Abstract
In this study we examined the survival, reproduction, and population size of the aquatic fern Salvinia natans (L.) All. during its expansion in the Vistula Delta (south Baltic Sea coast, northern Poland) with observations every 14 days for five consecutive years at 10 permanent plots in one river. Young sporophytes (genets from gametophytes and ramets resulting from vegetative propagation of sporophytes) appeared in the watercourses of the delta from April to October. The number of genets, which formed mainly in May, was positively correlated with April temperatures (r = 0.93; p = 0.026), and the number of ramets, which were produced in summer, with July temperatures (r = 0.91; p = 0.039). Population abundance in a given year depended mainly on the mortality rate of gametophytes in early spring and the intensity of vegetative reproduction in summer. Two types of vegetative propagation and ramet formation were observed: clone fragmentation and budding along the peripheral parts of the modules forming a clone (4.2 ± 3.7 buds/module). The intensity of each reproduction type was positively correlated with the mean monthly water temperature (r = 0.91 and r = 0.87, respectively).
Introduction
Salvinia natans (L.) All. is a Euro-Siberian and South Asian aquatic fern of the family Salviniaceae (Smith et al. Citation2006) which occurs in areas of temperate, subtropical and tropical climate (Rothmaler et al. Citation1986) in southern, southeastern, and southwestern Asia as well as eastern, southern, central, and southwestern Europe. Most populations of this species are in lowland regions in tributaries of the great Asian rivers, especially in eastern (the Amur, Huang He and Yangtze), southern (the Mekong, Ganges and Indus) and southwestern Asia (the Tigris, Euphrates, Syr Darya and Amu Darya). Outside Europe, Asia and Oceania there are sparse populations in northern Africa and North America (Mitchell Citation1986; Collinson Citation2002). In Europe it is found mainly in the catchments of the Caspian, Black, Adriatic, Mediterranean and Baltic Seas (Casper and Krausch Citation1980).
S. natans is an annual plant that reproduces by sexual and asexual (vegetative) means. The macrospore develops into the female gametophyte and the microspore into the male one. Fertilization takes place on the water surface, producing a zygote which becomes the sporophyte. An adult sporophyte of S. natans has a clonal structure formed in the process of adding new modules arranged radially around the central (oldest) part of the plant. The older the clone, the more complex is its structure. Clones are net-like and consist of modules that grow like lateral branches in vascular plants. Each module was linear in structure and made up of subunits called ramets. Clonal expansion consisted of an increase of the number and size of modules, as well as the number and size of ramets in the modules. The life cycle takes about 130 days from mid-May to late September (Gałka and Szmeja Citation2012).
Found in the Vistula Delta at varying abundance for at least 1500 years, S. natans is an indigenous species there. In the Gdańsk area, close to the western edge of the delta, the plant was sparse in the 5th and 6th centuries and massively abundant in the 7th and 8th centuries. These data come from palaeoecological studies carried out on archaeological sites distributed within the historical area of the city of Gdańsk (Święta-Musznicka et al. Citation2011). Temperatures along the southern coast of the Baltic Sea were higher in the latter than in the former period (Seppä and Poska Citation2004; Sillasoo et al. Citation2009). S. natans probably declined from the 9th century to the late 15th century and was only sporadic or absent during the so-called “Little Ice Age” from the 16th to 18th centuries (Święta-Musznicka et al. Citation2011). Studies by Markowski et al. (Citation2004) and Szmeja et al. (Citation2012) indicate that from the mid-19th century to the end of the 20th the plant was rare in the delta but that after 2005 it grew in every watercourse and formed very numerous populations. The population increase was so rapid that it completely clogged the delta's vast hydrological regulation system, construction of which began in the Middle Ages and was gradually expanded over the next centuries. During 2000–2004 the plant occupied 14 new watercourses in which there is evidence that the species had not occurred there for at least 150 years previously (Gałka and Szmeja Citation2012). The species was observed to expand in other regions of Europe as well, including Baden-Württemberg and Rhineland-Palatinate (Wolff and Schwarzer Citation2005) between 1999 and 2004, at the same time as in the study area.
The expansion of Salvinia in the Vistula Delta seems very likely to be due to climate warming (Szmeja et al. Citation2012) induced by an increase in the activity of the North Atlantic Oscillation (NAO). Since 1989, according to climatologists (e.g., Hurrell Citation1995, Citation1996; Marsz and Styszyńska Citation2010), warm and humid air has been flowing more often from the Atlantic to Northwestern Europe, Scandinavia and the Baltic Sea Region, especially in colder seasons. We think that the warming of these seasons affects the plant's survival rate, reproduction and population size. Here we test this idea and provide data enabling a comparison of S. natans expansion in other regions of Europe located within the area of influence of the NAO.
Methods
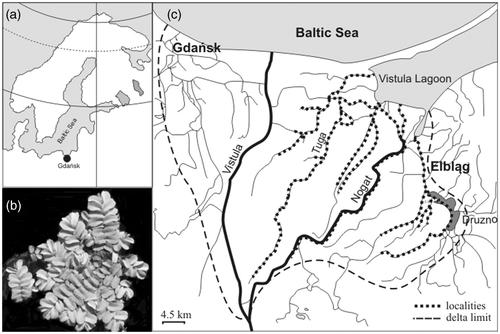
The studies were conducted in the delta of the Vistula River, northern Poland, which flows into the Baltic Sea (). The delta is a wedge with a surface area of 1700 km2, cut by a network of hydrologic regulation ditches and covered with fertile soil used for agriculture. The watercourses of the delta are shallow (2.2 ± 1.5 m), slow-flowing (0.1 ± 0.1 m/s), and rich in nitrogen (mean TN = 4.7 ± 4.2 mg/L) and phosphorus (mean TP = 0.7 ± 0.4 mg/L). The water in them is brackish (mean Cl− = 53.8 ± 21.3 mg/L), moderately calcium-rich (Ca2 + = 97.7 ± 18.5 mg/L), exhibits high conductivity (828 ± 212 µS/cm), and is neutral or alkaline pH (7.2–9.2). These analyses were conducted on river water samples collected in July 2006 from all 17 courses that contained populations of S. natans). The watercourses are for the most part well insolated. They warm up quickly in spring and are often overheated in summer, while in winter only the shallowest ones sometimes freeze to the bottom (Gałka and Szmeja Citation2012).
Figure 1. Location of the study area (•) in the Baltic Sea Region (A), Salvinia natans (B) and outline of the Vistula Delta (C).
From 1989 to 2010 the mean March and April temperature was 1.4°C higher than in the period 1951–1988. The standard deviation (SD) for the March and April temperature was 1.24 in 1989–2010, which was lower than for the same months in 1951–1988 (SD = 1.64). This means that spring in the Delta has been milder since 1989 (Marsz and Styszyńska Citation2010; Szmeja et al. Citation2012). We analyzed the time series of mean monthly air temperature from the stations in Kaliningrad (Königsberg until 1945; WMO Index 26 702) and in Elbląg (WMO Index 12 160). The datasets were obtained from the All-Russian Research Institute on Hydrometeorological Information–World Data Center (for Kaliningrad) and from NOAA Monthly Climatic Data of the World (for Elbląg). The observation data from Kaliningrad cover the period from 1851 to 2009, those from Elbląg begin with 1951 (Szmeja et al. Citation2012).
For five consecutive years (2006–2010) we measured the surface water temperature and counted and observed S. natans individuals every 14 days from April to November at 10 permanent plots (0.5 m × 0.5 m quadrats) established in the River Tuga. The plots were enclosed in wooden frames which projected from the water surface and prevented immigrants from entering. S. natans colonized the plot as a result of rapid divisions of clones. We determined reproduction on the basis of the number of sporophytes formed in the permanent plots in 14-day periods over the entire growing season. The fraction surviving each period was the measure of survival rate.
We estimated the number of macrospores on the water surface over the course of a year in the River Tuga on the basis of counts from 0.5 L water samples collected between March and October for five consecutive years (three samples each month). We estimated the gametophyte survival rate by comparing the cohort size of their late development stage with the early development stage in 24 surface water samples (0.5 L each). The samples were taken outside the permanent plots from April to June for five consecutive years. The early and late gametophyte stages were distinguished on the basis of features given by Schneller (Citation1976).
The population size was estimated based on 0.1 m2, randomly located samples from June to November every 14 days for 5 years. The surface aggregation of S. natans was determined every 14 days based on 30 randomly located samples, each of 0.25 m2. S. natans offspring can be divided into two groups: genets and ramets. The former are transformed gametophytes, while the latter are produced by vegetative propagation of sporophytes. The significance of differences between the analyzed features were determined by F tests, Student-t, Mann–Whitney or Kruskal–Wallis, according to the recommendations of Sokal and Rohlf (Citation1995).
Results
Meteorology
In the study years (2006–2010) the average air temperature in March (−0.9 ± 4.5°C) was lower than in April (7.3 ± 2.3°C; t = −7.21, df = 59, p < 0.001), the temperature in May (13.3 ± 2.9°C) was higher than in April (t = −9.3, df = 59, p < 0.001; ). During that period the years with the highest number of sub-zero March days were 2006 (26) and 2010 (17), followed by 2009 (13), 2008 (12), and 2007 (2). In April the temperature range was higher and narrower (−3.8 to + 25.3°C) and the number of days with ground frost lower (4.4 ± 2.2) than in March.
Table 1. Air temperature in the Vistula Delta (mean ± S.D., minimum–maximum, °C) in 2006–2010, in the phase of gametophyte and young sporophyte domination (March–May) in the Salvinia natans population and, for comparison, the spring, summer, autumn and annual means (from the Polish National Hydrological and Meteorological Service).
Reproduction
After the ice cover on the river melted, usually in late March or early April, the macrospores emerged on the water surface (), germinated and transformed into gametophytes. Following the mild winters of 2007/2008 and 2008/2009, gametophytes appeared at the beginning of April, and sporophytes at the turn of April/May. After the harsh winter of 2009/2010 we found gametophytes as late as mid-May and sporophytes did not appear until the end of May. The date of spore activation and gametophyte formation depended on the water temperature: the warmer the spring, the earlier the spore germination began. Also, the warmer the summer, the more intensive the vegetative propagation became.
Figure 2. Number of macrospores in 0.5 l water samples between March and September 2004–2010 in the Vistula Delta. Data from three samples per month, they represent means ± SE.
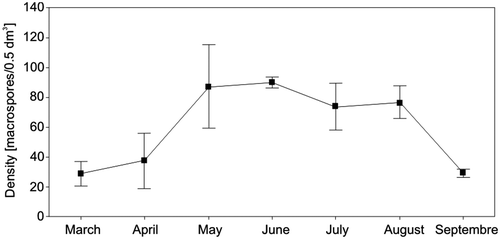
During 2006–2010, recruitment of offspring to the Salvinia population took place from April to October (). In total, 4683 individuals (sporophytes) were formed; 22% were genets and 78% ramets (data from surface water samples). In terms of timing, 28.1% of the offspring appeared in spring (April, May and June) and as much as 70.5% in summer (July and August) and only 1.4% in early autumn (September and October). Recruitment occurred mainly in summer.
Table 2. Number of genet/ramet offspring in the Salvinia natans population between April and October 2006–2010.
In spring, 56.7 ± 15.5% (range 37.2 – 83.3%) of the offspring were genets and 38.4 ± 17.9% (16.7–64%) were ramets (t = 5.6, df = 8, p < 0.001). In summer, 83.3 ± 17.0% (57.0–98.7%) were ramets and only 16.6 ± 16.8% (1.3–43.0%) were genets (t = 2.8, df = 18, p = 0.010). In early autumn, virtually all the offspring were ramets. Most offspring formed in the population over the course of a year were the result of vegetative propagation.
Analysis of the seasonality of formation showed that the number of genets produced in May was positively correlated with April temperature (r = 0.93, p = 0.026, n = 100 samples, 50 from each month), while the number of September ramets was related to July temperature (r = 0.91, p = 0.039, n = 100 samples, 50 from each month). There are two types of vegetative reproduction and ramet formation in S. natans: clone fragmentation and budding along the peripheral parts of the modules forming a clone. During the five years of research, each of these methods of propagation was positively correlated with water temperature (r = 0.91 and r = 0.87, respectively).
Complete disintegration of clones into modules took place within two weeks. Within the population this proceeded for about ten weeks and led to the formation of five module cohorts (generations). Each module is a vegetative offspring (ramet) and grows to become a new clone. Our observations of S. natans in the permanent plots indicate that a mature clone divided into 6.8 ± 4.5 ramets (data for n = 30 clones). One clone, undergoing a five divisions within a year, gave rise to 20–40 ramets (n = 30). To calculate the number of all ramet offspring, those formed by budding should also be added (4.2 ± 3.7 buds produced per clone, n = 30 clones). Five clonal generations formed 15–30 ramets. Together, fragmentation and budding yielded 35–70 ramets from one clone during a year. Fragmentation and budding occurred simultaneously and determined the population size in a given year.
Survival rate
In the Vistula Delta, the survival rate of S. natans depended on the seasonally variable temperature typical of this latitude (). The plant, especially its gametophytes and young sporophytes, was vulnerable to spring ground frost occurring from the end of March to mid-May. March was the coldest and most thermally variable spring month (−15.1 to + 20.1°C, 13.6 ± 8.0 days with ground frost). In April the temperature range was narrower (−3.8 to + 25.3°C) and there were fewer ground frost days than in March, and May was warmer and thermally more stable than April ().
The S. natans gametophytes were tiny and invisible to the naked eye. They were found from the beginning of April to mid-May, that is, during the period with ground frost. The early development stage of the gametophyte lasted from early April (mean water temperature 12.4 ± 0.2°C) to late April and the late development stage occurred in the first half of May (18.3 ± 1.5°C; t = −9.3, df = 59, p < 0.001). The mean water temperature for each of these periods was based on five measurements carried out in the permanent plots. The density of early gametophytes (2522 ± 3327 individuals/0.25 m2) was an order of magnitude higher than that of late ones (437 ± 326 indiv./0.25 m2; t = 2.7, df = 35, p = 0.01), reflecting the very high mortality of early gametophytes. They occurred in the surface water film and died en masse during spring ground frost.
The sporophytes appeared in May, tiny and floating on the water surface. In 2007 and 2008, 432.7 ± 413.4 early juvenile sporophytes grew in the permanent plots in May. By mid-June, however, only 9.6 ± 12.9 late juvenile ones were recorded. This suggests high mortality in the cohorts of early juvenile sporophytes. Observations under the microscope show that freezing air temperatures kills young gametophytes. In May 2007 and 2008 the cause of the mortality was ground frost.
The dominant stages in the population were mature sporophytes in July, and spore-producing ones in August: 95.7% of the mature sporophytes survived from June to the end of July, and 97.6% of the spore-producing ones survived from August to the end of September (Z = 3.74, p < 0.001, n = 39). The senescent stage of S. natans was dominant in the river from mid-October (water temperature 6.7 ± 1.2°C) to the beginning of November (2.0 ± 1.1°C). This is when the clones died back and disappeared. The plant persisted through the winter until April as spores.
Population size
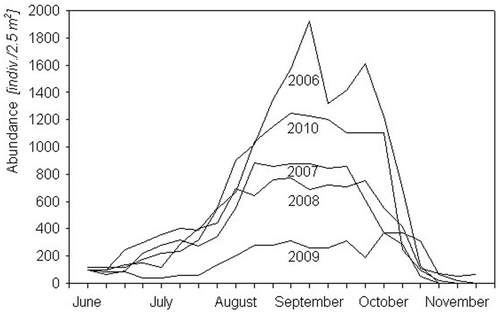
The total number of S. natans in the permanent plots varied from year to year (): 1936 in 2006, 959 in 2007, 865 in 2008, 422 in 2009, and 1124 in 2010. We associate the high numbers in 2006 and 2010 with the higher mean summer temperature in those years (), and consequently more intensive vegetative reproduction of S. natans. The very low number of individuals found in 2009 seems due mainly to the massive emergence of Hydrocharis morsus-ranae and Rhizoclonium sp. (Cladophoraceae) that year. The density of aggregations made up of only S. natans was 91.2 ± 43.7 individuals/0.25 m2, whereas that of S. natans combined with Rhizoclonium was lower (52.8 ± 26.8 individuals/0.25 m2; Z = 1.74, p < 0.001, n = 20), and in aggregations with Hydrocharis morsus-ranae it was 69.8 ± 20.2 individuals/0.25 m2, lower than that of pure aggregations of S. natans (Z = 2.04, p = 0.04, n = 20).
At the beginning of summer (in July), mature individuals dominated the population (10.0 ± 7.0/0.25 m2). They were clonal in structure and reproduced vegetatively; as a result, from mid-August to the end of September, the S. natans aggregations in the river had the largest area (31.3 ± 15.7 m2) and greatest density (29.0 ± 5.0 individuals/0.25 m2; F = 20.6, p = 0.002, n = 15). Plants moved with the river current, were transported by wind, and formed mats floating on the water surface. Mat disintegration began in late September. By the end of October the mats had mostly disintegrated (4.4 ± 2.9 individuals/0.25 m2) and had completely disappeared by the beginning of November when the water temperature dropped to 4.9 ± 1.8°C.
Discussion
Salvinia natans is an annual clonal plant; however, its architecture makes it more similar to perennials than to other annual plants. S. natans reproduces by sexual and asexual means, which is an intrinsic feature of the perennial life strategy (Grime and Mackey Citation2002; de Kroon et al. Citation2005), not the annual one. Low mortality of mature individuals (2.4–4.4%) and vegetative offspring (ramets) is another S. natans characteristic typical of clonal perennials. In August, 70.8% of the ramets survived from the beginning to the end of the month, similar to the survival of evergreen perennials such as Luronium natans (63.4%; Bazydło and Szmeja Citation2004). On the other hand, high seasonal variation of population size is a feature that makes S. natans different from clonal perennials and similar to annuals. The seasonal variability is higher than in aquatic perennials such as isoetids (Szmeja Citation1994a, Citation1994b) or mosses (Riis and Sand-Jensen Citation1997; Ilyashuk Citation2002; Szmeja Citation2010; Szmeja et al. Citation2010). Nevertheless, S. natans life strategy resembles that of perennial, more than annual, plants and this might favor its expansion under current conditions of climate warming.
Low temperatures during spring ground frost, which decimate the gametophytes and young sporophytes, and high temperatures in summer are the main regulators of population size variation in S. natans. High temperature boosted vegetative reproduction by fragmentation and budding. The gametophytes and young sporophytes appeared at low temperatures before other aquatic plants did, giving them unlimited access to light. Similar phenology has been noted in many plant species, for example forest ferns, which begin their development before the trees leaf out (Sawamura et al. Citation2009). In early spring, huge numbers of S. natans gametophytes died (94.2%) despite full insolation and the absence of other accompanying species. The gametophytes occurred in the surface film, which is very susceptible to temperature changes. Ground frost was the main cause of such high mortality of young S. natans in the Vistula Delta (). Mortality among seedlings of Lobelia dortmanna, an aquatic vascular plant occurring at the same latitude as the study location, was 26.4% (Szmeja Citation1994a), much lower than among the S. natans gametophytes.
Temperature is an important regulator of S. natans reproduction. Clone fragmentation and budding along the peripheral parts of clone-forming modules were the basic modes of offspring recruitment to the population. Each of these reproduction types was positively correlated with water temperature; high temperatures in spring and summer favored reproduction and survival and increased the population in the river (, ).
S. natans occupies slow-flowing watercourses (Longhi et al. Citation2008). The slow laminar flow facilitates distribution of phosphorus and nitrogen in the water as well as the uptake of these elements by plants (Howard and Harley Citation1998), including the ammonium form preferred by S. natans (Jampeetong and Brix Citation2009). The expansion of S. natans seems closely associated with the trends in these environmental factors in recent decades (Szmeja et al. Citation2012).
In the Vistula Delta, the combined mean temperature for March and April was +4.6°C in 1951–1988, with high interannual variation (SD = 1.64). During 1989–2010 the mean temperature for March and April rose to +5.7°C, and its interannual variation decreased (SD = 1.24; Szmeja et al. Citation2012). We suggest that the expansion of S. natans in the Vistula Delta was caused by climate warming, which we associate with an increase in the activity of the positive phase of the North Atlantic Oscillation (NAO) in this part of Europe (Hurrell Citation1995, Citation1996; Marsz and Styszyńska Citation2010). The expansion of this species in other European regions such as Silesia (Spałek Citation2008), Baden-Württemberg and Rhineland-Palatinate (Wolff and Schwarzer Citation2005) may have the same explanation. Temperature regulates not only the geographic distribution of aquatic macrophytes (Santamaría Citation2002) and their phenology (Szmeja and Bazydło Citation2005; Gałka and Szmeja Citation2012), but also the survival rate and reproduction of populations, especially in annual and perennial plants that undergo winter diapause (Szmeja Citation2010). Regulators such as grazing, pathogens and herbivores (Keane and Crawley Citation2002; Blumenthal Citation2005; Seastedt Citation2009) play a secondary role in the populations of this plant. The main factor behind the seasonal and long-term changes in S. natans populations to be temperature.
S. natans is an autochthonous component of the local flora. Since 1989 it has not migrated to neighboring areas, the inland hydrological network, or Poland's many lakes. It does not penetrate to the fresher bays of the southern Baltic Sea either, probably due to water salinity and strong wave action there. We think that it is in the first stage of its expansion, limited by the size and density of the local metapopulation. The second stage, if it ever comes, might involve occupation of different areas, as is the case with geographically foreign invasive species (Davis et al. Citation2000; Barrat-Segretain et al. Citation2002; Murphy Citation2002; Lockwood et al. Citation2007).
Conclusions
Our study shows that (1) the population size of the aquatic fern Salvinia natans is a function of gametophyte mortality in the spring and the intensity of vegetative reproduction in the summer; (2) the number of genets is positively correlated with temperature in April, while the ramets – with temperatures in July; (3) climate warming promotes vegetative propagation of S. natans and expansion of this species in the Vistula Delta.
Acknowledgements
We thank our colleagues for discussions and valuable comments on the manuscript. Michael Jacobs edited the manuscript for language. The paper is based on results obtained in work under Project N N304 411 638 funded by the Polish Ministry of Science and Higher Education.
References
- Barrat-Segretain , M H , Elger , A , Sagnes , P and Puijalon , S . 2002 . Comparison of three life-history traits of invasive Elodea canadensis Michx. and Elodea nuttallii (Planch.) H. St. John . Aquatic Botany , 74 : 299 – 313 . doi: 10.1016/S0304-3770(02)00106-7
- Bazydło , E and Szmeja , J . 2004 . The effect of pH, dissolved organic carbon and total phosphorus concentrations on selected life history traits of Luronium natans (L.) Raf . Polish Journal of Ecology , 52 : 191 – 200 .
- Blumenthal , D . 2005 . Interrelated causes of plant invasion . Science , 310 : 243 – 244 . doi: 10.1126/science.1114851
- Casper , S J and Krausch , H D . 1980 . Pteridophyta und Anthophyta , 72 – 74 . Lycopodiaceae bis Orchidaceae. Jena : Gustav Fischer Verlag .
- Collinson , M E . 2002 . The ecology of Cainozoic ferns . Review of Palaeobotany and Palynology , 119 : 51 – 68 . doi: 10.1016/S0034-6667(01)00129-4
- Davis , M A , Grime , J P and Thompson , J . 2000 . Fluctuating resources in plant communities: a general theory of invasibility . Journal of Ecology , 88 : 528 – 534 . doi: 10.1046/j.1365-2745.2000.00473.x
- de Kroon , H , Huber , H , Stuefer , J F and van Groenendael , J M . 2005 . A modular concept of phenotypic plasticity in plants . New Phytologist , 166 : 73 – 82 . doi: 10.1111/j.1469-8137.2004.01310.x
- Gałka , A and Szmeja , J . Forthcoming 2012. Phenology of the aquatic fern Salvinia natans (L.) All. in the Vistula Delta in the context of climate warming. Limnologica
- Grime , J P and Mackey , J ML . 2002 . The role of plasticity in resource capture by plants . Evolutionary Ecology , 16 : 299 – 307 . doi: 10.1023/A:1019640813676
- Howard , G W and Harley , K LS . 1998 . How do floating aquatic weeds affect wetland conservation and development? How can these effects be minimised? . Wetlands Ecology and Management , 5 : 215 – 225 . doi: 10.1023/A:1008209207736
- Hurrell , J W . 1995 . Decadal trends in the North Atlantic Oscillation: Regional temperatures and precipitation . Science , 269 : 676 – 679 . doi: 10.1126/science.269.5224.676
- Hurrell , J W . 1996 . Influence of variations in extratropical wintertime teleconnections on Northern Hemisphere temperature . Geophysical Research Letters , 23 : 665 – 668 . doi: 10.1029/96GL00459
- Ilyashuk , B P . 2002 . Growth and production of aquatic mosses in acidified lakes of Karelia Republic, Russia . Water, Air, and Soil Pollution , 135 : 285 – 290 . doi: 10.1023/A:1014742012441
- Jampeetong , A and Brix , H . 2009 . Nitrogen nutrition of Salvinia natans: Effects of inorganic nitrogen form on growth, morphology, nitrate reductase activity and uptake kinetics of ammonium and nitrate . Aquatic Botany , 90 : 67 – 73 . doi: 10.1016/j.aquabot.2008.06.005
- Keane , R M and Crawley , M J . 2002 . Exotic plant invasions and the enemy release hypothesis . Trends in Ecology and Evolution , 17 : 164 – 170 . doi: 10.1016/S0169-5347(02)02499-0
- Lockwood , J L , Hoopes , M F and Marchetti , M P . 2007 . Invasion ecology , Malden, Oxford, Carlton : Blackwell Publishing .
- Longhi , D , Bartoli , M and Viaroli , P . 2008 . Decomposition of four macrophytes in wetland sediments: Organic mater and nutrient decay and associated benthic processes . Aquatic Botany , 89 : 303 – 310 . doi: 10.1016/j.aquabot.2008.03.004
- Markowski , R , Żółkoś , K and Bloch-Orłowska , J . 2004 . Salvinia natans (L.) All. in Gdańsk Pomerania . Acta Botanica Cassubica , 4 : 187 – 196 .
- Marsz , A and Styszyńska , A . 2010 . “ Changes in sea surface temperature of the South Baltic Sea (1854–2005) ” . In The Polish Climate in the European Context: An Historical Overview , Edited by: Przybylak , R . 355 – 374 . Springer, monograph 18 .
- Mitchell , R S . 1986. A checklist of New York State plants. Contributions of Flora of New York State, Checklist III. New York State Bulletin No. 458
- Murphy , K J . 2002 . Plant communities and plant diversity in softwater lakes of northern Europe . Aquatic Botany , 73 : 287 – 324 . doi: 10.1016/S0304-3770(02)00028-1
- Riis , T and Sand-Jensen , K . 1997 . Growth reconstruction and photosynthesis of aquatic mosses: influence of light, temperature and carbon dioxide at depth . Journal of Ecology , 85 : 359 – 372 . doi: 10.2307/2960508
- Rothmaler , W , Schubert , R and Went , W . 1986 . Excursionsflora für die Gebiete der DDR und der BRD , Bd. 4, Kritischer Band. Berlin : Volk u. . Wissen Volkseigener Verlag
- Santamaría , L . 2002 . Why are most aquatic plants widely distributed? Dispersal, clonal growth and small-scale heterogeneity in a stressful environment . Acta Oecologica , 23 : 137 – 154 . doi: 10.1016/S1146-609X(02)01146-3
- Sawamura , M , Kawakita , A and Kato , M . 2009 . Fern–spore-feeder interaction in temperate forests in Japan: Sporing, phenology and spore-feeding insect community . American Journal of Botany , 96 : 594 – 604 . doi: 10.3732/ajb.0800256
- Schneller , J J . 1976 . The position of the megaprothallus of Salvinia natans . Fern Gaz , 11 : 217 – 219 .
- Seastedt , T . 2009 . Traits of plant invaders . Nature , 459 : 783 – 784 . doi: 10.1038/459783a
- Seppä , H and Poska , A . 2004 . Holocene annual mean temperature changes in Estonia and their relationship to solar insolation and atmospheric circulation patterns . Quaternary Research , 61 : 22 – 31 . doi: 10.1016/j.yqres.2003.08.005
- Sillasoo , Ü , Poska , A , Seppä , H , Blaauw , M and Chambers , F M . 2009 . Linking past cultural developments to palaeoenvironmental changes in Estonia . Vegetation History and Archaeobotany , 18 : 315 – 327 . doi: 10.1007/s00334-008-0210-6
- Smith , A R , Pryer , K M , Schuettpelz , E , Korall , P , Schneider , H and Wolf , P G . 2006 . A classification for extant ferns . Taxon , 55 : 705 – 731 . doi: 10.2307/25065646
- Sokal , R R and Rohlf , F J . 1995 . Biometry: the principles and practice of statistics in biological research. , New York : W.H. Freeman and Co .
- Spałek , K . 2008 . “ Salvinia natans (L.) All. in fishponds and oxbow lakes in Lower and Opole Silesia (SW Poland) ” . In Club mosses, horsetails and ferns in Poland – resources and protection , Edited by: Szczęśniak , E and Gola , E . 147 – 160 . Wroclaw University Press .
- Święta-Musznicka , J , Latałowa , M , Szmeja , J and Badura , M . 2011 . Salvinia natans in medieval wetland deposits in Gdańsk, northern Poland: Evidence for the early medieval climate warming . Journal of Paleolimnology , 45 : 369 – 383 . doi: 10.1007/s10933-011-9505-1
- Szmeja , J . 1994a . An individual's status in populations of isoetid species . Aquatic Botany , 48 : 203 – 224 . doi: 10.1016/0304-3770(94)90016-7
- Szmeja , J . 1994b . Dynamics of the abundance and spatial organisation of isoetids populations in an oligotrophic lake . Aquatic Botany , 49 : 19 – 32 . doi: 10.1016/0304-3770(94)90003-5
- Szmeja , J . 2010 . Changes in the aquatic moss Sphagnum denticulatum Brid. population abundance in a softwater lake over a period of three years . Acta Societatis Botanicorum Poloniae , 79 : 167 – 173 .
- Szmeja , J and Bazydło , E . 2005 . The effect of water conditions on the phenology and age structure of Luronium natans (L.) Raf. populations . Acta Societatis Botanicorum Poloniae , 74 : 253 – 262 .
- Szmeja , J , Bociąg , K and Merdalski , M . 2010 . Effect of light competition with filamentous algae on the population dynamics and development of the moss species Warnstorfia exannulata in a softwater lake . Polish Journal of Ecology , 58 : 221 – 230 .
- Szmeja , J , Gałka , A , Styszyńska , A and Marsz , A . Forthcoming 2012. Is climate change responsible for expansion of the aquatic fern Salvinia natans (L.) All. in the Vistula Delta (south Baltic Sea coast)? Oceanological and Hydrobiological Studies
- Wolff , P and Schwarzer , A . 2005 . Der Schwimmfarn Salvinia natans (L.) All. (Salviniaceae) in der Pfalz . Pollichia , 91 : 83 – 96 .