Abstract
Gizzard shad (Dorosoma cepedianum) is an ecologically important species in many waters of the USA. Their versatile feeding and nutrient-recycling capabilities and importance as prey, combined with their commonly high biomasses, result in widespread ecosystem effects. Despite their importance, few studies have focused on variables that influence their population dynamics, especially in mid-latitude waters. The objectives of this study were to: (1) describe population dynamics including size and age structure, growth, and mortality and (2) determine potential environmental influences on annual variations in growth increments of gizzard shad. Gizzard shad populations were sampled annually in three eutrophic reservoirs in Missouri, over a successive 5-year period. Size and age structure were temporally variable owing to variable year-class strength and differing growth rates. Strong year-classes arose from age-0 cohorts that experienced relatively high overwinter survival. Annual variations in growth increments across all cohorts and reservoirs appeared to be affected by the initial length of fish at the beginning of the growing season and by food availability. Annual mortality estimates ranged from 0.38 to 0.70 for ages 3–7 among the reservoirs. Due to their dynamic nature, effects of these populations on their ecosystems also must be temporally variable and complex. Future studies should focus on the importance of these temporal effects on ecosystem dynamics.
Introduction
Gizzard shad (Dorosoma cepedianum) is an important fish species in many lakes and reservoirs in the USA. They can influence trophic levels both below and above them through their nutrient recycling, feeding, and availability as prey (see reviews in Stein et al. Citation1995; Vanni et al. Citation2005). They can feed on pelagic phytoplankton, zooplankton, and detritus by pump-filter feeding (Drenner et al. Citation1978, Citation1982a) and by grazing on bottom sediments (Mundahl and Wissing Citation1987; Schaus et al. Citation2002). Gizzard shad are also important nutrient recyclers, transporting nutrients associated with benthic substrates into the water column (Vanni et al. Citation2005), and are important prey species for piscivorous fishes (Noble Citation1981; Storck Citation1986; Michaletz Citation1997b). Their versatile feeding and nutrient-recycling capabilities and importance as prey, combined with their commonly high biomasses, result in widespread ecosystem effects (Stein et al. Citation1995; Vanni et al. Citation2005).
Though considerable work has been done on the ecosystem effects of gizzard shad, far fewer studies have focused on population dynamics of this species other than during their first year of life. Populations on the northern edge of their range appear to be strongly regulated by overwinter mortality, and are typically comprised of only a few year classes, except in water bodies with thermal refugia (Ward et al. Citation2006; Wuellner et al. Citation2008). Northern populations exhibit fast growth and attain large sizes (Bodola Citation1965; Ward et al. Citation2006; Wuellner et al. Citation2008). Populations in more southerly locations like Missouri usually have more consistent recruitment, slower growth, and smaller maximum sizes (DiCenzo et al. Citation1996; Michaletz Citation1998; Clayton and Maceina Citation2002). In southern waters, population dynamics are influenced by reservoir productivity, with populations in eutrophic reservoirs exhibiting more consistent recruitment, higher abundance, slower growth after age 0, and higher mortality than populations in oligo-mesotrophic reservoirs (DiCenzo et al. Citation1996; Michaletz Citation1998; Clayton and Maceina Citation2002).
Although general trends in population dynamics of gizzard shad across latitudinal and trophic gradients have been identified, populations can also vary substantially among trophically similar water bodies (DiCenzo et al. Citation1996; Michaletz Citation1998), as well as among years within a water body (Storck Citation1986; Schaus et al. Citation2002). With few exceptions, these variations and the environmental variables influencing them have not been well studied. In perhaps the most studied system, Acton Lake, Ohio, gizzard shad biomass varied by over two orders of magnitude among years, affecting food resources and growth rates of gizzard shad (Schaus et al. Citation2002). During years when gizzard shad biomass was lower and zooplankton biomass was higher, gizzard shad fed more heavily on zooplankton and grew faster than during years when gizzard shad biomass was higher, zooplankton biomass was lower, and gizzard shad consumed mostly detritus. Other studies have noted changes in gizzard shad size and age structure within a water body over time (e.g., Storck Citation1986; Willis Citation1987) but did not explicitly examine causes for these changes, although they attributed them to variable abundance and overwinter mortality of young.
In this study, gizzard shad population dynamics were examined in Long Branch, Mark Twain, and Thomas Hill lakes. These highly eutrophic reservoirs are located in the fertile Glacial Till Plains physiographic section of northern Missouri, USA, where land use consists primarily of cropland with lesser amounts of pasture and forest. These reservoirs are often turbid, especially during periods of high inflow when large amounts of sediment are imported (Knowlton and Jones Citation1995). Mark Twain Lake is deeper (mean depth = 8.9 m) and larger (surface area = 7550 ha) than Long Branch Lake (mean depth = 4.4 m, surface area = 980 ha) and Thomas Hill Lake (mean depth = 4.9 m, surface area = 1781 ha). Long Branch and Mark Twain lakes are operated by the USA Army Corps of Engineers for flood control and Mark Twain Lake for hydropower generation. Thomas Hill Lake functions as a cooling reservoir for a coal-fired power plant, which results in elevated water temperatures. The reservoirs are thermally stratified from June through mid-September, during which time the hypolimnions become hypoxic. During stratification, access to bottom sediments by gizzard shad is limited to shallow, littoral areas.
I examined temporal changes in gizzard shad populations in these three eutrophic reservoirs over a 5-year period. Although similar in productivity and within 65 km of one another, these reservoirs contain gizzard shad populations that exhibit different population characteristics, particularly growth rates during young ages (Michaletz Citation1998). The objectives of this study were to: (1) describe gizzard shad size and age structure, growth, and mortality and (2) determine potential environmental influences on annual variations in growth increments of gizzard shad. This study provides insight into important environmental variables that affect gizzard shad populations in mid-latitude, eutrophic reservoirs.
Method
Population dynamics
Gizzard shad populations were sampled along shorelines during the spring spawning season (late May-early June) with nighttime electrofishing. Yearling and older gizzard shad were collected usually for 20 min at six fixed sites (three in an uplake area, three in a downlake area) in each reservoir during 2004–2008. All captured fish were placed on ice and later frozen. In the laboratory, fish were thawed, measured (TL, total length, nearest mm), and sagittal otoliths were removed from 10 individuals per 10-mm length group for each reservoir to estimate ages (Clayton and Maceina Citation1999). The fish selected for aging were also individually weighed (nearest g). Fish ages were estimated by counting annuli on images of sectioned otoliths. Fish that had ages assigned were used to develop an age-length key which was then applied to the entire sample to estimate age composition and mean length at age.
Using the information collected from electrofishing samples, relative abundance, size and age structure, length–weight relationships, growth, and mortality of gizzard shad were estimated. Catch-per-unit-effort (CPUE, number/h of electrofishing), length frequencies, length–weight relationships, and age composition were determined for each reservoir and year. Linear regression was used to predict weight for a given length using log10-transformed data. A single von Bertalanffy growth equation was computed for each reservoir by averaging mean lengths for each age across all years. Averaging across years was done to provide an overall growth comparison among the reservoirs. Similarly, annual mortality (A) was estimated by averaging CPUE of each age across years to reduce the effects of variable recruitment (Miranda and Bettoli Citation2007) and sampling variability. Catch-curve analysis was then used to compute instantaneous mortality (Z) by regressing these average CPUE estimates against age. Annual mortality was computed as A = 1 − e− Z (Ricker Citation1975). Only ages 3 through 7 were used because fish were not fully vulnerable to the sampling gear until age 3 and few fish older than age 7 were present. Both von Bertalanffy growth equations and catch-curve analyses were computed using FAST software (Slipke and Maceina Citation2001).
Growth analysis
Annual growth increments of gizzard shad were determined by subtracting the mean TL and weight (determined from length–weight relationships) for a given year class in year i from the mean TL and weight for that same year class in year i + 1. Linear regression models were used to explain variations in growth increments using separate models for length and weight. Independent variables () incorporated into these models for consideration were initial mean TL or weight of gizzard shad in year i, cumulative water temperatures above 15°C (degree days), mean total phosphorus concentration (µg/L), mean total nitrogen concentration (µg/L), mean chlorophyll concentration (µg/L), mean turbidity (NTU), mean Secchi depth (m), and mean zooplankton biomass (µg/L, dry weight). Mean values for the environmental variables were computed for periods when water temperatures were 15°C or higher between electrofishing samples collected in year i and year i + 1 because feeding, and presumably growth, of gizzard shad rapidly declines below this temperature (Salvatore et al. Citation1987). Growth increments for ages 1–4 for all three reservoirs were included in a single model to increase the sample size and the range of values for environmental variables. Thus, one observation in the model consisted of the observed annual mean growth increment for a particular age group and reservoir. Fish older than age 4 were not included because of their generally low sample sizes. Competing models with up to four independent variables were compared using the information-theoretic approach (Burnham and Anderson Citation2002). Models were restricted to four independent variables to avoid over fitting the data. Akaike's information criterion (AIC) was used to assess the fit of each candidate model. The model with the lowest AIC value was considered the most parsimonious. However, models within two units of the best model were assumed to have similar support. Because models for length increments and weight increments indicated similar results, only length-increment models are presented.
Table 1. Summary statistics (grand mean, range given within parentheses) for environmental variables used to assess growth of gizzard shad.
Water temperatures at 1 m deep were measured at a single downlake site every 4 h in each reservoir throughout the study with recording thermographs. Measurements were averaged for each day to determine an average daily temperature. Water quality and zooplankton data were collected in each of two areas (uplake and downlake) biweekly during April through October or early November, but only data taken when water temperatures exceeded 15°C were used to compute mean values. Chlorophyll, turbidity, Secchi depth, and zooplankton biomass were measured at two fixed sites per area, whereas nutrients were measured at only one fixed site per area. Chlorophyll, turbidity, total phosphorus, and total nitrogen were quantified from depth-integrated water samples collected from the photic zone (defined to be 1.5 × Secchi depth, Bremigan and Stein Citation1999). Turbidity was measured on site using a turbidity meter. Chlorophyll a concentration was measured using ethanol extraction and spectrophotometry (APHA Citation1985) and total phosphorus and total nitrogen were measured using methods described in Prepas and Rigler (Citation1982) and Crumpton et al. (Citation1992), respectively. Zooplankton were collected at each site with a single vertical tow of a conical plankton net (30 cm in diameter × 150 cm long with 63 -µm mesh). Tows were made from 9 m deep (or less in shallow water) to the surface. Zooplankton samples were preserved in 10% formalin solution. Zooplankton samples were processed by identifying and counting all organisms within three subsamples for each sample to estimate densities. Zooplankton were identified as rotifers, copepod nauplii, cyclopoid copepods, calanoid copepods, or to genus for cladocerans. Body lengths of 100 randomly selected crustacean zooplankton (excluding copepod nauplii), 10 copepod nauplii, and 10 rotifers were measured per area using procedures in Culver et al. (Citation1985). These body lengths were used for both sites within an area to estimate individual dry weights using equations in Dumont et al. (Citation1975), Rosen (Citation1981), and Culver et al. (Citation1985). Zooplankton biomass was estimated by multiplying mean individual dry weight times density for each taxon and then summing over all taxa. Calanoid copepods were excluded from zooplankton biomass calculations because they can evade pump-filter feeding by gizzard shad (Drenner et al. Citation1978). Values for water quality and zooplankton variables were averaged across all sites for each date.
Results
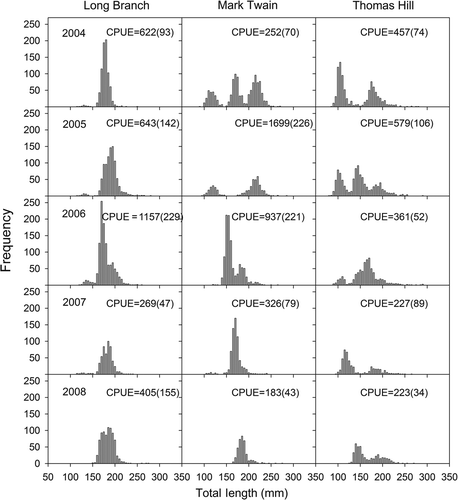
Size structure and CPUE of gizzard shad varied among years and reservoirs (). Length frequencies were mostly unimodal in Long Branch Lake but frequently multimodal in Mark Twain and Thomas Hill lakes. Most fish ranged in length from 150 to 230 mm in all reservoirs. Electrofishing CPUE varied nearly 10 fold among years in Mark Twain Lake, ranging from 183 fish/h in 2008 to 1699 fish/h in 2005. Electrofishing CPUE was most consistent in Thomas Hill Lake where CPUE varied about two fold among years.
Figure 1. Length frequencies of gizzard shad collected from the study reservoirs during 2004–2008. Mean (SE) electrofishing (CPUE, number/h) is also shown.
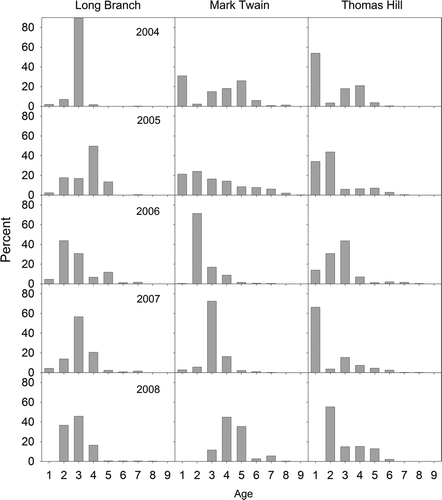
Age structures were also highly variable among years and reservoirs (). Populations consisted mostly of fish less than age 6 with typically three or fewer abundant year classes. However, fish as old as 9 years were collected in Long Branch and Mark Twain lakes. Mostly age-3 fish (2001 year class) were present in Long Branch Lake in 2004 and this year class remained relatively abundant for the next 2 years. Other dominant year classes included the 2004 year class for Long Branch and Mark Twain lakes and the 2003 and 2006 year classes for Thomas Hill Lake. Age-1 fish were captured every year except 2008 when they were absent from samples in all three reservoirs. Annual mortality estimates for ages 3–7 were 0.70 for Long Branch Lake (catch-curve r 2 = 0.90; p = 0.01), 0.38 for Mark Twain Lake (r 2 = 0.99; p = 0.0005), and 0.60 for Thomas Hill Lake (r 2 = 0.92; p = 0.009).
Mean TL at age 1 was largest in Long Branch Lake and smallest in Thomas Hill Lake, but by age 4 mean TLs were similar among reservoirs (). Regression analysis indicated that variations in annual growth increments were best explained by the initial total length of gizzard shad in year i and Secchi depth (). Three-variable models containing these two variables plus an additional variable showed similar support. However, in every case, the addition of the third variable did not explain more than 0.5% of additional variance and coefficients for these variables were never significant at p < 0.05. Annual growth increments decreased with both increasing initial total length and Secchi depth (, ). Initial total length explained most of the variation with a partial r 2 of 0.72. Secchi depths in Mark Twain Lake were sometimes greater than those in the other reservoirs and in those situations, gizzard shad experienced slower growth for a given initial length than in the other reservoirs.
Figure 3. Von Bertalanffy growth curves for gizzard shad in Long Branch (LOB), Mark Twain (MTL), and Thomas Hill (THL) lakes. Equations are lt = 245.533(1–e −0.284( t + 1.863)) for LOB (r 2 = 0.98; p < 0.0001), lt = 233.473(1–e −0.412( t + 0.830)) for MTL (r 2 = 0.99; p < 0.0001), and lt = 244.282(1–e −0.346( t + 0.755)) for THL (r 2 = 0.99; p < 0.0001). Mean length for age 7 in Thomas Hill Lake was not computed due to very low sample size.
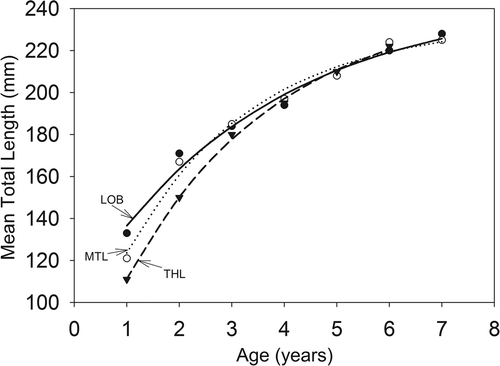
Figure 4. Three-dimensional scatter plot showing the influence of initial total length of gizzard shad and Secchi depth on growth increments of gizzard shad in Long Branch (solid circle), Mark Twain (open circle), and Thomas Hill (solid, upside-down triangle) lakes.
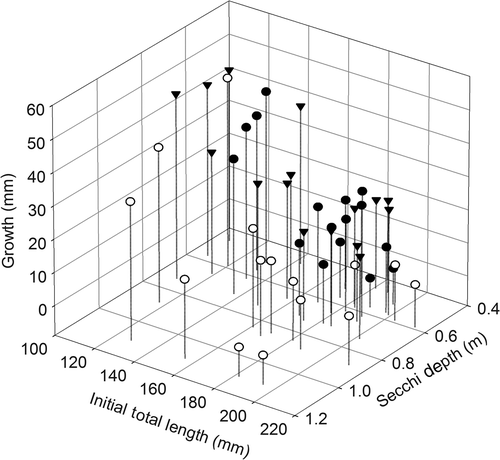
Table 2. Regression models explaining variation in growth increments (mm) of gizzard shad using initial mean total length (ITL) of gizzard shad, cumulative water temperature (Temp), total phosphorus concentration (TP), total nitrogen concentration (TN), chlorophyll concentration (Chla), turbidity (Turb), Secchi depth (Secchi), and zooplankton biomass (Zoop) as explanatory variables (n = 47).
Discussion
Variation in size structure among reservoirs and years was caused by differing year-class strength and growth rates of gizzard shad. Each reservoir had one or two dominant year classes during the 5-year study. The dominant year classes of 2004 for Long Branch and Mark Twain lakes and of 2003 and 2006 in Thomas Hill Lake arose from age-0 cohorts that experienced relatively high overwinter survival due to their large body size or because winters were mild (Michaletz Citation2010). Information on the overwinter survival for the strong 2001 year class for Long Branch Lake was not available. Thus, survival during the first winter of life strongly influenced population age and size structure in these systems, similar to populations in northern waters (Ward et al. Citation2006; Wuellner et al. Citation2008), and in other Midwestern USA reservoirs (Storck Citation1986; Willis Citation1987). However, unlike northern waters, year classes were rarely missing because some young survived their first winter nearly every year. While electrofishing CPUE for yearlings may not be representative of their actual abundance (Michaletz Citation1996), yearlings were sampled every year in each reservoir except for in 2008. No yearlings were sampled in any reservoir during 2008 owing to high overwinter mortality of that cohort (Michaletz Citation2010).
Variable growth rates also influenced the size structure of these populations. While the range of adult (age 3 and older, Michaletz Citation1998) sizes was generally similar among reservoirs and similar to those for populations in other eutrophic reservoirs (DiCenzo et al. Citation1996), size structures were typically unimodal in Long Branch Lake but multimodal in the other two reservoirs. Gizzard shad in Long Branch Lake grew faster during their first two growing seasons and more quickly reached adult sizes than in the other reservoirs, which led to the unimodal length distributions for this reservoir. For Mark Twain and Thomas Hill lakes, the modal lengths for age-1 and age-2 fish were usually distinct from those of the older ages. However, for age-4 and older fish, mean lengths were similar among the three reservoirs, indicating that age-2 and age-3 fish in Mark Twain and Thomas Hill lakes grew faster than these age groups in Long Branch Lake.
The large changes in CPUE among years within a reservoir may not be reflective of actual changes in abundance. Although large numbers of fish can be collected with spring electrofishing, CPUE estimates can be temporally variable (Michaletz Citation1990), possibly due to changes in spawning behavior in response to changes in water temperatures and water levels (Michaletz Citation1997a). Size structure appears to be more consistent than CPUE over the spawning season (Michaletz Citation1990). Additionally, electrofishing CPUE for gizzard shad can be imprecise (Van Den Avyle et al. Citation1995; Michaletz Citation1996). Therefore, caution should be used when comparing electrofishing CPUE among years and reservoirs.
Annual mortality rates for the three populations were similar to those reported for other eutrophic waters but higher than those for oligo-mesotrophic waters. Annual mortality is commonly about 60% or higher in eutrophic waters but can be as low as 20–30% in oligotrophic waters (Michaletz Citation1998). Mortality estimates for populations in Long Branch and Thomas Hill Lakes were very similar to rates previously reported for these reservoirs (Michaletz Citation1998). However, the estimate of 0.38 for the population in Mark Twain Lake in this study is considerably lower than the previous estimate of 0.77 (Michaletz Citation1998), indicating possible changes in population dynamics in this reservoir. Fish as old as age 9 were collected in both Long Branch and Mark Twain lakes but these older fish were somewhat more common in the latter reservoir. Nevertheless, populations in all three reservoirs consisted mostly of fish age 6 and younger, consistent with age structures for populations in other eutrophic reservoirs (DiCenzo et al. Citation1996; Clayton and Maceina Citation2002).
Regression analysis indicated that annual variations in growth increments of gizzard shad among the reservoirs were best explained by the initial size of the fish and Secchi depth. Most of the variation in growth increments was explained by the initial length of the fish. Smaller fish have a greater growth potential than larger fish because growth commonly decreases asymptotically with increases in fish length (Isely and Grabowski Citation2007). Secchi depth was likely an indirect measure of food abundance for gizzard shad. Secchi depths decrease with increases in phytoplankton abundance and suspended solid concentrations, which are both important food sources for gizzard shad (Drenner et al. Citation1982b, Citation1986; Mundahl and Wissing Citation1987; Schaus et al. Citation2002). Because of reduced access to bottom sediments during summer stratification, gizzard shad in these reservoirs probably fed mostly on suspended plankton and sediments as they were commonly captured with trawls in offshore locations in the epilimnion (P.H. Michaletz, personal observation). Reservoir populations of gizzard shad appear to benefit from allochthonous inputs of sediment and nutrients which are associated with water inflows from agricultural watersheds (Vanni et al. Citation2005; Pilati et al. Citation2009). During periods of drought, these inputs are much reduced (Knowlton and Jones Citation1995) and may lead to slower growth of gizzard shad. Gizzard shad, especially in Mark Twain Lake, grew slower during years with higher water transparency () and presumably reduced food resources.
Surprisingly, zooplankton biomass was not correlated with growth rates of gizzard shad in these reservoirs. Other studies have shown that growth rates of gizzard shad increased when these fish included zooplankton in their diets (Mundahl and Wissing Citation1987; Schaus et al. Citation2002). In my study reservoirs, zooplankton biomass peaked during the spring spawning season for gizzard shad and rapidly declined to low levels by early summer (P.H. Michaletz, unpublished data). Pierce et al. (Citation1981) found that food intake by gizzard shad decreased markedly during their spawning season. Thus, when zooplankton were most abundant, adult gizzard shad probably fed very little, which may have reduced the importance of zooplankton on growth of gizzard shad. Perhaps, zooplankton may have been more important for growth of young fish. Mean length at age 1 for gizzard shad in Long Branch Lake was higher than for age-1 fish in the other two reservoirs, coinciding with the higher biomass of zooplankton in this reservoir. Zooplankton biomass was positively associated with growth of age-0 gizzard shad in two Ozark reservoirs (Michaletz Citation1997a).
Cumulative water temperature was also not correlated with growth rates of gizzard shad. Due to power-plant cooling effects, water temperatures were warmer and the growing seasons longer in Thomas Hill Lake than in the other reservoirs. However, growth rates in Thomas Hill Lake were not usually higher than in the other reservoirs, and mean lengths at age 1 were lowest in this reservoir. Temperature influences were either masked by effects of other variables or perhaps temperatures reached suboptimally high levels for growth of gizzard shad. Sebring (Citation2002) estimated that the optimal and maximum temperatures for consumption for gizzard shad were 25°C and 32.4°C, respectively. While summer temperatures for all three reservoirs exceeded 25°C, temperatures in Thomas Hill were consistently higher and occasionally exceeded 32.4°C. Thus, in Thomas Hill Lake the potential positive effects of a longer growing season on growth may have been negated by suboptimally high summer temperatures.
In summary, this study documents the dynamic nature of gizzard shad populations in mid-latitude, eutrophic reservoirs. Temporal changes in size and age structure of these populations were primarily a result of variable year-class strength and differing growth rates. Strong year-classes arose from age-0 cohorts that experienced relatively high overwinter survival. Annual variations in growth increments appeared to be affected by the initial length of fish and by food availability. Due to their dynamic nature, effects of these populations on their ecosystems also must be temporally variable and complex. Future studies should focus on the importance of these temporal effects on ecosystem dynamics.
Acknowledgments
I thank Dean Nicks, Earl Buckner, Jamey Decoske, Mike Colvin, Steffanie Abel, Rachel Wright, Karen Brickey, Bill Mabee, and numerous seasonal employees for their assistance with field and laboratory work. I thank staff from the Limnology Laboratory, University of Missouri, for analyzing nutrient samples. Mike Roell, Mike Siepker, and an anonymous reviewer provided helpful comments on previous drafts of the manuscript. This study was partially funded through Federal Aid in Sport Fish Restoration, Project F-1-R, Study I-38, to the Missouri Department of Conservation.
References
- APHA . 1985 . Standard methods for the examination of water and wastewater, , 16th ed , New York , NY : American Public Health Association .
- Bodola , A . 1965 . Life history of the gizzard shad, Dorosoma cepedianum (LeSueur), in western Lake Erie . United States Fish and Wildlife Service, Fisheries Bulletin , 65 : 391 – 425 .
- Bremigan , MT and Stein , RA . 1999 . Larval gizzard shad success, juvenile effects, and reservoir productivity: toward a framework for multi-system management . Transactions of the American Fisheries Society , 128 : 1106 – 1124 .
- Burnham , KP and Anderson , DR . 2002 . Model selection and multimodel inference: a practical information-theoretic approach, , 2nd ed , New York , NY : Springer .
- Clayton , DL and Maceina , MJ . 1999 . Validation of annulus formation in gizzard shad otoliths . North American Journal of Fisheries Management , 19 : 1099 – 1102 .
- Clayton , DL and Maceina , MJ . 2002 . Trophic state differences in population characteristics of gizzard shad in two Tennessee River impoundments . Lake and Reservoir Management , 18 : 109 – 117 .
- Crumpton , WG , Isenhart , TM and Mitchell , PD . 1992 . Nitrate and organic N analysis with second-derivative spectroscopy . Limnology and Oceanography , 37 : 907 – 913 .
- Culver , DA , Boucherle , MM , Bean , DJ and Fletcher , JW . 1985 . Biomass of freshwater crustacean zooplankton from length-weight regressions . Canadian Journal of Fisheries and Aquatic Sciences , 42 : 1380 – 1390 .
- DiCenzo , VJ , Maceina , MJ and Stimpert , MR . 1996 . Relations between reservoir trophic state and gizzard shad population characteristics in Alabama reservoirs . North American Journal of Fisheries Management , 16 : 888 – 895 .
- Drenner , RW , de Noyelles Jr. , F and Kettle , D . 1982a . Selective impact of filter-feeding gizzard shad on zooplankton community structure . Limnology and Oceanography , 27 : 965 – 968 .
- Drenner , RW , O’Brien , WJ and Mummert , JR . 1982b . Filter-feeding rates of gizzard shad . Transactions of the American Fisheries Society , 111 : 210 – 215 .
- Drenner , RW , Strickler , JR and O’Brien , WJ . 1978 . Capture probability: the role of zooplankter escape in the selective feeding of planktivorous fish . Journal of the Fisheries Research Board of Canada , 35 : 1370 – 1373 .
- Drenner , RW , Threlkeld , ST and McCracken , MD . 1986 . Experimental analysis of the direct and indirect effects of an omnivorous filter-feeding clupeid on plankton community structure . Canadian Journal of Fisheries and Aquatic Sciences , 43 : 1935 – 1945 .
- Dumont , HJ , Van de Velde , I and Dumont , S . 1975 . The dry weight estimate of biomass in a selection of cladocera, copepod, and rotifer from the plankton, periphyton, and benthos of continental waters . Oecologia , 19 : 75 – 97 .
- Isely , JJ and Grabowski , TB . 2007 . “ Age and growth ” . In Analysis and interpretation of freshwater fisheries data , Edited by: Guy , CS and Brown , ML . 187 – 228 . Bethesda , MD : American Fisheries Society .
- Knowlton , MF and Jones , JR . 1995 . Temporal and spatial dynamics of suspended sediment, nutrients, and algal biomass in Mark Twain Lake, Missouri . Archiv für Hydrobiologie , 135 : 145 – 178 .
- Michaletz , PH . 1990 . Comparison of electrofishing and gill netting for sampling gizzard shad in two Ozark impoundments , Jefferson City : Missouri Department of Conservation, D-J Project F-1-R-39, Study I-28, Job 2, Final Report, Part 1 .
- Michaletz , PH . 1996 . Comparison of electrofishing and gill netting for sampling gizzard shad . Proceedings of the Annual Conference Southeastern Association of Fish and Wildlife Agencies , 48 ( 1994 ) : 533 – 541 .
- Michaletz , PH . 1997a . Factors affecting abundance, growth, and survival of age-0 gizzard shad . Transactions of the American Fisheries Society , 126 : 84 – 100 .
- Michaletz , PH . 1997b . Influence of abundance and size of age-0 gizzard shad on predator diets, diet overlap, and growth . Transactions of the American Fisheries Society , 126 : 101 – 111 .
- Michaletz , PH . 1998 . Population characteristics of gizzard shad in Missouri reservoirs and their relation to reservoir productivity, mean depth, and sport fish growth . North American Journal of Fisheries Management , 18 : 114 – 123 .
- Michaletz , PH . 2010 . Overwinter survival of age-0 gizzard shad in Missouri reservoirs spanning a productivity gradient: roles of body size and winter severity . Transactions of the American Fisheries Society , 139 : 241 – 256 .
- Miranda , LE and Bettoli , PW . 2007 . “ Mortality ” . In Analysis and interpretation of freshwater fisheries data , Edited by: Guy , CS and Brown , ML . 229 – 277 . Bethesda , MD : American Fisheries Society .
- Mundahl , ND and Wissing , TE . 1987 . Nutritional importance of detritivory in the growth and condition of gizzard shad in an Ohio reservoir . Environmental Biology of Fishes , 20 : 129 – 142 .
- Noble , RL . 1981 . Management of forage fishes in impoundments of the southern United States . Transactions of the American Fisheries Society , 110 : 738 – 750 .
- Pierce , RJ , Wissing , TE and Megrey , BA . 1981 . Aspects of the feeding ecology of gizzard shad . Transactions of the American Fisheries Society , 110 : 391 – 395 .
- Pilati , A , Vanni , MJ , González , MJ and Gaulke , AK . 2009 . Effects of agricultural subsidies of nutrients and detritus on fish and plankton of shallow-reservoir ecosystems . Ecological Applications , 19 : 942 – 960 .
- Prepas , EE and Rigler , FA . 1982 . Improvements in quantifying the phosphorus concentration in lake water . Canadian Journal of Fisheries and Aquatic Sciences , 39 : 822 – 829 .
- Ricker , WE . 1975 . Computation and interpretation of biological statistics of fish populations , Vol. 191 , 2 – 6 . Ottawa, Canada : Fisheries Research Board of Canada Bulletin .
- Rosen , RA . 1981 . Length-dry weight relationships of some freshwater zooplankton . Journal of Freshwater Ecology , 1 : 225 – 229 .
- Salvatore , SR , Mundahl , ND and Wissing , TE . 1987 . Effect of water temperature on food evacuation rate and feeding activity of age-0 gizzard shad . Transactions of the American Fisheries Society , 116 : 67 – 70 .
- Schaus , MH , Vanni , MJ and Wissing , TE . 2002 . Biomass-dependent diet shifts in omnivorous gizzard shad: implications for growth, food web, and ecosystem effects . Transactions of the American Fisheries Society , 131 : 40 – 54 .
- Sebring , SH . 2002 . Development and application of a bioenergetics model for gizzard shad [master's thesis] , [Lubbock] : Texas Tech University .
- Slipke , JW and Maceina , MJ . 2001 . Fishery analysis and simulation tools (FAST 2.0) , Auburn , AL : Auburn University, Department of Fisheries and Allied Aquacultures .
- Stein , RA , DeVries , DR and Dettmers , JM . 1995 . Food-web regulation by a planktivore: exploring the generality of the trophic cascade hypothesis . Canadian Journal of Fisheries and Aquatic Sciences , 52 : 2518 – 2526 .
- Storck , TW . 1986 . Importance of gizzard shad in the diet of largemouth bass in Lake Shelbyville, Illinois . Transactions of the American Fisheries Society , 115 : 21 – 27 .
- Van Den Avyle , MJ , Boxrucker , J , Michaletz , P , Vondracek , B and Ploskey , GR . 1995 . Comparison of catch rate, length distribution, and precision of six gears used to sample reservoir shad populations . North American Journal of Fisheries Management , 15 : 940 – 955 .
- Vanni , MJ , Arend , KK , Bremigan , MT , Bunnell , DB , Garvey , JE , González , MJ , Renwick , WH , Soranno , PA and Stein , RA . 2005 . Linking landscapes and food webs: effects of omnivorous fish and watersheds on reservoir ecosystems . BioScience , 55 : 155 – 167 .
- Ward , MJ , Willis , DW and Galinat , GF . 2006 . Gizzard shad recruitment patterns in a western South Dakota irrigation reservoir . Journal of Freshwater Ecology , 21 : 201 – 207 .
- Willis , DW . 1987 . Reproduction and recruitment of gizzard shad in Kansas reservoirs . North American Journal of Fisheries Management , 7 : 71 – 80 .
- Wuellner , MR , Graeb , BDS , Ward , MJ and Willis , DW . 2008 . “ Review of gizzard shad population dynamics at the northwestern edge of its range ” . In Balancing fisheries management and water uses for impounded river systems , Edited by: Allen , MS , Sammons , S and Maceina , MJ . Vol. 62 , 637 – 653 . Bethesda , MD : American Fisheries Society, Symposium .