Abstract
The effects of culture and extract solutions of six macrophytes on the growth of three common phytoplankton were investigated under laboratory conditions. Culture solutions were taken from a Hoagland nutrient solution that contained live plants growing for 7 days and the extract solution was made from a solution with fresh plant pieces immersed in Hoagland nutrient solution for 48 h. The algae were grown in the culture or extract solution for 15 days and the algal density was determined every 2 days. The results showed that the culture solution of the macrophytes both inhibited and stimulated algae growth depending on the macrophytes and the algae. All extract solutions of the macrophytes exhibited a stimulatory effect on the growth of the algae that also varied with the macrophytes and the phytoplankton. The results indicated that culture solutions of macrophytes may be more suitable for the control of algal blooms in eutrophic waters than their extract solutions.
Introduction
The rapid growth of algae in eutrophic waters (algae blooms) has created great problems in aquatic ecosystems. As a result, finding effective methods for control and elimination of algae blooms has become a mandatory task for most water resource managers. Amongst the measures adopted to control algal blooms, biological methods were widely accepted owing to their higher levels of environmental safety and lower operating costs to implement (Chang et al. Citation2007). The presence of macrophytes is considered to be of great importance for maintaining clear water in shallow lakes (Donk and Bund Citation2002). Recently, macrophytes have received a great amount of attention globally because of their ability to promote the restoration of eutrophic waters. Firstly, macrophytes can absorb nutrients directly and thus can compete for these nutrients and resources with the algae to decrease nutrient availability for phytoplankton growth substantially. As a result, the growth of phytoplankton can be inhibited and water transparency improved (Donk and Bund Citation2002). Secondly, macrophytes can reduce the recruitment of seston into water by reducing wind- and fish-induced resuspension of bottom sediments (Gulati and Van Donk Citation2002; Horppila and Nurminen 2003) and influence the light environment for phytoplankton. Finally, macrophytes can excrete allelopathic substances to inhibit the growth of phytoplankton (Nan et al. Citation2004; Hilt and Gross Citation2008).
Allelopathy was originally defined as both a stimulatory and inhibitory biochemical interaction (Molisch Citation1937) but most studies have focused on the inhibitory effect of allelopathic substances (Gross Citation2003). Many macrophytes, such as Myriophyllum spicatum Linn. (Gross et al. Citation1996; Nakai et al. Citation1996, Citation2000; Leu et al. Citation2002) and Stratiotes aloides Linn. (Mulderij et al. Citation2006), were reported to have an inhibiting effect on algal growth, whereas some macrophytes, such as Elodea canadensis Michx., Potamogeton pectinatus Linn. (Ozimek et al. Citation1991), Chara globularis var. globularis Thuillier, and Chara contraria var. contraria A. Braun ex Kützing (Mulderij et al. Citation2003) were reported to have an inhibitory effect on phytoplankton biomass. Some macrophytes, such as S. aloides (Mulderij, Mooij, Smolders et al. Citation2005), have an inhibitory effect on phytoplankton composition or on both biomass and composition of the phytoplankton. Erhard and Gross (Citation2006) studied the allelopathic activity of E. canadensis and Elodea nuttallii (Planch.) St. John against epiphytes and phytoplankton. Wang et al. (Citation2007) reported the effects of macroalgae Ulva pertusa Kjellm and Gracilaria lemaneiformis (Bory) Dawson on the growth of four bloom-forming dinoflagellate species. Both studies indicated that the inhibitory effect on algae varied amongst aquatic macrophytes. However, little information is available on the specific differences in effects of macrophytes on alga growth.
Culture solutions (solutions originating from a liquid culture of living plants) of many macrophytes were reported to have allelopathic effects, such as S. aloides (Mulderij, Mooij, Smolders et al. Citation2005; Mulderij, Mooij, and Donk Citation2005), M. spicatum (Hilt Citation2006), Ulva lactuca Linn. (Nan et al. Citation2004), and Myriophyllum aquaticum (Vell.) Verdc. (Wu et al. Citation2008). Besides the studies on culture solutions from macrophytes, other reports focused on the effects of extract solutions from macrophytes (solutions in which dry or fresh plant pieces were immersed for a period of time), such as Ceratophyllum demersum Linn., Vallisneria spiralis Linn. (Xian et al. Citation2006), Hordeum vulgare Linn. (Ferrier et al. Citation2005), Ambrosia artemisiifolia Linn. (Brückner et al. Citation2003), E. canadensis, E. nuttallii (Erhard and Gross Citation2006), and Oryza sativa Linn. (Park et al. Citation2009). Extract solutions of all of these macrophytes were demonstrated to have inhibitory effects on algal growth. Some allelochemicals were extracted from macrophytes to test which compound inhibited phytoplankton and it was found that the effects on algae varied depending on different compounds used in the experiment (Gross Citation2003). Additionally, except for the growth of phytoplankton, chlorophyll a content, photosystem (PS) II activity (Hilt Citation2006), phycocyanin (PC) content and allophycocyanin (APC) content (Wu et al. Citation2008), and the activity of photosynthesis in phytoplankton (Sukenik et al. Citation2002) also varied greatly with different macrophyte species. The studies above indicated that the inhibitory effect on algal growth of both culture and extract solutions involved allelopathic effects (Nan et al. Citation2004). However, there have been comparisons between extract solutions and culture solutions from the same macrophyte. We hypothesized that there are significant differences in algal growth amongst different macrophytes and between culture solutions and extract solutions. In the present study, effects of culture and extract solutions of six macrophytes on the growth of three common algae in eutrophic waters were investigated under laboratory conditions. The aim of the present study was to compare (i) the differences of the inhibitory effects amongst solutions derived from macrophytes and (ii) the inhibitory effect between culture solutions and extract solutions of the same macrophyte.
Methods
Solution preparation
Six macrophytes (Hydrocotyle sibthorpioides Lam., Jussiaea repens Linn., Hydrilla verticillata (Linn. f) Royle, Ipomoea aquatica Forsk., Myriophyllum verticillatum Linn. and Vallisneria natans (Lour.) Hara), two algae (Chlorella pyrenoidosa Chick. and Scenedesmus obliquus (Turp.) Kützing), and a cyanobacterium (Microcystis aeruginosa Kützing) were used in the present study. H. sibthorpioides is an amphibious macrophyte, J. repens and I. aquatica are floating plants, and H. verticillata, M. verticillatum, and V. natans are submerged plants. The macrophytes are commonly used in ecological restoration in China. M. aeruginosa is a dominant photosynthetic species in most eutrophic lakes and C. pyrenoidosa and S. obliquus are also common in the eutrophic lakes in South China. The macrophytes were collected from the botanical garden in South China Normal University, and the three phytoplankton strains were provided from Jinan University's algal culture laboratory.
First, macrophytes were washed with tap water and then transferred to distilled water for 5 minutes. Approximately 200 g of washed plants were put into a white plastic container (50 × 30 × 20 cm, L × W × H) with 3 L of Hoagland nutrient solution and cultured in an incubator for 7 d (25°C, 4200 lx, 12 h; 22°C, 800 lx, 12 h). The culture solutions were then filtered through a 0.45 -µm acetyl cellulose membrane to exclude protozoa and microbes and the filtered solutions were stored in a refrigerator at 4°C until use.
For each species, approximately 100 g of healthy plant material was cut into pieces (including roots, stems, and leaves) and put into a flask with 2 L of Hoagland nutrient solution. The flask was transferred to an incubator for 48 h (25°C, 4200 lx, 12 h; 22°C, 800 lx, 12 h) and then the solution was filtered using a 0.45 µm acetyl cellulose membrane.
After a week, the three phytoplankton species were enriched in a BG-11 culture medium, trained in Hoagland solution, and transferred to new medium successively several times in order to achieve pure strains and high cell density. Three replicate test cultures were made for each phytoplankton species.
Phytoplankton growth experiment
Five millilitres of phytoplankton culture were transferred to a flask, along with 100 mL of Hoagland solution and 20 mL of either culture solution or extract solution. Three control flasks with the same amount of phytoplankton culture were also prepared. All the treated and control flasks were incubated (25°C, 4200 lx, 12 h; 22°C, 800 lx, 12 h) for 15 d. The cell density was determined every 2 d by sampling 10 mL of the incubation solution from each flask, after which 10 mL of fresh nutrient solution was supplied to each flask. The sample was tested for optical density (OD) using an ultraviolet-visible spectrophotometer (UV-2450, Shimadzu, Japan) at 650 nm and the corresponding cell quantity was determined using a plankton counting chamber at 400 × magnification (0.1 mL of sample per chamber, 30 fields per chamber, three chambers per sample). Finally, the number of phytoplankton cells was calculated based on the OD value (Li and Qin Citation2005). The relative growth rate (RGR) was used to indicate phytoplankton growth according to the following formula:
Data analysis
All statistics were performed using Excel 2003 (Microsoft, Inc.). A two-way ANOVA was used to test for significant differences between the two solutions and among macrophyte species. The Least significant difference (LSD) multiple comparison test was used to test the significance among the different macrophytes. Student's t-test was used to compare the difference between the culture and extract solutions for the same macrophyte.
Results
The growth rates of all three phytoplankton species were significantly influenced by macrophyte species, type of solution, and the interaction of species and solutions (two-way ANOVA, ).
Table 1. Results of the two-way ANOVA on the relative growth rate of three common algae.
Effects on the growth of C. pyrenoidosa
During the first five days, culture solutions of all macrophytes except V. natans showed an inhibiting effect on the growth of C. pyrenoidosa, whilst the culture solution of V. natans exhibited a stimulating effect. In the subsequent days, all macrophyte solutions inhibited growth (). The RGR of C. pyrenoidosa was the lowest in the H. sibthorpioide culture solution, which was significantly lower than the RGR in the control and the culture solutions of M. verticillatum, I. aquatica, and V. natans ().
Figure 1. The effect of culture solutions (a) and extract solutions (b) of six macrophytes on the growth of Chlorella pyrenoidosa (a: H. sibthorpioides; b: H. verticillata; c: V. natans; d: I. aquatica; e: M. verticillatum; f: J. repens; g: control).
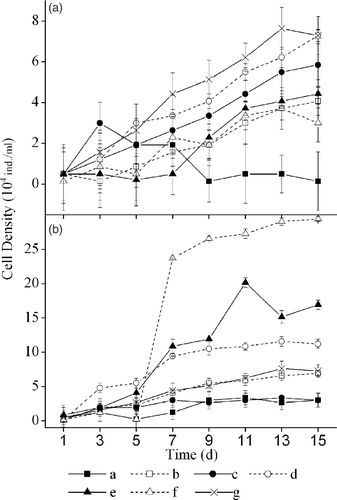
Table 2. Mean relative growth rate of C. pyrenoidosa grown for 15 d in culture and extract solutions derived from six macrophytes (mean ± SE, n = 3).
The growth of C. pyrenoidosa was slower in the extract solutions than in the control solution during the first five days for all macrophytes except I. aquatica (). In the following days, C. pyrenoidosa grew significantly faster in the extract solutions of J. repens, M. verticillatum, and I. aquatica than in the control solution, whilst growing more slowly in the extract solutions of H. verticillata, V. natans, and H. sibthorpioides.
Generally, C. pyrenoidosa grew faster in the extract solutions than in the culture solutions. C. pyrenoidosa had a significantly higher RGR in the extract solutions of J. repens (p < 0.01), I. aquatica (p < 0.01), H. verticillata (p < 0.01), and H. sibthorpioides (p < 0.05) than in the corresponding culture solutions.
Effects on the growth of S. obliquus
During the initial five days, the culture solutions of all macrophytes exhibited an inhibiting effect on S. obliquus. During the subsequent days, M. verticillatum and H. sibthorpioides solutions exhibited a stimulating effect rather than an inhibiting effect, with the former showing stronger stimulation (). The RGR of S. obliquus was the lowest in J. repens culture solution, which was significantly lower than that in the culture solutions of M. verticillatum and H. sibthorpioides (). S. obliquus also exhibited a lower RGR in the control solution than in the culture solutions of M. verticillatum and H. sibthorpioides.
Figure 2. The effect of culture solutions (a) and extract solutions (b) of six macrophytes on the growth of Scenedesmus obliquus. The legends are the same as in .
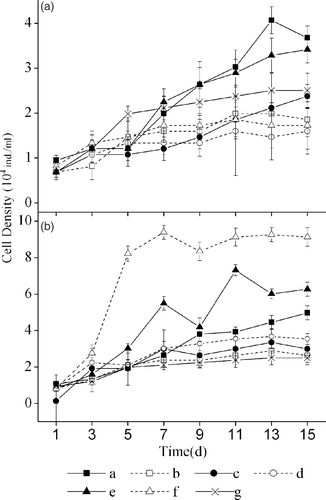
Table 3. Mean relative growth rate of S. obliquus grown for 15 d in culture and extract solutions derived from six macrophytes (mean ± SE, n = 3).
S. obliquus grew more rapidly in the extract solutions of all macrophytes than in the control solution, except for V. natans, where S. obliquus grew more slowly than in the control solution (). S. obliquus exhibited the highest RGR in J. repens extract solution, which was significantly higher than that in the extract solutions of H. sibthorpioides, I. aquatica, V. natans, H. verticillata, and M. verticillatum and in the control solution.
Generally, S. obliquus grew faster in the extract solutions than in the culture solutions. S. obliquus had a significantly higher RGR in the extract solutions of J. repens (p < 0.01) and I. aquatica (p < 0.05) than that in the culture solutions of these species.
Effects on the growth of M. aeruginosa
The culture solutions of all six macrophytes except M. verticillatum inhibited M. aeruginosa, with H. sibthorpioides showing the strongest inhibition. In contrast, the culture solution of M. verticillatum exhibited a stimulating effect after the 13th d (). The RGR of M. aeruginosa was the lowest in H. sibthorpioide culture solution, which was significantly lower than that in the control the culture solutions of the other five species (). The RGR in the culture solutions of V. natans and H. verticillata were also significantly lower than those in the culture solutions of J. repens, I. aquatic, and M. verticillatum.
Figure 3. The effect of culture solutions (a) and extract solutions (b) of six macrophytes on the growth of Microcystis aeruginosa. The legends are the same as in .
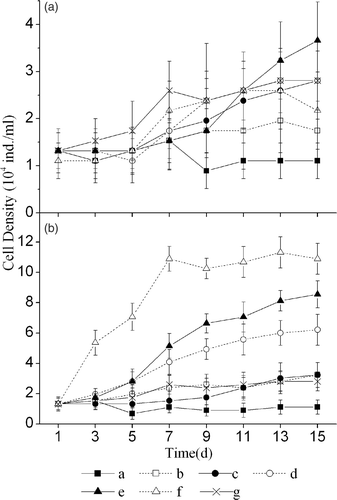
Table 4. Mean relative growth rate of M. aeruginosa grown for 15 d in culture and extract solutions derived from six macrophytes (mean ± SE, n = 3).
M. aeruginosa grew more rapidly in the extract solutions of J. repens, M. verticillatum, and I. aquatica than in the control solution, whereas it grew more slowly in the extract solutions of H. sibthorpioides, V. natans, and H. verticillata than in the control solution (). M. aeruginosa exhibited the highest RGR in the extract solution of J. repens, which was significantly higher than that in the extract solutions of H. sibthorpioides, H. verticillata, V. natans, and I. aquatica and in the control solution. The lowest RGR occurred in the extract solution of H. sibthorpioides, which was significantly lower than that in the extract solutions of the other five macrophytes and the control.
Generally, M. aeruginosa grew faster in the extract solutions than in the culture solutions. M. aeruginosa had a significantly higher RGR in the extract solutions of J. repens (p < 0.01), M. verticillatum (p < 0.01), and I. aquatica (p < 0.05) than in the corresponding culture solutions.
Discussion
It was confirmed that macrophytes affected phytoplankton growth through allelopathic effects (Brückner et al. Citation2003; Nan et al. Citation2004; Mulderij, Mooij, and Donk Citation2005). Previous research indicated that J. repens had an allelopathic inhibiting effect on radish (Hong et al. 2003). Macrophyte species V. natans (Xian et al. Citation2006), M. verticillatum (Hilt Citation2006; Bauer et al. Citation2009; Zhu et al. Citation2010; Wu et al. Citation2010), H. verticillata (Wu et al. Citation2010), and I. aquatica (Nelson et al. Citation2003) were also reported to have some allelopathic effect on algae. In the present study, macrophyte chemicals or residues significantly influenced phytoplankton growth; therefore, allelopathic effects may play an important role regardless of the influence of nutrients and predation by zooplankton. The culture solution of H. sibthorpioides had a stronger inhibiting effect on the growth of C. pyrenoidosa and M. aeruginosa compared with the culture solutions of the other five macrophytes. This result suggests that H. sibthorpioides may have an allelopathic effect on phytoplankton growth and can be regarded as a candidate plant for inhibiting algae blooms in eutrophic waters.
The response of phytoplankton to allelopathic substances from macrophytes is likely to be species dependent. Jasser (Citation1995) found that cyanobacteria were more sensitive to allelochemicals from Ceratophyllum than green algae. Similar results were also found in other studies (Sabine and Andreas Citation2002; Mulderij et al. Citation2003). The present study also showed that the culture solution of H. sibthorpioides had a significant inhibitory effect on the growth of C. pyrenoidosa and M. Aeruginosa but had a significant stimulatory effect on the growth of S. obliquus.
The present study indicated that extract solutions of most macrophytes tested had no significant effect on phytoplankton growth compared to their culture solutions. This result has three possible explanations: (i) during the process of immersion, some nutrients may dissolve from the plant material into the solution, which may be favorable for the growth of algae; (ii) some allelochemicals from living plants may be inactive when the plant dies; and (iii) the initial weight of the macrophytes in culture solutions was two times that of the plant material used in the extract solutions, which may have caused differences in the concentrations of inhibiting compounds between the culture and extract solutions. Men et al. (Citation2006) and Li et al. (Citation2009) discovered that the extract solutions of macrophytes had definite allelopathic effects on algal growth depending on their concentration. Nakai et al. (Citation1999) demonstrated that the inhibition of cyanobacteria growth by the macrophyte M. spicatum required a continuous secretion of allelopathic compounds that were unstable outside the plants. They found that the growth of M. aeruginosa was not inhibited by the initial addition of M. spicatum culture solution. Schagerl et al. (Citation2002) also found that the living biomass of some cyanobacteria clearly inhibited other phytoplankton but no allelopathic activity was detected in the test using culture filtrates. In the present study, we adopted an initial addition of solution instead of a continuous addition which may be another important factor affecting phytoplankton growth. The reason why extract solutions stimulated algal growth in the present experiment needs further study.
Amongst the three common phytoplankton tested, S. obliquus was most sensitive to culture solutions; however, its growth was not inhibited but stimulated by culture solutions of most of the macrophytes. Eichhornia crassipes (Mart.) Solms (Sun et al. Citation1988), Acorus tatarinowii Schott (He and Ye Citation1999), S. aloides (Mulderij, Mooij, Smolders et al. Citation2005; Mulderij, Mooij, and Donk Citation2005), and Phragmitis communis Trin. (Men et al. Citation2006) were reported to have certain inhibiting effects on S. obliquus growth. Men et al. (Citation2006) reported that growth of S. obliquus was inhibited only during the first several days, whilst growing more rapidly during the subsequent days of study. In the present experiment, the rapid growth of S. obliquus in the culture solutions of six macrophytes may be related to: (i) lack of sufficient and continuous addition of extract solutions during the experiment; and (ii) lack of sensitivity to the macrophytes selected for this experiment.
Based on our results of algae inhibition and those from the literature (Nelson et al. Citation2003; Wang et al. Citation2007; Hilt and Gross Citation2008), it is obvious that macrophytes are possible candidates for controlling algae blooms. However, there is a gap between the theoretical possibility and its practical application and this requires further investigation. Notwithstanding the studies on allelopathy of macrophytes under controlled laboratory conditions, more evidence is needed for allelopathy in situ as well as the actual allelopathic mechanism.
In summary, the culture solutions of aquatic macrophytes generally had significant inhibitory effects, whereas extract solutions from the macrophytes typically had stimulatory effects or non-significant effects on the growth of common phytoplankton. The observed effects of culture and extract solutions were dependent on both the macrophyte species used to a make the solutions and phytoplankton species.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (30470346), the Natural Science Foundation of Guangdong Province (06025056), and the project ‘Ecological Rehabilitation of Eutrophic Lakes’ from the Bureau of Water Resources, Baiyun District, Guangzhou.
References
- Bauer , N , Blaschke , U , Beutler , E , Gross , EM , Jenett-Siems , K , Siems , K and Hilt , S . 2009 . Seasonal and interannual dynamics of polyphenols in Myriophyllum verticillatum and their allelopathic activity on Anabaena variabilis . Aquatic Botany , 91 ( 2 ) : 110 – 116 .
- Brückner , DJ , Lepossa , A and Herpai , Z . 2003 . Inhibitory effect of ragweed (Ambrosia artemisiifolia L.)-inflorescence extract on the germination of Amaranthus hypochondriacus L. and growth of two soil algae . Chemosphere , 51 ( 6 ) : 515 – 519 .
- Chang , XX , Wu , C and Zhao , J . 2007 . “ Advance of allelopathic inhibitory effects of aquatic macrophytes on algae and its application ” . In Advance in ecological science , Edited by: Duan , CQ . 147 – 174 . Beijing : Higher Education Press . (in Chinese)
- Donk , E and Bund , WJ . 2002 . Impact of submerged macrophytes including charophytes on phyto- and zooplankton communities: allelopathy versus other mechanisms . Aquatic Botany , 72 ( 3–4 ) : 261 – 274 .
- Erhard , D and Gross , EM . 2006 . Allelopathic activity of Elodea canadensis and Elodea nuttallii against epiphytes and phytoplankton . Aquatic Botany , 85 ( 3 ) : 203 – 211 .
- Ferrier , MD , Butler , BR , Sr., Terlizzi , DE and Lacouture , RV . 2005 . The effects of barley straw (Hordeum vulgare) on the growth of freshwater algae . Bioresource Technology , 96 ( 16 ) : 1788 – 1795 .
- Gross , EM . 2003 . Allelopathy of aquatic autotrophs . Critical Reviews in Plant Sciences , 22 ( 3–4 ) : 313 – 339 .
- Gross , EM , Meyer , H and Schilling , G . 1996 . Release and ecological impact of algicidal hydrolysable polyphenols in Myriophyllum spicatum . Phytochemistry , 41 ( 1 ) : 133 – 138 .
- Gulati , RD and Van Donk , E . 2002 . Lakes in the Netherlands, their origin, eutrophication and restoration: state-of-the-art review . Hydrobiologia , 478 ( 1–3 ) : 73 – 106 .
- He , CQ and Ye , JX . 1999 . Inhibitory effects of Acorus tatarinowii on algae growth . Acta Ecologica Sinica , 19 ( 5 ) : 754 – 758 . (in Chinese)
- Hilt , S . 2006 . Allelopathic inhibition of epiphytes by submerged macrophytes . Aquatic Botany , 85 ( 3 ) : 252 – 256 .
- Hilt , S and Gross , EM . 2008 . Can allelopathically active submerged macrophytes stabilise clear-water states in shallow lakes? . Basic and Applied Ecology , 9 ( 4 ) : 422 – 432 .
- Hong , NH , Xuan , TD , Eiji , T , Hiroyuki , T , Mitsuhiro , M and Khanh , TD . 2003 . Screening for allelopathic potential of higher plants from Southeast Asia . Crop Protection , 22 ( 6 ) : 829 – 836 .
- Horppila , J and Nurminen , L . 2003 . Effects of submerged macrophytes on sediment resuspension and internal phosphorus loading in Lake Hiidenvesi (southern Finland) . Water Research , 37 : 4468 – 4474 .
- Jasser , I . 1995 . The influence of macrophytes on a phytoplankton community in experimental conditions . Hydrobiologia , 306 ( 1 ) : 21 – 32 .
- Leu , E , Liszkay , AK , Goussias , C and Gross , EM . 2002 . Polyphenolic allelochemicals from the aquatic angiosperm Myriophyllum spicatum inhibit photosystem II . Plant Physiology , 130 ( 4 ) : 2011 – 2018 .
- Li , QH , Guo , PY , Tian , MY and Jiang , ZY . 2009 . Allelopathy of Salix babylonica leaf extracts on Chlorella pyrenoidosa . Chinese Journal of Applied Ecology , 28 ( 5 ) : 884 – 888 . (in Chinese)
- Li , Y and Qin , JG . 2005 . Comparison of growth and lipid content in three Botryococcus braunii strains . Journal of Applied Phycology , 17 ( 6 ) : 551 – 556 .
- Men , YJ , Hu , HY and Li , FM . 2006 . Effects of an allelopathic fraction from Phragmitis communis Trin on the growth characteristics of Scenedesmus obliquus . Ecology and the Environment , 15 ( 5 ) : 925 – 929 . (in Chinese)
- Molisch , H . 1937 . Der Einfluss einer Pflanze auf die andere: Allelopathie , Jena : Fisher Verlag .
- Mulderij , G , Donk , EV and Roelofs , JGM . 2003 . Differential sensitivity of green algae to allelopathic substances from Chara . Hydrobiologia , 491 ( 1–3 ) : 261 – 271 .
- Mulderij , G , Mooij , WM , Smolders , AJP and Donk , EV . 2005 . Allelopathic inhibition of phytoplankton by exudates from Stratiotes aloides . Aquatic Botany , 82 ( 4 ) : 284 – 296 .
- Mulderij , G , Mooij , WM and Donk , EV . 2005 . Allelopathic growth inhibition and colony formation of the green alga Scenedesmus obliquus by the aquatic macrophyte Stratiotes aloides . Aquatic Ecology , 39 ( 1 ) : 11 – 21 .
- Mulderij , G , Smolders , AJP and Donk , EV . 2006 . Allelopathic effect of the aquatic macrophyte, Stratiotes aloides, on natural phytoplankton . Freshwater Biology , 51 : 554 – 561 .
- Nan , CR , Zhang , HZ and Zhao , GQ . 2004 . Allelopathic interactions between the macroalga Ulva pertusa and eight microalgal species . Journal of Sea Research , 52 ( 4 ) : 259 – 268 .
- Nakai , S , Hosomi , M , Okada , M and Murakami , A . 1996 . Control of algal growth by macrophytes and macrophyte extracted bioactive compounds . Water Science and Technology , 34 ( 7–8 ) : 227 – 235 .
- Nakai , S , Inoue , Y , Hosomi , M and Murakami , A . 1999 . Growth inhibition of blue-green algae by allelopathic effects of macrophytes . Water Science and Technolgy , 39 ( 8 ) : 47 – 53 .
- Nakai , S , Inoue , Y , Hosomi , M and Murakami , A . 2000 . Myriophyllum spicatum released allelopathic polyphenols inhibiting growth of blue-green alga Microcystis aeruginosa . Water Research , 34 ( 11 ) : 3026 – 3032 .
- Nelson , TA , Lee , DJ and Smith , BC . 2003 . Are ‘green tides’ harmful algal blooms? Toxic properties of water-soluble extracts from two bloom-forming macroalgae, Ulva fenestrata and Ulvaria obscura (Ulvophyceae) . Journal of Physiocology , 39 : 874 – 879 .
- Ozimek , T , Ska , EP and Hankiewicz , A . 1991 . Effects of filamentous algae on submerged macrophyte growth: a laboratory experiment . Aquatic Botany , 41 ( 4 ) : 309 – 315 .
- Park , MH , Chung , IM , Ahmad , A , Kim , BH and Hwang , SJ . 2009 . Growth inhibition of unicellular and colonial Microcystis strains (Cyanophyceae) by compounds isolated from rice (Oryza sativa) hulls . Aquatic Botany , 90 ( 4 ) : 309 – 314 .
- Sabine , K and Andreas , N . 2002 . Allelopathic growth inhibition of selected phytoplankton species by submerged macrophytes . Journal of Phycology , 38 ( 5 ) : 862 – 871 .
- Schagerl , M , Unterrieder , I and Angeler , DG . 2002 . Allelopathy among cyanoprokaryota and other algae originating from Lake Neusiedlersee (Austria) . International Review of Hydrobiology , 87 ( 4 ) : 365 – 374 .
- Sukenik , A , Eshkol , R , Livne , A , Hadas , O , Rom , M , Tchenov , D , Vardi , A and Kaplan , A . 2002 . Inhibition of growth and photosynthesis of the dinoflagellate Peridinium gatunense by Microcystis sp. (cyanobacteria): a novel allelopathic mechanism . Limnology and Oceanography , 47 ( 6 ) : 1656 – 1663 .
- Sun , WH , Yu , ZW and Yu , SW . 1988 . Inhibitory effect of Eichhornia crassipes (Mart.) Solms on algae . Acta Phytophysiologica Sinica , 14 ( 3 ) : 294 – 300 . (in Chinese)
- Wang , Y , Yu , ZM , Song , XX , Tang , XX and Zhang , SD . 2007 . Effects of macroalgae Ulva pertusa (Chlorophyta) and Gracilaria lemaneiformis (Rhodophyta) on growth of four species of bloom-forming dinoflagellates . Aquatic Botany , 86 ( 2 ) : 139 – 147 .
- Wu , C , Chang , XX , Dong , HJ , Li , DF and Liu , JY . 2008 . Allelopathic inhibitory effect of Myriophyllum aquaticum (Vell.) Verdc. on Microcystis aeruginosa and its physiological mechanism . Acta Ecologica Sinica , 28 ( 6 ) : 2595 – 2603 .
- Wu , YH , Kerr , PG , Hu , ZY and Yang , LZ . 2010 . Removal of cyanobacterial bloom from a biopond–wetland system and the associated response of zoobenthic diversity . Bioresource Technology , 101 ( 11 ) : 3903 – 3908 .
- Xian , Q , Chen , H , Liu , H , Zou , H and Yin , D . 2006 . Isolation and identification of antialgal compounds from the leaves of Vallisneria spiralis L. by activity-guided fractionation . Environmental Science and Pollution Research , 13 ( 4 ) : 233 – 237 .
- Zhu , JY , Liu , BY , Wang , J , Gao , YN and Wu , ZB . 2010 . Study on the mechanism of allelopathic influence on cyanobacteria and chlorophytes by submerged macrophyte (Myriophyllum spicatum) and its secretion . Aquatic Toxicology , 98 : 196 – 203 .