Abstract
The stocking of western mosquitofish Gambusia affinis for mosquito control negatively impacts native fishes with similar ecological requirements. In this study, a series of laboratory microcosm experiments was used to examine intra and interspecific agonistic behavioral interactions (e.g., chases and nips) between western mosquitofish and northern starhead topminnow Fundulus dispar, northern studfish F. catenatus, blackstripe topminnow F. notatus, and banded killifish F. diaphanous at three fish densities and in the presence/absence of vegetation. Western mosquitofish exhibited more agonistic behaviors than the four topminnow species and caused a change in topminnow behavior in mixed-species microcosms. Mosquitofish were aggressive toward conspecifics, with most of the chases and nips occurring at the highest densities and when vegetation was absent. Topminnows exhibited few agonistic behaviors toward conspecifics, but intraspecific chasing and nipping did occur when exposed to mosquitofish. Agonistic behaviors by topminnows toward mosquitofish occurred infrequently, and mosquitofish initiated almost all of the chases and nips. While all four topminnow species were attacked by mosquitofish, northern starhead topminnow and banded killifish were chased and nipped more frequently than the other topminnow species. These two topminnows exhibited the most behavioral changes and fin damage and one northern starhead topminnow died following mosquitofish attacks. Based on these results, it appears that the stocking of western mosquitofish into primary and connecting waterways could have negative impacts on native topminnow species that occur in these systems.
Introduction
The western mosquitofish Gambusia affinis is the most widely distributed larvivorous fish in the world. Previous research suggests that western mosquitofish (and its congener eastern mosquitofish G. holbrooki) prefer mosquito larvae over other prey taxa; therefore, the stocking of these fishes is often considered to be a viable alternative to pesticide use (Krumholz Citation1948; Hoy and Reed Citation1971; Bence Citation1988). As a result, both species have been stocked outside their natural range for mosquito control and now occupy waterways throughout much of the United States (Fuller et al. Citation1999). However, the introduction of non-native fishes, such as mosquitofish, may have long-term negative impacts on aquatic communities, resulting in declines in abundance and extirpation of native species (Li and Moyle Citation1999; Everett and Sherfy Citation2001; Gale et al. Citation2004; Leyse et al. Citation2004; Pyke Citation2005; Rieman et al. Citation2006).
Mosquitofish become successfully established in aquatic systems in part due to their aggressive behaviors. Agonistic behaviors, such as chasing and nipping, can negatively impact less aggressive fishes. Nipping causes fin damage, which may lead to secondary infection, reduce the ability to forage effectively or escape predators, and cause mortality (Meffe et al. Citation1983; Abbott and Dill Citation1985). In laboratory studies, more than half of Sonoran topminnows Poeciliopsis occidentalis sonoriensis had fin damage in the presence of western mosquitofish; however, no fish had fin damage in aquaria without mosquitofish (Meffe Citation1985). Barrens topminnow Fundulus julisia had all their fins eaten within 24 h after the introduction of western mosquitofish, causing mortality of all but one fish (Laha and Mattingly Citation2007).
Although behavioral responses of fish to environmental stimuli must be flexible, a shift in habitat use for fish trying to escape agonistic encounters with other fishes may lead to deleterious effects. For example, juvenile bluegill Lepomis macrochirus and pumpkinseed sunfish L. gibbosus become restricted to vegetated areas with fewer trophic resources in the presence of predators such as largemouth bass Micropterus salmoides (Mittelbach Citation1984). When vegetation or trophic resources are limited, these species may experience a competitive bottleneck that limits recruitment (Werner et al. Citation1983). Least chub Lotichthys phlegethontis sought refuge in aquatic vegetation in the presence of western mosquitofish and remained stationary to avoid attacks, causing declines in growth and abundance (Mills et al. Citation2004). When Sonoran topminnows were exposed to western mosquitofish in laboratory experiments, the topminnows ceased to feed, retreated to areas with structure to escape aggression and predation, and experienced slower growth, greater mortality, and reduced reproductive potential (Schoenherr Citation1981).
In this study, we evaluated behavioral interactions between western mosquitofish and four species of topminnow (northern starhead topminnow Fundulus dispar, northern studfish F. catenatus, blackstripe topminnow F. notatus, and banded killifish F. diaphanous). All four topminnows have the potential to be negatively impacted by western mosquitofish due to their extensive niche overlap (Zeiber Citation2007). A series of laboratory experiments was used to quantify: (1) intra and interspecific interactions of western mosquitofish and the four topminnow species at three fish density combinations and in the presence–absence of vegetation and (2) percent fin damage and survival following these interactions. These results will provide a greater understanding of the potential impacts of western mosquitofish stockings on native topminnows.
Materials and methods
Field collections
The five fish species used in the laboratory experiments for this study were collected from Indiana waters in May 2005 and 2006. Sampling locations by species were as follows: (1) western mosquitofish (Martell Forest Pond; Tippecanoe County; 40.4534° N, 87.0542° W); (2) northern studfish (Sugar Creek; Johnson County; 39.3043° N, 85.5812° W); (3) northern starhead topminnow (Loomis Lake; Porter County; 41.5200° N, 87.0550° W); (4) blackstripe topminnow (Moots Creek; White County; 40.5378° N, 86.7804° W); and (5) banded killifish (Silver Lake; Steuben County; 41.6303° N, 85.0644° W). None of the topminnow species were collected from systems containing western mosquitofish. The sampling gears used for fish collections included a 3.18-mm knotless mesh seine (length: 3.05 m; depth: 1.22 m) or a 3.18-mm knotless mesh dip net (diameter: 40 mm). Following collections, fish of each species were placed into separate 8.93-L buckets of water containing aquatic vegetation and transported to Purdue University (West Lafayette, IN). In the laboratory, fish of each species were placed into separate, 30-L plastic aquaria containing artificial vegetation constructed from 16 to 45-cm long green plastic strips attached to a 3.18-cm hex nut. Water temperature and photoperiod were maintained at 22°C and 14:10 h (light:dark), respectively. Fish were daily fed bloodworms (Chironomus spp.) ad libitum and allowed to acclimate to laboratory conditions for a minimum of 1 week.
Microcosm experiments
A series of experiments was conducted in June and July 2005 and 2006 to assess behavioral interactions between western mosquitofish and topminnows. Mixed-species experiments were conducted in twenty-four 110-L static glass aquaria and a plastic divider was used to initially separate fish species. Half of the aquaria contained two clusters of artificial vegetation constructed as described previously, while the other half contained no vegetation. Density treatments consisted of eight fish per aquarium at three density combinations: (1) two mosquitofish and six topminnows; (2) four mosquitofish and four topminnows; and (3) six mosquitofish and two topminnows. Thus, a 2 × 3 (vegetation × fish density) block design was conducted and each treatment had three replicates (18 aquaria total). In addition, eight fish were randomly allocated to each single-species control aquarium (three replicates of each; six aquaria per species) to assess intraspecific interactions only.
Three days prior to the experiment, fish were placed in the aquaria. Water temperature and photoperiod were maintained at 22°C and 14:10 h (light:dark), respectively, for the duration of the experiment. All fish were fed bloodworms ad libitum each day to decrease the likelihood that agonistic behaviors were due to hunger.
At the onset of the experiment, the divider in each aquarium was removed to allow fish to interact. Each microcosm was observed for a 5-min period, five times each day for seven consecutive days (a total of 175 min of observation per microcosm). Agonistic interactions that were recorded included the number of chases and nips, both between species and among conspecifics. Chases were defined as a behavior where one fish followed another individual closely, forcing that fish to move further away to avoid a direct encounter. A chase was considered completed once the pursuer broke away. Nips occurred when one fish physically bit another individual. At the end of each observation period, any dead fish were removed from the aquarium and examined for fin damage. At the end of the experimental period, all fish were sacrificed, measured for total length (nearest 1 mm) and wet weight (nearest 0.01 g), and the percentage of each fin that was consumed and/or damaged (if any) was recorded for each fish.
Data analyses
A one-way analysis of variance (ANOVA) was conducted to determine if there was a difference in the number of chases and nips by vegetation treatment among fish species in control (single species) aquaria. A t-test was also used to determine if vegetation influenced the number of chases and nips for each species in control aquaria. A two-way ANOVA was used to determine if there was a difference in the number of chases and nips initiated by each species in mixed-species (mosquitofish plus one of the topminnows) aquaria among fish density and vegetation treatments. When significant differences (α = 0.05) were detected for ANOVAs, a Tukey's test determined the location of the differences. A 3 × 5 contingency table was used to determine if there was a difference in percent fin damage by fin type for each species in single and mixed species aquaria. Although graphical and tabular results represent untransformed data, all percentage data were arcsine transformed prior to statistical analyses. All analyses were conducted using SigmaStat 3.5 software (Systat Software, Inc., San Jose, California), and followed procedures as described in Zar (Citation1990). The reported test statistics for each of the statistical analyses were designated with a subscript C or N for chases and nips, respectively.
Results
The mean total length and wet weight of western mosquitofish and the four topminnows varied by species (). Western mosquitofish was the smallest species, although northern starhead topminnow was similar in size. Blackstripe topminnow and banded killifish were larger than mosquitofish, as was northern studfish which was the largest topminnow species.
Table 1. Mean total length and wet weight (range in parentheses) of fish used in behavioral observations involving western mosquitofish and topminnows.
Intraspecific interactions
The mean number of intraspecific chases and nips per observation period in control aquaria was greater for western mosquitofish than for topminnows (). Mosquitofish chased and nipped conspecifics significantly more than topminnows chased and nipped their conspecifics in both the presence (FC = 24.3, p ≤ 0.001; FN = 56.2, p ≤ 0.001) and absence (FC = 30.1, p ≤ 0.001; FN = 16.3, p ≤ 0.01) of vegetation. While the absence of vegetation yielded a greater number of intraspecific chases for mosquitofish (tC = −2.66, p = 0.02) and blackstripe topminnow (tC = −4.76, p ≤ 0.001), there was no difference in the number of nips for either species (mosquitofish: tN = 1.99, p = 0.07; blackstripe topminnow: tN = −1.62, p = 0.13). There were no differences in the number of intraspecific chases and nips for northern starhead topminnow (tC = −1.87, p = 0.09; tN = −1.17, p = 0.27), northern studfish (tC = −1.00, p = 0.34; tN = −1.92, p = 0.08), and banded killifish (tC = −2.52, p = 0.08; tN = 0.87, p = 0.4) between the vegetation treatments.
Figure 1. Mean number (SE) of intraspecific chases and nips per observation period for each species in control treatments with and without vegetation.
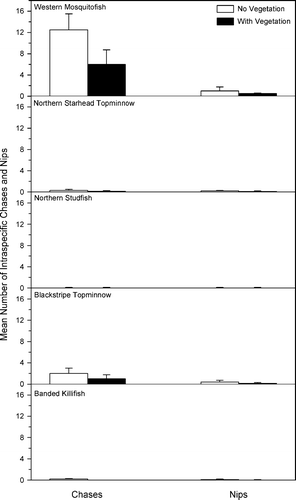
Fin damage caused by conspecifics in control microcosms was significantly higher for western mosquitofish than for topminnows (χ 2 = 3.33, p ≤ 0.001; ). Fourteen percent of western mosquitofish had damage to at least one fin (primarily the caudal fin), while 3% of northern starhead topminnows (primarily the caudal fin) and northern studfish (most commonly the dorsal fin) had fin damage. While no blackstripe topminnows incurred fin damage, 9% of banded killifish had damage, primarily to the caudal fin.
Figure 2. Percent of fish of each species with caudal, dorsal, and anal fin damage in control and experimental treatments.
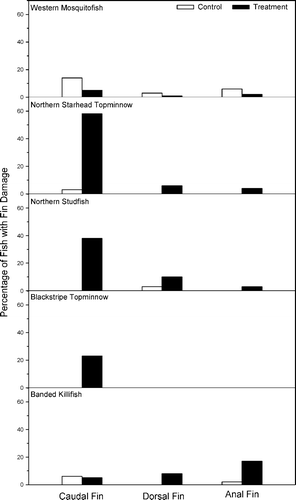
Interspecific interactions
The percent of western mosquitofish with fin damage from topminnows was significantly lower than the converse encounter (χ 2 = 2.23, p ≤ 0.001; ). Seven percent of mosquitofish had fin damage (primarily to the caudal fin) from topminnow nips. However, 58% of northern starhead topminnows incurred fin damage (primarily on the caudal fin) from mosquitofish. In one instance, a northern starhead topminnow had all fins nipped off within 24 h and died due to mosquitofish attacks. Caudal fin damage occurred for northern studfish (38%) and blackstripe topminnows (23%), while banded killifish (30%) exhibited damage primarily to the anal fin.
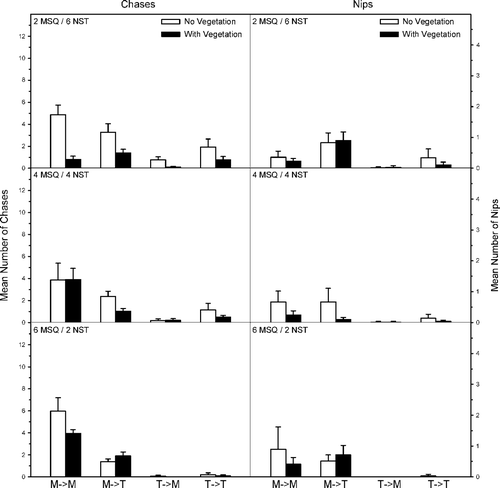
Agonistic behaviors between western mosquitofish and northern starhead topminnow were dependent on mosquitofish-topminnow density and the presence–absence of vegetation (). Mosquitofish chased and nipped conspecifics significantly more at higher mosquitofish densities (FC = 17.2, p ≤ 0.001; FN = 5.2, p = 0.04) and in the absence of vegetation (FC = 6.2, p ≤ 0.01; FN = 8.7, p ≤ 0.001); however, the interaction terms were only significant for chases (FC = 5.9, p ≤ 0.01; FN = 0.08, p = 0.59). There were no significant differences in the number of mosquitofish chases and nips toward topminnows, regardless of topminnow density (FC = 1.5, p = 0.23; FN = 1.5, p = 0.24) or vegetation (FC = 1.3, p = 0.21; FN = 0.2, p = 0.66), and the interaction terms were also not significant (FC = 2.0, p = 0.3; FN = 1.2, p = 0.34). Topminnow chases and nips toward conspecifics were more frequent at greater topminnow densities (FC = 9.0, p ≤ 0.001; FN = 4.2, p = 0.02) and in the absence of vegetation (FC = 7.2, p = 0.01; FN = 4.5, p = 0.04); however, the interaction terms were not significant (FC = 2.4, p = 0.1; FN = 0.9, p = 0.41). Fewer topminnow chases toward mosquitofish occurred at lower topminnow densities (FC = 8.9, p ≤ 0.001) and in the presence of vegetation (FC = 8.2, p ≤ 0.01), and the interaction terms were also significant (FC = 6.8, p ≤ 0.01). There were no differences in the number of nips by topminnows toward mosquitofish, regardless of topminnow density (FN = 1.6, p = 0.22), vegetation (FN = 0.10, p = 0.95), or their interaction (FN = 0.04, p = 0.98).
Figure 3. Mean number (SE) of chases and nips per observation period within and between western mosquitofish (MSQ) and northern starhead topminnows (NST). The direction of aggressive behavior is shown as an arrow (i.e., M → T means MSQ chasing NST). Density treatments included two MSQ and six NST (top), four MSQ and four NST (middle), and six MSQ and two NST (bottom).
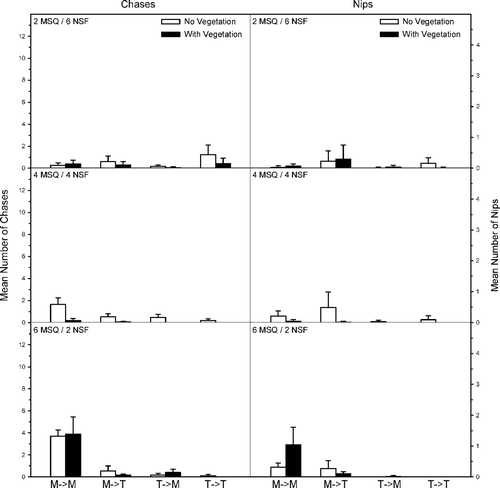
Mosquitofish-topminnow density and the presense-absence of vegetation influenced behavioral interactions between western mosquitofish and northern studfish (). While there was no difference in mosquitofish chases toward conspecifics based on vegetation (FC = 2.0, p = 0.17), significantly more chases occurred at higher mosquitofish densities (FC = 63.5, p ≤ 0.001) and the interaction term was significant (FC = 4.1, p = 0.03). Significantly fewer nips were observed by mosquitofish at lower conspecific densities (FN = 21.6, p ≤ 0.001) and in the presence of vegetation (FN = 5.5, p = 0.02). Their interaction term was also significant (FN = 10.1, p ≤ 0.001). No differences existed in mosquitofish chases and nips toward studfish for mosquitofish density (FC = 0.6, p = 0.54; FN = 0.1, p = 0.87), vegetation (FC = 8.3, p ≤ 0.01; FN = 2.3, p = 0.14), and their interactions (FC = 0.1, p = 0.89; FN = 1.5, p = 0.23). A significantly greater number of chases and nips occurred for studfish toward conspecifics at higher studfish densities (FC = 14.4, p ≤ 0.001; FN = 7.0, p = 0.01) and in the absence of vegetation (FC = 7.6, p ≤ 0.01; FN = 2.9, p = 0.04); however, the interaction terms were not significant (FC = 2.7, p = 0.08; FN = 2.1, p = 0.14). The number of chases and nips toward mosquitofish by studfish did not differ by studfish density (FC = 1.5, p = 0.25; FN = 0.68, p = 0.51) or vegetation (FC = 1.8, p = 0.18; FN = 0.1, p = 0.76). While the interaction term for chases was significant (FC = 6.3, p = 0.01), the interaction term was not significant for nips (FN = 1.3, p = 0.3).
Figure 4. Mean number (SE) of chases and nips per observation period within and between western mosquitofish (MSQ) and northern studfish (NSF). The direction of aggressive behavior is shown as an arrow (i.e., M → T means MSQ chasing NSF). Density treatments included two MSQ and six NSF (top), four MSQ and four NSF (middle), and six MSQ and two NSF (bottom).
Behavioral interactions between western mosquitofish and blackstripe topminnow were influenced by mosquitofish–topminnow density and presence–absence of vegetation (). Chases and nips by mosquitofish toward conspecifics was significantly lower at the lowest mosquitofish density (FC = 63.2, p = 0.01; FN = 15.8, p ≤ 0.001) and in the presence of vegetation (FC = 7.4, p = 0.01; FN = 3.9, p = 0.04); however, the interaction terms were only significant for chases (FC = 8.0, p = 0.001;FN = 2.6, p = 0.09). The number of chases and nips initiated by mosquitofish toward topminnows was not different by fish density (FC = 1.2, p = 0.32; FN = 3.0, p = 0.06), vegetation (FC = 0.48, p = 0.49; FN = 1.8, p = 0.19), and the interaction terms were not significant (FC = 1.8, p = 0.19; FN = 0.1, p = 0.87). The number of chases by topminnows toward conspecifics was significantly lower at lower topminnow densities (FC = 105.0, p ≤ 0.001) and in the presence of vegetation (FC = 15.2, p ≤ 0.001). However, the interaction term was not significant (FC = 1.9, p = 0.17). No differences were detected in the number of intraspecific nips by topminnows by topminnow density (FN = 3.0, p = 0.06), vegetation (FN = 0.67, p = 0.42), and the interaction term (FN = 2.3, p = 0.11). Chases by topminnows toward mosquitofish were significantly fewer at lower topminnow densities (FC = 3.9, p = 0.03) and in the presence of vegetation (FC = 12.5, p = 0.001), but the interaction term was not significant (FC = 1.8, p = 0.18). There were significantly fewer nips by topminnows toward mosquitofish in the presence of vegetation (FN = 8.3, p ≤ 0.01), but there were no differences in the number of nips by topminnow density (FN = 3.1, p = 0.06) and the interaction term was not significant (FN = 1.6, p = 0.22).
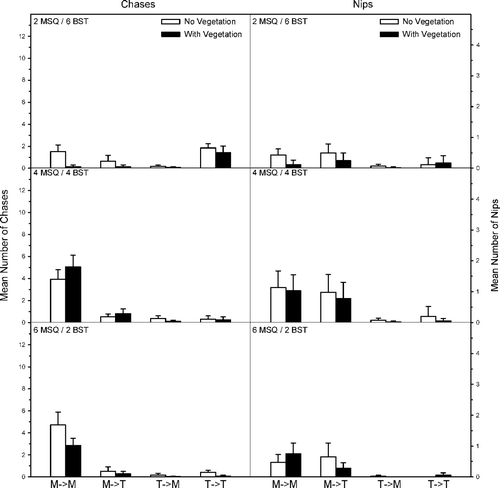
Figure 5. Mean number (SE) of chases and nips per observation period within and between western mosquitofish (MSQ) and blackstripe topminnows (BST). The direction of aggressive behavior is shown as an arrow (i.e., M → T means MSQ chasing BST). Density treatments included two MSQ and six BST (top), four MSQ and four BST (middle), and six MSQ and two BST (bottom).
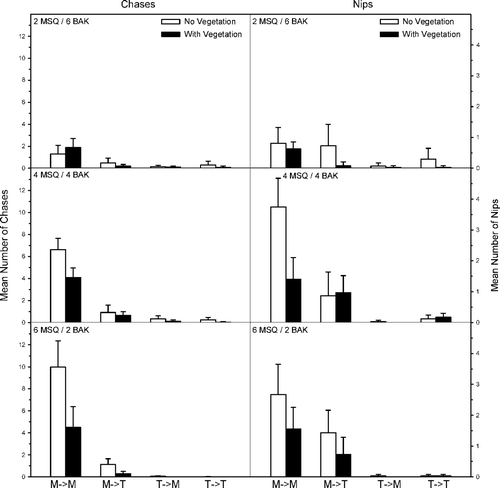
For aquaria containing western mosquitofish and banded killifish, agonistic behaviors were influenced by mosquitofish–killifish density and the presence–absence of vegetation (). There were significantly fewer chases and nips by mosquitofish toward conspecifics at lower mosquitofish densities (FC = 46.0, p ≤ 0.001; FN = 18.9, p ≤ 0.001) and in the presence of vegetation (FC = 30.8, p ≤ 0.001; FN = 21.4, p ≤ 0.001). In both cases, the interaction terms were significant (FC = 14.7, p ≤ 0.001; FN = 5.5, p ≤ 0.01). There were no differences in mosquitofish chases and nips toward killifish by mosquitofish density (FC = 2.6, p = 0.09; FN = 3.0, p = 0.06). Chases and nips were significantly fewer in the presence of vegetation (FC = 9.7, p ≤ 0.01; FN = 4.4, p = 0.04), but the interaction terms were not significant (FC = 2.1, p = 0.13; FN = 1.7, p = 0.2). While there were no differences in the number of chases and nips among killifish conspecifics by killifish density (FC = 2.6, p = 0.09; FN = 1.6, p = 0.22), there were significantly fewer interactions in the presence of vegetation (FC = 5.5, p = 0.02; FN = 2.1, p = 0.04), but no differences in interaction terms (FC = 1.2, p = 0.31; FN = 0.6, p = 0.94). Chases by killifish toward mosquitofish were significantly fewer at lower killifish densities (FC = 4.7, p = 0.02) and in the presence of vegetation (FC = 4.6, p = 0.04), but the interaction term was not significant (FC = 1.2, p = 0.30). There was no significant difference in killifish nips toward mosquitofish among killifish densities (FN = 1.6, p = 0.22), presence–absence of vegetation (FN = 1.6, p = 0.22), or the interaction term (FN = 0.1, p = 0.94).
Figure 6. Mean number (SE) of chases and nips per observation period within and between western mosquitofish (MSQ) and banded killifish (BAK). The direction of aggressive behavior is shown as an arrow (i.e., M → T means MSQ chasing BAK). Density treatments included two MSQ and six BAK (top), four MSQ and four BAK (middle), and six MSQ and two BAK (bottom).
Discussion
Western mosquitofish exhibited more intra and interspecific agonistic behaviors than the four topminnow species and caused a change in topminnow behavior in mixed-species microcosms. Mosquitofish were aggressive toward conspecifics, with most of the chases and nips occurring at the highest mosquitofish densities and when vegetation was absent. Topminnows exhibited few agonistic behaviors toward conspecifics, but intraspecific chasing and nipping did occur in the presence of mosquitofish. Chasing and nipping by topminnows toward mosquitofish occurred infrequently, and mosquitofish initiated almost all chases and nips toward conspecifics and topminnows. While all four topminnows were attacked by mosquitofish, northern starhead topminnows and banded killifish were chased and nipped more frequently than the other killifish species and exhibited the most behavioral changes and fin damage.
Mosquitofish are frequently aggressive toward other fishes and have caused population declines through competition and predation. For example, the Barrens topminnow, Sonoran topminnow, and White Sands pupfish Cyprinodon tularosa all experienced declines in abundance following the introduction of western mosquitofish into aquatic systems (Schoenherr Citation1981; Meffe Citation1985; Rogowski and Stockwell Citation2006; Laha and Mattingly Citation2007). Eastern mosquitofish have also been shown to prey on larval and juvenile sailfin molly P. latipinna in the Florida Everglades (Taylor et al. Citation2001). Predation by eastern mosquitofish caused a shift in size structure for least killifish Heterandria formosa, where juveniles and small males were consumed, leaving only larger females. This change in size structure could reduce reproduction rates and cause population declines (Schaefer et al. Citation1994). Although predation by western mosquitofish on topminnows was not observed in our study, cannibalism on juvenile mosquitofish occurred during experimental trials. While the extent of mosquitofish predation on early life stages of topminnows is unknown, the intensity of agonistic behaviors on adult topminnows that was observed clearly demonstrates their aggressive nature.
The behavior of the four topminnow species in this study was strongly influenced by the presence–absence of western mosquitofish. In microcosms without mosquitofish, topminnows utilized the entire tank, regardless of whether vegetation was present or absent. Conversely, topminnows in aquaria with mosquitofish often sought refuge to escape attacks. When vegetation was not present, fish often remained motionless to avoid attracting the attention of mosquitofish. Similar results have been observed in studies involving other fishes. For example, largemouth bass were unable to locate, and subsequently chased fewer, bluegills at higher vegetation densities (Savino and Stein Citation1989) and greater habitat complexity reduced the frequency of aggressive acts, such as chasing, for zebra fish Danio rerio (Basquill and Grant Citation1998). Although vegetation reduced the number of chases and nips among conspecifics for topminnows, it did not mitigate aggressive acts by mosquitofish as they chased topminnows into and within vegetation and then out into open areas where they were nipped by mosquitofish.
Even when fish are able to successfully utilize vegetation as refugia to minimize direct interactions with an aggressive species, there may be negative consequences to the recipient species. Many fish will remain in vegetation and adapt to resource limitations, which can lead to reductions in growth, development, and reproductive potential (Sih Citation1992). For example, small bluegills restricted to vegetation to escape largemouth bass predation exhibited greater intraspecific competition for food and reduced growth rates (Mittelbach Citation1986). Density-dependent effects on growth may lead to competitive bottlenecks and negatively affect recruitment and abundance (Werner et al. Citation1983). The extent to which topminnows in our study utilize vegetation in the wild to avoid mosquitofish attacks is unclear, but changes in their behavior could cause reduced growth, reproduction, and abundance.
Higher fish densities increase aggression within and among fish species. For example, aggressive intraspecific interactions were positively correlated to fish density for age-0 Atlantic salmon Salmo salar and adult parrotfish Sparisoma viride (Mumby and Wabnitz Citation2002; Blanchet et al. Citation2006). Further, Spanish toothcarp Aphanius iberus were chased and nipped more at higher eastern mosquitofish densities (Rinco et al. Citation2002). Similar behaviors were observed among western mosquitofish conspecifics at higher fish densities in our study, where the greatest number of chases and nips occurred at the highest density of fish. Although topminnows exhibited lower levels of intraspecific aggression, there were greater numbers of chases and nips at higher densities.
Although the extent of fin damage varied on a species-specific basis, more fin damage occurred in mixed-species than single-species aquaria. Many mosquitofish chases toward topminnows ended in a fin nip, and because the caudal fin was closest during the chase, this fin typically received the most damage. Approximately 69% of Sonoran topminnows exhibited caudal fin damage in systems with western mosquitofish, while individuals collected prior to mosquitofish introductions had no fin damage (Meffe Citation1985). Similar results were found in laboratory experiments; within one hour of exposure to mosquitofish, most Sonoran topminnows had damaged fins from attacks, which led to mortality for some individuals (Schoenherr Citation1981; Meffe et al. Citation1983). In our study, one northern starhead topminnow had all its fins bitten off by western mosquitofish and was unable to escape subsequent attacks. Although this was the only topminnow mortality, similar outcomes occurred in pilot studies. In natural systems, fish that receive fin damage may be more susceptible to disease and predation or may be unable to forage effectively, thus leading to mortality from mosquitofish attacks (Laha and Mattingly Citation2007).
The results of behavioral research conducted in a laboratory setting can be difficult to apply to natural systems. However, direct observations in aquatic systems are often not possible due to the presence of aquatic vegetation, poor water clarity, and/or deep-water depths (Dolloff et al. 1996). Because our research required the quantification of chases, nips, and fin damage, behavioral observations of mosquitofish and topminnows in natural systems were not feasible. However, fish behaviors observed in confined laboratory microcosms may not be representative or may exaggerate behaviors in aquatic systems. For example, Martin (1975) concluded that aggressive behaviors exhibited by eastern mosquitofish were more intense and frequent in laboratory microcosms than in open-water areas in a natural system. However, Sutton et al. (Citation2009) documented that northern starhead topminnows were extirpated over a 110-day period from large outdoor mesocosms containing western mosquitofish. We believe that the agonistic behaviors observed in our study also occur in natural systems.
Due to the aggressive agonistic behaviors, that western mosquitofish directed toward topminnows in our study, we do not recommend the stocking of this species into aquatic systems that support native topminnows. Further, western mosquitofish should not be introduced into wetlands, farm ponds, woodland pools, or ornamental ponds where the possibility of escaping into nearby waterways exists. In some cases, western mosquitofish may already inhabit these systems. Therefore, additional research is required to determine the potential impacts of western mosquitofish on topminnows in systems where they co-occur.
Acknowledgments
We would like to thank L. Edenfield, N. Richardson, M. León, R. Cripe, H. Patrick, J. Hoffmeister, A. McAlexander, J. Van Dame, and C. A. Charlton for their assistance in the field and laboratory. The experimental procedures used in this research were approved by the Purdue University Animal Care and Use Committee as protocol 01-058. Fish collection permits were provided by the Indiana Department of Natural Resources (#s 06-0005 and 06-0022). This project was funded through the Indiana Nongame Fund and State Wildlife Grants T-7-R-1 through the Indiana Department of Natural Resources. Additional funding for this research was provided by the American Fisheries Society Hutton Junior Fishery Biologist program.
References
- Abbott , J C and Dill , L M . 1985 . Patterns of aggressive attack in juvenile steelhead trout (Salmo gairdneri) . Canadian Journal of Fisheries and Aquatic Sciences , 42 : 1702 – 1706 . doi: 10.1139/f85-213
- Basquill , S P and Grant , J WA . 1998 . An increase in habitat complexity reduces aggression and monopolization of food by zebra fish (Danio rerio) . Canadian Journal of Zoology , 76 : 770 – 772 .
- Bence , J R . 1988 . Indirect effects and biological control of mosquitoes by mosquitofish . Journal of Applied Ecology , 25 : 505 – 521 . doi: 10.2307/2403840
- Blanchet , S , Dodson , J J and Brosse , S . 2006 . Influence of habitat structure and fish density on Atlantic salmon Salmo salar L. territorial behavior . Journal of Fish Biology , 68 : 951 – 957 . doi: 10.1111/j.0022-1112.2006.00970.x
- Dolloff , A , Kershner , J and Thurow , R . 1996 . “ Underwater observation ” . In Fisheries techniques , 2 , Edited by: Murphy , B R and Willis , D W . 533 – 554 . Bethesda (MD) : American Fisheries Society .
- Everett , R A and Sherfy , M H . 2001 . The Chesapeake Bay: a model for regional approaches to the prevention and control of aquatic non-indigenous species . Transactions of the North American Wildlife and Natural Resources Conference . 2001 , Vol. 66 , pp. 611–624.
- Fuller , P L , Nico , L G and Williams , J D . 1999 . Nonindigenous fishes introduced into inland waters of the United States , Bethesda (MD) : American Fisheries Society .
- Gale , W L , Hill , M S and Zydlewski , G B . 2004 . Physiological and behavioral differences of hatchery and wild-reared steelhead Oncorhynchus mykiss smolts of the same origin . Journal of Fish Biology , 65 : 328 – 329 . doi: 10.1111/j.1095-8649.2004.559ah.x
- Hoy , J B and Reed , D E . 1971 . The efficacy of mosquitofish for control of Culex tarsalis in California rice fields . Mosquito News , 31 : 567 – 572 .
- Krumholz , L A . 1948 . Reproduction in the western mosquitofish (Gambusia affinis) and its use in mosquito control . Ecological Monographs , 18 : 1 – 47 . doi: 10.2307/1948627
- Laha , M and Mattingly , H T . 2007 . Ex situ evaluation of impacts of invasive mosquitofish on the imperiled Barrens topminnow . Environmental Biology of Fishes , 78 : 1 – 11 . doi: 10.1007/s10641-006-9040-5
- Leyse , K E , Lawler , S P and Strange , T . 2004 . Effects of an alien fish, Gambusia affinis, on an endemic California fairy shrimp, Linderiella occidentalis: implications for conservation of diversity in fishless waters . Biological Conservation , 118 : 57 – 65 . doi: 10.1016/j.biocon.2003.07.008
- Li , H W and Moyle , P B . 1999 . “ Management of introduced fishes ” . In Inland fisheries management in North America , 2nd ed , Edited by: Kohler , C C and Hubert , W A . 345 – 374 . Bethesda (MD) : American Fisheries Society .
- Martin , R G . 1975 . Sexual and aggressive behavior, density and social structure in a natural population of mosquitofish, Gambusia affinis holbrooki . Copeia , 3 : 445 – 454 . doi: 10.2307/1443641
- Meffe , G K . 1985 . Predation and species replacement in American southwest fishes: a case study . Southwestern Naturalist , 30 : 173 – 187 . doi: 10.2307/3670732
- Meffe , G K , Hendrickson , D A , Minckley , W L and Rinne , J N . 1983 . Factors resulting in the decline of the endangered Sonoran topminnow Poeciliopsis occidentalis (Atheriniformes: Poeciliidae) in the United States . Biological Conservation , 25 : 135 – 159 . doi: 10.1016/0006-3207(83)90057-5
- Mills , M D , Rader , R B and Belk , M C . 2004 . Complex interactions between native and invasive fish: the simultaneous effects of multiple negative interactions . Oecologia , 141 : 713 – 721 . doi: 10.1007/s00442-004-1695-z
- Mittelbach , G . 1984 . Predation and resource partitioning in two sunfishes (Centrarchidae) . Ecology , 65 : 499 – 513 . doi: 10.2307/1941412
- Mittelbach , G . 1986 . Predator-mediated habitat use: some consequences for species interactions . Environmental Biology of Fishes , 16 : 159 – 169 . doi: 10.1007/BF00005168
- Mumby , P J and Wabnitz , C CC . 2002 . Spatial patterns of aggression, territory size, and harem size in five sympatric Caribbean parrotfish species . Environmental Biology of Fishes , 63 : 265 – 279 . doi: 10.1023/A:1014359403167
- Pyke , G H . 2005 . A review of the biology of Gambusia affinis and G. holbrooki . Reviews in Fish Biology and Fisheries , 15 : 339 – 365 . doi: 10.1007/s11160-006-6394-x
- Rieman , B E , Peterson , J T and Myers , D L . 2006 . Have brook trout (Salvelinus fontinalis) displaced bull trout (Salvelinus confluentus) along longitudinal gradients in central Idaho streams? . Canadian Journal of Fisheries and Aquatic Sciences , 63 : 63 – 78 . doi: 10.1139/f05-206
- Rinco , P A , Correas , A M , Morcillo , F , Risuen , P and Lobon-Cervia , J . 2002 . Interaction between the introduced eastern mosquitofish and two autochthonous Spanish toothcarps . Journal of Fish Biology , 61 : 1560 – 1585 . doi: 10.1111/j.1095-8649.2002.tb02498.x
- Rogowski , D L and Stockwell , C A . 2006 . Assessment of population impacts of exotic species on populations of a threatened species, White Sands pupfish, Cyprinodon tularosa . Biological Invasions , 18 : 79 – 87 . doi: 10.1007/s10530-005-0238-9
- Savino , J F and Stein , R A . 1989 . Behavioral interactions between fish predators and their prey: effects of plant density . Animal Behavior , 37 : 311 – 321 . doi: 10.1016/0003-3472(89)90120-6
- Schaefer , J F , Heulett , S T and Farrell , T M . 1994 . Interactions between two poeciliid fishes (Gambusia holbrooki and Heterandia formosa) and their prey in a Florida marsh . Copeia , 2 : 516 – 520 . doi: 10.2307/1447002
- Schoenherr , A A . 1981 . “ The role of competition in the replacement of native fishes by introduced species ” . In Fishes in North American deserts , Edited by: Naiman , R J and Soltz , D L . 173 – 203 . New York (NY) : John Wiley .
- Sih , A . 1992 . Prey uncertainty and the balancing of antipredator and feeding needs . American Naturalist , 139 : 1052 – 1069 . doi: 10.1086/285372
- Sutton , T M , Zeiber , R A and Fisher , B E . 2009 . Mesocosm evaluation of western mosquitofish impacts on northern starhead topminnows . Proceedings of the Indiana Academy of Sciences . 2009 , Vol. 118 , pp. 88–95.
- Taylor , R C , Trexler , J C and Loftus , W F . 2001 . Separating the effects of intra- and interspecific age-structured interactions in an experimental fish assemblage . Oecologia , 127 : 143 – 152 . doi: 10.1007/s004420000575
- Werner , E E , Gilliam , J F , Hall , D J and Mittelbach , G G . 1983 . An experimental test of the effects of predation risk on habitat use in fish . Ecology , 64 : 1540 – 154 . doi: 10.2307/1937508
- Zar , J H . 1990 . Biostatistical analysis , 4th ed. , Upper Saddle River (NJ) : Prentice Hall .
- Zeiber , R A . 2007. Population-level effects of western mosquitofish on native killifishes and larval amphibians in Indiana waters [MS thesis]. [West Lafayette (IN)]: Purdue University