Abstract
Ammonia is one of the most common pollutants in aquaculture systems and its elevated concentration in surface water is a critical concern in fish larvae production. The purpose of this study was to assess the effects of un-ionized ammonia (NH3) on hatch success of fertilized eggs of silver carp Hypophthalmichthys molitrix and bighead carp Hypophthalmichthys nobilis, two ecologically and commercially important species in freshwater ecosystems. The results showed total hatch rates and viability of newly hatched larvae 24 h post-hatch decreased significantly with increasing NH3–N concentration for both silver carp and bighead carp, whereas abnormal rate increased significantly with increasing NH3–N concentration. When fitted to the data of total hatch rates and viability of newly hatched larvae 24 h post-hatch, it is clear that the logistic model with three parameters can be fitted to ecotoxicological data well and can describe dose-dependent effects of un-ionized ammonia on hatching success of fertilized eggs of both silver carp and bighead carp. According to the model, the EC50 for the hatch rates of silver carp and bighead carp was 0.98 and 0.86 mg L−1, respectively. Our results should be useful to hatchery managers and fingerling producers in establishing acceptable water quality parameters for the hatchery and nursery ponds.
Introduction
Ammonia is a naturally occurring product of biological metabolism but high concentrations are often associated with anthropogenic discharge. It is one of the most common pollutants in aquaculture systems and its mechanisms of toxicity and lethal concentrations to various fishes and crustaceans of commercial importance are relatively well documented (Cavalli et al. Citation2000). Ammonia exists in solution primarily as un-ionized ammonia (NH3) and ion in equilibrium, which depends on pH and temperature of the water. Un-ionized ammonia can be toxic to fish because it diffuses easily through fish respiratory membranes, damaging gill epithelium thereby impairing gas exchange, disrupting the osmoregulatory system, targeting the central nervous system, and inducing reactive oxygen species (Ip and Chew Citation2010), although toxic concentrations vary among species (Tomasso Citation1994; Harcke and Daniels Citation1999; Foss et al. Citation2009; Yang et al. Citation2010; Chen et al. Citation2011).
Silver carp Hypophthalmichthys molitrix and bighead carp Hypophthalmichthys nobilis, both are commercially important species distributed and cultured extensively in developing countries and also are important components of freshwater ecosystems (Xie Citation2001; Sun et al. Citation2011). Usually, their artificial reproduction to mass produce larvae is essential for meeting mass aquaculture demand. It is well known that early life stages are more sensitive than adult fish to many pollutants because of the thinner epithelial layer combined with a relatively larger body surface-to-volume ratio, high metabolic rate, and limited mobility (Amado and Monserrat Citation2010). Normally, ammonia is a critical water quality parameter in fish larvae production. Especially with industrial development, more and more ammonia-containing waste water is discharged into natural waters. The increasing concentrations of ammonia in surface waters are becoming a worldwide concern. The elevated concentration of ammonia in aquatic ecosystems could directly cause damage to fish. In this experiment, therefore, we used silver carp and bighead carp as test organisms to determine responses of development of embryos to different un-ionized ammonia levels. Dose–response functions of the relevant indices were fitted where appropriate. Information on the toxicity of ammonia to fertilized eggs of silver carp and bighead carp would be useful to hatchery managers and fingerling producers to establish acceptable water quality parameters for hatchery and nursery ponds.
Methods
Artificially fertilized eggs of silver carp and bighead carp were obtained by induced ovulation of cultured broodstock by injections of [D-Ala6-Pro9-Net]-luteinizing hormone releasing hormone analogue. The eggs were stripped manually and artificially fertilized. In order to assess real effects of un-ionized ammonia on normally developed embryos, we selected the embryos at the middle blastula stage, at which point the fertilized eggs are considered normal and will likely develop into larvae. When embryos were at the middle blastula stage (i.e., around 5 h post-fertilization) dead and physically damaged embryos were removed and only normally developing embryos were randomly selected for transfer to different concentrations of un-ionized ammonia.
Ammonia test solutions were prepared by dissolving ammonium chloride (NH4Cl) (Sinopharm, Shanghai, China) in de-chlorinated tap water. NH3–N concentrations were calculated using the general equation of bases (Emerson et al. Citation1975):
The calculation of pK a is based on the equation developed by Emerson et al. (Citation1975):
The nominal concentrations of NH3–N used in the incubation experiments were 0, 0.19, 0.37, 0.56, 0.74, 0.93, 1.12, and 1.49 mg L−1 for silver carp and 0, 0.37, 0.74, 1.12, 1.49, 1.67, 2.05, and 2.23 mg L−1 for bighead carp based on preliminary tests. An incubation unit consisted of a glass Petri dish (11.5 cm × 2 cm) filled with 100 mL of test solution. One hundred embryos at the middle blastula stage were placed in Petri dishes, in triplicate, with different NH3–N concentrations to determine the total hatch rate and abnormal rate. Dead embryos were removed and counted every 8 h until all the larvae hatched or all the embryos died. Total hatch rate was determined as the percentage of experimental embryos that hatched, regardless of viability.
Abnormal rate was determined as the percentage of abnormally developed larvae out of the total number of hatched larvae. Abnormal larvae were defined as those with abnormal body curvature or tail flexure. Newly hatched larvae were removed and held in static containers for a further 24 h to observe their continued normal development and immediate post-hatch viability. After 24 h, deformed and moribund larvae were counted. Viability of newly hatched larvae 24 h post-hatch (V24ph) was determined as the percentage of total hatch that was alive and normally developed at 24 h post-hatch, which has been used widely to describe viability of newly hatched larvae (Kamler Citation2002; Yang and Yang Citation2004; Yang and Chen Citation2006). The experiments were conducted at a water temperature of 23 ± 1°C, a dissolved oxygen concentration above 5.0 mg L−1, 20 µmol photons m−2 s−1light intensity, 14 L:10 D photoperiod, and pH of 7.6 ± 0.2. To maintain constant NH3–N concentrations, test solutions were replaced with fresh ones every day according to the preliminary experiment. The pH of the solution was measured twice each day and adjusted using 0.5 M H2SO4or 1 M NaOH when necessary.
All data are presented as mean ± 1 SE and were evaluated by one-way analysis of variance (ANOVA) followed by Duncan's multiple range test (α = 0.05). To describe the dose-dependent effect of ammonia, a three-parameter logistic model, Y = a/(1 + (X/X 0) b ), was chosen to fit the data of total hatch rate and V24ph, where Y is value of the index, X is the NH3–N concentration, a is the value of the index at zero NH3–N concentration (theoretic maximum value at zero NH3–N concentration), X 0 is the concentration that reduces the value of the index by 50% (i.e., EC50), b is the form parameter determining shape of curve. The no-observed-effect concentration (NOEC) is defined as the highest tested toxicant concentration that yields no statistically significant from the control (Chao and Chen Citation2000). NOEC values are determined by using the analysis of variance followed by multiple comparison tests. All statistical analyses, including the three-parameter logistic model to fit the data, and determining NOEC were carried out with SigmaPlot 11.0.
Results and discussion
The total hatch rate of fertilized eggs of silver carp in the control (0 mg L−1NH3–N) was 80%, whereas the total hatch rate in 1.49 mg L−1 of NH3–N was 24.44% (). As for fertilized eggs of bighead carp, the total hatch rate in the control was 87.78%, whereas in the 2.23 mg L−1 of NH3–N the total hatch rate was 7.78% (). For fertilized eggs of both silver carp and bighead carp, the total hatch rates decreased significantly with increasing NH3–N concentration (p < 0.001), but there were no significant differences between the control and the lowest NH3–N treatments (0.19 mg L−1 for silver carp and 0.37 mg L−1for bighead carp). The three-parameter logistic model fitted the hatch rate well; according to the model, the EC50 for the hatch rates of silver carp and bighead carp was 0.98 ± 0.09 and 0.86 ± 0.16 mg L−1 (), respectively. In the NH3–N treatments, there were some abnormal embryos observed during development () and abnormal larvae were hatched. The abnormal rate increased significantly with increasing NH3–N concentration and there were significant differences between the control and the NH3–N treatment ().
Figure 1. Total hatch rates of fertilized eggs of silver carp and bighead carp incubated under different NH3–N concentrations. Vertical bars represent ± 1 SE. A curve was fit using the logistic model Y = a/(1 + (X/X0 ) b ).
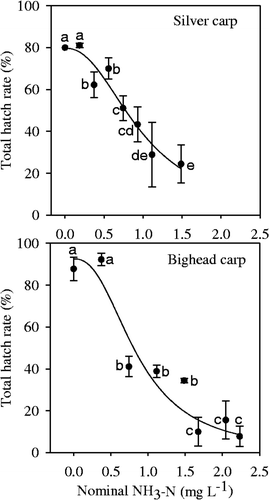
Figure 2. Fertilized eggs of silver carp incubated for 24 h under different NH3–N concentrations: a) normal embryos under 0 mg L−1 NH3–N, b) abnormal embryos under 1.49 mg L−1 NH3–N. The arrows show abnormal embryos with twist tail or without tail.
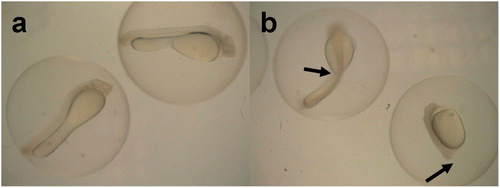
Figure 3. Abnormal rates of fertilized eggs of silver carp and bighead carp incubated under different NH3–N concentrations. Vertical bars represent ± 1 SE.
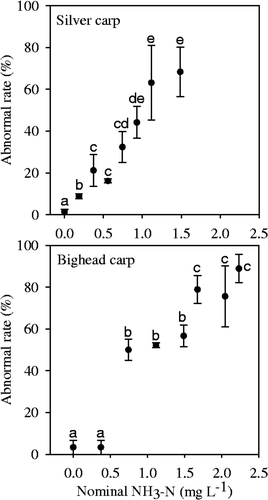
Table 1. The parameters in the logistic model Y = a/(1 + (X/X0 ) b ) for total hatch rate and viability of newly hatched larvae 24 h post-hatch (V24ph) of silver carp and bighead carp.
V24ph in the control was 96.7%, and decreased significantly with increasing NH3–N concentration (); in the 1.49 mg N L−1 treatment, no newly hatched larvae survived in 24 h. For bighead carp larvae, V24ph was 100% in the NH3–N concentrations lower than 0.74 mg L−1 NH3–N, and decreased significantly above this concentration (). Furthermore, no newly hatched larvae survived after 24 h in the 1.49 mg N L−1 treatment and above.
Figure 4. Viability of newly hatched larvae of silver carp and bighead carp incubated under different NH3–N concentrations in 24 h. Vertical bars represent ± 1 SE. The curve was fit using a logistic model Y = a/(1 + (X/X0 ) b ).
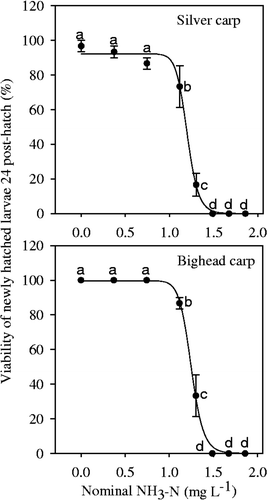
These results indicate that, although embryos can hatch to larvae under elevated NH3–N conditions, the larvae were severely harmed by NH3–N and thus died soon. The three-parameter logistic model fit the data well; according to the model, the EC50 for V24ph of silver carp and bighead carp was 1.20 ± 0.01 and 1.25 ± 0.01 mg N L−1 (), respectively. As ammonia–N concentrations were nominal and not actually measured in the experiments, we cannot exclude the possibility that the actual values deviate somewhat from our results.
In this study, using the ecologically and commercially important species, silver carp and bighead carp, we investigated the impact of un-ionized ammonia. According to the data of the total hatch rate, abnormal rate, and V24ph, it is clear that un-ionized ammonia is toxic to fertilized eggs and larvae of silver carp and bighead carp, which support an increasing body of literature on ammonia toxicity (Tomasso Citation1994; Harcke and Daniels Citation1999; Foss et al. Citation2009; Yang et al. Citation2010; Chen et al. Citation2011). The NOEC of NH3–N was about 0.19 mg L−1 for silver carp embryos and 0.37 mg L−1 for bighead embryos (). Generally speaking, tolerance to ammonia was similar between silver carp and bighead carp. The EC50 values for larvae viability of the two species were higher than those of their hatch rate (), which indicates the embryos were less tolerant of NH3–N than the larvae. However, a low sensitivity towards NH3–N is generally observed during the embryonic stage compared to the larval stage in both marine and freshwater species (Holt and Arnold Citation1983; Daniels and Boyd Citation1987). Our results are inconsistent with those from some other fish species, such as hybrid striped bass Morone chrysops × Morone saxatilis (Harcke and Daniels Citation1999), smallmouth bass Micropterus dolomieui (Broderius et al. Citation1985), and sunfish Lepomis cyanellus (McCormick et al. Citation1984). The larvae of these species were much less NH3–N tolerant than embryos. The differences between our results and the literature suggest the NH3–N tolerance of embryos and larvae of fish may be species-specific or those experiments conducted under different conditions as ammonia toxicity changes with temperature and pH (Emerson et al. Citation1975). Based on the above toxicological data, embryos and newly hatched larvae of silver carp and bighead carp seem to be susceptible to ammonia-containing polluted water.
When fitted to the data of hatching and viability in our study, the logistic model with three parameters produced good fits without any transformation and revealed the dose-dependent effect of un-ionized ammonia on hatching success of fertilized eggs of silver carp and bighead carp and get robust results as used in other studies (Xiang et al. Citation2012). Generally speaking, the model is suitable for describing the concentration–response function and ultimately leads to a better understanding of the relationship between ammonia concentration and hatch parameters of fish.
In conclusion, un-ionized ammonia can decrease the hatch rate and viability of newly hatched larvae, and increase the abnormal rate. When fitted to the data presented in this work, it is clear that the logistic model with three parameters can be fitted to ecotoxicological data well without any transformation, and can describe dose-dependent effect of un-ionized ammonia on hatching success of fertilized eggs.
Acknowledgements
The authors thank Hanjiang Fish Breeding Farm for providing fertilized eggs. This study was supported by the State Key Fundamental Research and Development Program (2008CB418102).
References
- Amado , L L and Monserrat , J M . 2010 . Oxidative stress generation by microcystins in aquatic animals: why and how . Environment International , 36 ( 2 ) : 226 – 235 . doi: 10.1016/j.envint.2009.10.010
- Broderius , S , Drummond , R , Fiandt , J and Russom , C . 1985 . Toxicity of ammonia to early life stages of the smallmouth bass at four pH values . Environmental Toxicology and Chemistry , 4 ( 1 ) : 87 – 96 . doi: 10.1002/etc.5620040111
- Cavalli , R O , Berghe , E V , Lavens , P , Thuy , N TT , Wille , M and Sorgeloos , P . 2000 . Ammonia toxicity as a criterion for the evaluation of larval quality in the Prawn, Macrobrachium rosenbergii . Comparative Biochemistry and Physiology. Part C , 125 ( 3 ) : 333 – 343 .
- Chao , M-R and Chen , C-Y . 2000 . No-observed-effect concentrations in batch and continuous algal toxicity tests . Environmental Toxicology and Chemistry , 19 ( 6 ) : 1589 – 1596 . doi: 10.1002/etc.5620190616
- Chen , Y F , Sun , H J , Yang , W and Yang , Z . 2011 . Oxidative stress responses of grass carp Ctenopharyngodon idella larvae exposed to purified microcystin under different ammonia concentrations . Fresenius Environmental Bulletin , 20 ( 11 ) : 2869 – 2874 .
- Daniels , H V and Boyd , C E . 1987 . Acute toxicity of ammonia and nitrite to spotted seatrout . Progressive Fish-Culturist , 49 ( 4 ) : 260 – 263 . doi: 10.1577/1548-8640(1987)49<260:ATOAAN>2.0.CO;2
- Emerson , K R , Russo , R C , Lund , R E and Thurston , R V . 1975 . Aqueous ammonia equilibrium calculations: effect of pH and temperature . Journal of the Fisheries Research Board of Canada , 32 ( 12 ) : 2379 – 2383 . doi: 10.1139/f75-274
- Foss , A , Imsland , A K , Roth , B , Schram , E and Stefansson , S O . 2009 . Effects of chronic and periodic exposure to ammonia on growth and blood physiology in juvenile turbot (Scophthalmus maximus) . Aquaculture , 296 ( 1–2 ) : 45 – 50 . doi: 10.1016/j.aquaculture.2009.07.013
- Harcke , J E and Daniels , H V . 1999 . Acute toxicity of ammonia and nitrite to reciprocal cross hybrid striped bass Morone chrysops X M. saxatilis eggs and larvae . Journal of the World Aquaculture Society , 30 ( 4 ) : 496 – 500 . doi: 10.1111/j.1749-7345.1999.tb00998.x
- Holt , G J and Arnold , C R . 1983 . Effects of ammonia and nitrite on growth and survival of red drum eggs and larvae . Transactions of the American Fisheries Society , 112 ( 2B ) : 314 – 318 . doi: 10.1577/1548-8659(1983)112<314:EOAANO>2.0.CO;2
- Ip , Y K and Chen , S F . 2010 . Ammonia production, excretion, toxicity, and defense in fish: a review . Frontiers in Physiology , 1 : 134 doi: 10.3389/fphys.2010.00134
- Kamler , E . 2002 . Ontogeny of yolk-feeding fish: an ecological perspective . Reviews in Fish Biology and Fisheries , 12 ( 1 ) : 79 – 103 . doi: 10.1023/A:1022603204337
- McCormick , J H , Broderius , S J and Fiandt , J T . 1984 . Toxicity of ammonia to early life stages of the green sunfish Lepomis cyanellus . Environmental Pollution – Series A , 36 ( 2 ) : 147 – 163 . doi: 10.1016/0143-1471(84)90096-5
- Sun , H J , Yang , W , Chen , Y F and Yang , Z . 2011 . Effect of purified microcystin on oxidative stress of silver carp Hypophthalmichthys molitrix larvae under different ammonia concentrations . Biochemical Systematics and Ecology , 39 ( 4–6 ) : 536 – 543 . doi: 10.1016/j.bse.2011.08.001
- Tomasso , J R . 1994 . Toxicity of nitrogenous wastes to aquaculture animals . Reviews in Fisheries Science , 2 ( 4 ) : 291 – 314 . doi: 10.1080/10641269409388560
- Xiang , F H , Geng , L L , Lü , K , Zhang , J , Minter , E JA and Yang , Z . 2012 . Effect of long-term nitrite exposure on the cladoceran Daphnia obtusa: survival, moults, and reproduction . Biochemical Systematics and Ecology , 41 ( 1 ) : 98 – 103 . doi: 10.1016/j.bse.2011.12.006
- Xie , P . 2001 . Gut contents of bighead carp (Aristichthys nobilis) and the processing and digestion of algal cells in the alimentary canal . Aquaculture , 195 ( 1–2 ) : 149 – 161 . doi: 10.1016/S0044-8486(00)00549-4
- Yang , W , Xiang , F H , Liang , L and Yang , Z . 2010 . Toxicity of ammonia and its effects on oxidative stress mechanisms of juvenile crucian carp (Carassius auratus) . Journal of Freshwater Ecology , 25 ( 2 ) : 297 – 302 . doi: 10.1080/02705060.2010.9665080
- Yang , Z and Chen , Y F . 2006 . Salinity tolerance of embryos of obscure puffer Takifugu obscurus . Aquaculture , 253 ( 1–4 ) : 393 – 397 . doi: 10.1016/j.aquaculture.2005.08.014
- Yang , Z and Yang , J X . 2004 . Effect of photoperiod on the embryonic development of obscure puffer (Takifugu obscurus) . Journal of Freshwater Ecology , 19 ( 1 ) : 53 – 58 . doi: 10.1080/02705060.2004.9664512