Isle Bay, Lake Erie . Journal of Great Lakes Research , 31 ( 4 ) : 397 – 404 .
- Badzinski , SS and Petrie , SA . 2006 . Diets of lesser and greater scaup during autumn and spring on the lower Great Lakes . Wildlife Society Bulletin , 34 ( 3 ) : 664 – 674 .
- Barton , DR , Johnson , RA , Campbell , L , Petruniak , J and Patterson , M . 2005 . Effects of round gobies (Neogobius melanostomus) on dreissenid mussels and other invertebrates in eastern Lake Erie, 2002-2004 . Journal of Great Lakes Research , 31 ( 2 ) : 252 – 261 .
- Bartsch , MR , Bartsch , LA and Gutreuter , S . 2005 . Strong effects of predation by fishes on an invasive macroinvertebrate in a large floodplain river . Journal of the North American Benthological Society , 24 ( 1 ) : 168 – 177 .
- Bauer , CR , Bobeldyk , AM and Lamberti , GA . 2007 . Predicting habitat use and trophic interactions of Eurasian ruffe, round gobies, and zebra mussels in nearshore areas of the Great Lakes . Biological Invasions , 9 ( 6 ) : 667 – 678 .
- Bhagat , Y and Ruetz III , CR . 2011 . Temporal and fine-scale spatial variation in fish assemblage structure in a drowned river mouth system of Lake Michigan . Transactions of the American Fisheries Society , 140 ( 6 ) : 1429 – 1440 .
- Bowers , RW and de Szalay , FA . 2007 . Fish predation of zebra mussels attached to Quadrula quadrula (Bivalvia: Unionidae) and benthic molluscs in a Great Lakes coastal wetland . Wetlands , 27 ( 1 ) : 203 – 208 .
- Bulté , G and Blouin-Demers , G . 2008 . Northern map turtles (Graptemys geographica) derive energy from the pelagic pathway through predation on zebra mussels (Dreissena polymorpha) . Freshwater Biology , 53 ( 3 ) : 497 – 508 .
- Campbell , LM , Thacker , R , Barton , D , Muir , DCG , Greenwood , D and Hecky , RE . 2009 . Re-engineering the eastern Lake Erie littoral food web: the trophic function of non-indigenous Ponto-Caspian species . Journal of Great Lakes Research , 35 ( 2 ) : 224 – 231 .
- Carlton , JT . 2008 . The zebra mussel Dreissena polymorpha found in North America in 1986 and 1987 . Journal of Great Lakes Research , 34 ( 4 ) : 770 – 773 .
- Carman , SM , Janssen , J , Jude , DJ and Berg , MB . 2006 . Diel interactions between prey behaviour and feeding in an invasive fish, the round goby, in a North American river . Freshwater Biology , 51 ( 4 ) : 742 – 755 .
- Chotkowski , MA and Marsden , JE . 1999 . Round goby and mottled sculpin predation on lake trout eggs and fry: field predictions from laboratory experiments . Journal of Great Lakes Research , 25 ( 1 ) : 26 – 35 .
- Clapp , DF , Schneeberger , PJ , Jude , DJ , Madison , G and Pistis , C . 2001 . Monitoring round goby (Neogobius melanostomus) population expansion in eastern and northern Lake Michigan . Journal of Great Lakes Research , 27 ( 3 ) : 335 – 341 .
- Cooper , MJ , Ruetz III , CR , Uzarski , DG and Burton , TM . 2007 . Distribution of round gobies in coastal areas of Lake Michigan: are wetlands resistant to invasion? . Journal of Great Lakes Research , 33 ( 2 ) : 303 – 313 .
- Cooper , MJ , Ruetz III , CR , Uzarski , DG and Shafer , BM . 2009 . Habitat use and diet of the round goby (Neogobius melanostomus) in coastal areas of Lake Michigan and Lake Huron . Journal of Freshwater Ecology , 24 ( 3 ) : 477 – 488 .
- Corkum , LD , Sapota , MR and Skora , KE . 2004 . The round goby, Neogobius melanostomus, a fish invader on both sides of the Atlantic Ocean . Biological Invasions , 6 ( 2 ) : 173 – 181 .
- Coulter , DP , Murry , BA , Webster , WC and Uzarski , DG . 2011 . Effects of dreissenid mussels, chironomids, fishes, and zooplankton on growth of round goby in experimental aquaria . Journal of Freshwater Ecology , 26 ( 2 ) : 155 – 162 .
- Diggins , TP , Kaur , J , Chakraborti , RK and DePinto , JV . 2002 . Diet choice by the exotic round goby (Neogobius melanostomus) as influenced by prey motility and environmental complexity . Journal of Great Lakes Research , 28 ( 3 ) : 411 – 420 .
- Djuricich , P and Janssen , J . 2001 . Impact of round goby predation on zebra mussel size distribution at Calumet Harbor, Lake Michigan . Journal of Great Lakes Research , 27 ( 3 ) : 312 – 318 .
- Fraleigh , PC , Klerks , PL , Gubanich , G , Matisoff , G and Stevenson , RC . 1993 . “ Abundance and settling of zebra mussels (Dreissena polymorpha) veligers in western and central Lake Erie ” . In Zebra mussels: biology, impacts, and control , Edited by: Nalepa , TF and Schloesser , DW . 129 – 142 . Ann Arbor , MI : Lewis Publishers .
- French III , JRP and Jude , DJ . 2001 . Diets and diet overlap of nonindigenous gobies and small benthic native fishes co-inhabiting the St. Clair River, Michigan . Journal of Great Lakes Research , 27 ( 3 ) : 300 – 311 .
- Garton , DW and Haag , WR . 1993 . “ Seasonal reproductive cycles and settlement patterns of Dreissena polymorpha in western Lake Erie ” . In Zebra mussels: biology, impacts, and control , Edited by: Nalepa , TF and Schloesser , DW . 111 – 128 . Ann Arbor , MI : Lewis Publishers .
- Ghedotti , MJ , Smihula , JC and Smith , GR . 1995 . Zebra mussel predation by round gobies in the laboratory . Journal of Great Lakes Research , 21 ( 4 ) : 665 – 669 .
- Hamilton , DJ , Ankney , CD and Bailey , RC . 1994 . Predation of zebra mussels by diving ducks: an exclosure study . Ecology , 75 ( 2 ) : 521 – 531 .
- Janssen , J and Jude , DJ . 2001 . Recruitment failure of mottled sculpin Cottus bairdi in Calumet Harbor, southern Lake Michigan, induced by the newly introduced round goby Neogobius melanostomus . Journal of Great Lakes Research , 27 ( 3 ) : 319 – 328 .
- Johnson , TB , Allen , M , Corkum , LD and Lee , VA . 2005 . Comparison of methods needed to estimate population size of round gobies (Neogobius melanostomus) in western Lake Erie . Journal of Great Lakes Research , 31 ( 1 ) : 78 – 86 .
- Jude , DJ and DeBoe , SF . 1996 . Possible impact of gobies and other introduced species on habitat restoration efforts . Canadian Journal of Fisheries and Aquatic Sciences , 53 ( Suppl. 1 ) : 136 – 141 .
- Jude , DJ , Janssen , J and Crawford , G . 1995 . “ Ecology, distribution, and impact of the newly introduced round and tubenose gobies on the biota of the St. Clair and Detroit rivers ” . In The Lake Huron ecosystem: ecology, fisheries and management , Edited by: Munawar , M , Edsall , T and Leach , J . 447 – 460 . Amsterdam : SPB Academic .
- Jude , DJ , Reider , RH and Smith , GR . 1992 . Establishment of Gobiidae in the Great Lakes basin . Canadian Journal of Fisheries and Aquatic Sciences , 49 ( 2 ) : 416 – 421 .
- Kuhns , LA and Berg , MB . 1999 . Benthic invertebrate community responses to round goby (Neogobius melanostomus) and zebra mussel (Dreissena polymorpha) invasion in southern Lake Michigan . Journal of Great Lakes Research , 25 ( 4 ) : 910 – 917 .
- Lederer , AM , Janssen , J , Reed , T and Wolf , A . 2008 . Impact of the introduced round goby (Apollonia melanostoma) on dreissenids (Dreissena polymorpha and Dreissena bugensis) and on macroinvertebrate community between 2003 and 2006 in the littoral zone of Green Bay, Lake Michigan . Journal of Great Lakes Research , 34 ( 4 ) : 690 – 697 .
- Lederer , A , Massart , J and Janssen , J . 2006 . Impact of round gobies (Neogobius melanostomus) on dreissenids (Dreissena polymorpha and Dreissena bugensis) and the associated macroinvertebrate community across an invasion front . Journal of Great Lakes Research , 32 ( 1 ) : 1 – 10 .
- Lindeman , PV . 2006 . Zebra and quagga mussels (Dreissena spp.) and other prey of a Lake Erie population of common map turtles (Emydidae: Graptemys geographica) . Copeia , 2006 ( 2 ) : 268 – 273 .
- Magoulick , DD and Lewis , LC . 2002 . Predation on exotic zebra mussels by native fishes: effects on predator and prey . Freshwater Biology , 47 ( 10 ) : 1908 – 1918 .
- Martin , GW and Corkum , LD . 1994 . Predation of zebra mussels by crayfish . Canadian Journal of Zoology , 72 ( 11 ) : 1867 – 1871 .
- May , B and Marsden , JE . 1992 . Genetic identification and implications of another invasive species of dreissenid mussel in the Great Lakes . Canadian Journal of Fisheries and Aquatic Sciences , 49 ( 7 ) : 1501 – 1506 .
- Montgomery , DC . 1991 . Design and analysis of experiments , 3rd , New York : Wiley .
- Perry , WL , Lodge , DM and Lamberti , GA . 1997 . Impact of crayfish predation on exotic zebra mussels and native invertebrates in a lake-outlet stream . Canadian Journal of Fisheries and Aquatic Sciences , 54 ( 1 ) : 120 – 125 .
- Polačik , M , Janáč , M , Jurajda , P , Adámek , Z , Ondračková , M and Vassilev , M . 2009 . Invasive gobies in the Danube: invasion success facilitated by availability and selection of superior food resources . Ecology of Freshwater Fish , 18 ( 4 ) : 640 – 649 .
- Power , ME . 1992 . Habitat heterogeneity and the functional significance of fish in river food webs . Ecology , 73 ( 5 ) : 1675 – 1688 .
- Rahel , FJ . 2000 . Homogenization of fish faunas across the United States . Science , 288 ( 5467 ) : 854 – 856 .
- Ray , WJ and Corkum , LD . 1997 . Predation of zebra mussels by round gobies, Neogobius melanostomus . Environmental Biology of Fishes , 50 ( 3 ) : 267 – 273 .
- Ray , WJ and Corkum , LD . 2001 . Habitat and site affinity of the round goby . Journal of Great Lakes Research , 27 ( 3 ) : 329 – 334 .
- Ricciardi , A . 2001 . Facilitative interactions among aquatic invaders: is an “invasional meltdown” occurring in the Great Lakes? . Canadian Journal of Fisheries and Aquatic Sciences , 58 ( 12 ) : 2513 – 2525 .
- Ruetz III , CR , Earl , BM and Kohler , SL . 2006 . Evaluating passive integrated transponder tags for marking mottled sculpins: effects on growth and mortality . Transactions of the American Fisheries Society , 135 ( 6 ) : 1456 – 1461 .
- Ruetz III , CR , Newman , RM and Vondracek , B . 2002 . Top-down control in a detritus-based food web: fish, shredders, and leaf breakdown . Oecologia , 132 ( 2 ) : 307 – 315 .
- Ruetz III , CR , Strouse , DL and Pothoven , SA . 2009 . Energy density of introduced round goby compared with four native fishes in a Lake Michigan tributary . Transactions of the American Fisheries Society , 138 ( 4 ) : 938 – 947 .
- Ruetz III , CR , Uzarski , DG , Krueger , DM and Rutherford , ES . 2007 . Sampling a littoral fish assemblage: comparison of small-mesh fyke netting and boat electrofishing . North American Journal of Fisheries Management , 27 ( 3 ) : 825 – 831 .
- Schaeffer , JS , Bowen , A , Thomas , M , French III , JRP and Curtis , GL . 2005 . Invasion history, proliferation, and offshore diet of the round goby Neogobius melanostomus in western Lake Huron, USA . Journal of Great Lakes Research , 31 ( 4 ) : 414 – 425 .
- Sietman , BE , Dunn , HL , Tucker , JK and Kelner , DE . 2003 . Muskrat (Ondatra zibethicus) predation on zebra mussels (Dreissena polymorpha) attached to unionid bivalves . Journal of Freshwater Ecology , 18 ( 1 ) : 25 – 32 .
- Skora , KE and Rzeznik , J . 2001 . Observations on diet composition of Neogobius melanostomus Pallas 1811 (Gobiidae, Pisces) in the Gulf of Gdansk (Baltic Sea) . Journal of Great Lakes Research , 27 ( 3 ) : 290 – 299 .
- Stammler , KL and Corkum , LD . 2005 . Assessment of fish size on shelter choice and intraspecific interactions by round gobies Neogobius melanostomus . Environmental Biology of Fishes , 73 ( 2 ) : 117 – 123 .
- Strayer , DL . 2009 . Twenty years of zebra mussels: lessons from the mollusk that made headlines . Frontiers in Ecology and the Environment , 7 ( 3 ) : 135 – 141 .
- Thorp , JH , DeLong , MD and Casper , AF . 1998 . In situ experiments on predatory regulation of a bivalve mollusc (Dreissena polymorpha) in the Mississippi and Ohio rivers . Freshwater Biology , 39 ( 4 ) : 649 – 661 .
- Tucker , JK , Cronin , FA , Soergel , DW and Theiling , CH . 1996 . Predation on zebra mussels (Dreissena polymorpha) by common carp (Cyprinus carpio) . Journal of Freshwater Ecology , 11 ( 3 ) : 363 – 372 .
- Vanderploeg , HA , Nalepa , TF , Jude , DJ , Mills , EL , Holeck , KT , Liebig , JR , Grigorovich , IA and Ojaveer , H . 2002 . Dispersal and emerging ecological impacts of Ponto-Caspian species in the Laurentian Great Lakes . Canadian Journal of Fisheries and Aquatic Sciences , 59 ( 7 ) : 1209 – 1228 .
- Vitousek , PM , D’Antonio , CM , Loope , LL , Rejmánek , M and Westbrooks , R . 1997a . Introduced species: a significant component of human-caused global change . New Zeeland Journal of Ecology , 21 ( 1 ) : 1 – 16 .
- Vitousek , PM , Mooney , HA , Lubchenco , J and Melillo , M . 1997b . Human domination of Earth's ecosystems . Science , 277 ( 5325 ) : 494 – 499 .