Abstract
Information regarding nest site selection for bluegill (Lepomis macrochirus) is limited. Therefore, our study identified important characteristics of bluegill nest sites in a shallow, natural lake - West Long Lake, Nebraska, USA, on the Valentine National Wildlife Refuge. Bluegill nest colonies were identified visually from a boat and nine abiotic and seven vegetative variables were measured at both nest colony sites and randomly selected sites (i.e., no active bluegill colonies at random sites). Measurements of six variables differed between nest and random sites, suggesting that these variables may be influential in the nest selection process for bluegill. These variables included submersed macrophyte species, submersed macrophyte coverage, distance to nearest submersed macrophytes, water temperature, distance to shore and maximum southern fetch. In contrast to other studies, nest site substrate composition was not different between nesting sites and random sites. Our results indicate that nesting substrate may not be limiting to bluegill in West Long Lake. Rather, other characteristics (e.g., submersed macrophytes and protection from wind) appear to play a larger role in determining bluegill nest site selection in our study lake.
Introduction
Bluegill (Lepomis macrochirus) often serve a dual role in fisheries, acting as a prey species and a valued sport fish. Owing to the importance of bluegill in many fisheries, several studies have focused specifically on understanding bluegill recruitment with most focusing at the larval or juvenile stages (Miner and Stein Citation1993; Cargnelli and Gross Citation1996; Partridge and Devries Citation1999; Santucci and Wahl Citation2003; Shoup and Wahl Citation2008, Citation2011; Kaemingk et al. Citation2012). These studies have highlighted the importance of ultraviolet light (Olson et al. Citation2006), turbidity (Miner and Stein Citation1993), hatching date (Cargnelli and Gross Citation1996; Garvey et al. Citation2002; Santucci and Wahl Citation2003), predation (Shoup and Wahl Citation2008), prey size (Bremigan and Stein Citation1994) and competition (Kaemingk et al. Citation2012) in the recruitment process for bluegill populations. While this information is vitally important in our understanding of the recruitment process, comparatively less attention has been given to the nest selection process or habitat requirements needed for bluegill prior to reaching the larval or juvenile stages.
Early work on bluegill nest site selection primarily focused on qualitative observations without measuring other environmental factors that may be important in this process (Richardson Citation1913; Coggeshall Citation1924). More recently, Gosch et al. (Citation2006) realized this information gap in our understanding of the bluegill recruitment process and assessed bluegill nest site selection in Lake Cochrane, a small glacial lake in eastern South Dakota. While their study provided an important baseline for examining bluegill reproduction, the shorelines of their study lake were highly developed (e.g., shoreline modification and protection, boat docks), subject to considerable levels of anthropogenic disturbance (Stohr and Redlin Citation2005), and protected from wind due to the natural geologic formation (i.e., depression) of the lake and surrounding trees. Therefore, information on bluegill nest site selection is needed on lakes with minimal anthropogenic disturbance across different geographic regions and lake morphologies to improve our understanding of the recruitment process in bluegill.
The objective of this study was to quantify specific habitat factors associated with bluegill nest site selection within a natural lake in the Nebraska Sandhills. Based on information from previous studies we hypothesized that bluegill would select a sand or gravel substrate and reduced levels of submersed macrophytes (aquatic vegetation) when constructing a nest. However, because physical lake attributes (natural, shallow, shoreline not developed) differed in our study compared to previous studies we were uncertain if these factors would remain important in our study.
Methods
West Long Lake (31 ha) is a shallow (mean depth = 1.3 m) natural lake located within the Valentine National Wildlife Refuge of the Sandhills region in north-central Nebraska, USA. Using the methods outlined by Paukert et al. (Citation2002), total macrophyte coverage observed in West Long Lake during late July of 2010 was 29.4% emergent (dominant taxa included common reed Phragmites australis, softstem bulrush Schoenoplectus tabernaemontani and common cattail Typha latifolia), 58.9% submersed (dominant taxa included clasping-leaf pondweed Potamogeton richardsonii, coontail Ceratophyllum demersum, and star duckweed Lemna trisulca), and 11.7% open water (void of macrophytes). Water transparency is typically high except during excessive sustained wind periods and often the bottom of the lake is visible at maximum depths (2.0 m). The lake watershed is mostly mid- and tall-grass prairie and limited cattle grazing is allowed (Bleed and Flowerday Citation1989). The fish assemblage is relatively simple, comprised of bluegill, yellow perch (Perca flavescens), largemouth bass (Micropterus salmoides), northern pike (Esox lucius), and black bullhead (Ameiurus melas).
All bluegill colonies present within West Long Lake were located by visually inspecting the entire littoral shoreline by boat on 13 June 2011. Each bluegill colony where at least five male bluegills were actively guarding their nests (Gosch et al. Citation2006) and depressions in the substrate were visible (Phelps et al. Citation2009) were marked during the study. Angling was used to identify the species and sex of some of the individuals within the colony (Gosch et al. Citation2006; Phelps et al. Citation2009). At some colonies, no fish were captured but it was readily apparent that fish nesting on the colony were male bluegills as water transparency was high and no other fish species in the lake exhibits this type of behavior during spawning. Upon locating a bluegill colony, a handheld global positioning system (GPS) unit was used to mark the location so habitat variables could be measured at a later date.
Habitat characteristics were measured at the center of each nesting colony on 23 June 2011 using the methods outlined by Pope and Willis (Citation1997). Surface water temperature (°C) and dissolved oxygen (mg L−1) were measured using a portable meter (model HQ30d, Hach Co., Loveland, Colorado, USA). Conductivity (μS cm−1) was recorded using a portable meter (model PCS Testr 35, Oakton Instruments, Vernon Hills, Illinois, USA). Substrate firmness (cm) was determined by placing a 9.0 kg, 4.1-cm diameter metal pole on the lake bottom and measuring the distance it moved into the substrate (Mitzner Citation1987; Gosch et al. Citation2006; Kaemingk et al. Citation2011a). Water depth (m) was also recorded at the center of each bluegill nesting colony. Distance to shore (m), maximum fetch (distance to farthest shore in m), and south fetch (distance to south shore in m) were measured using ArcGIS® software (ESRI Citation2008).
The nearest emergent and submersed macrophyte species were identified and distance (cm) from the colony to the nearest macrophyte species was recorded. Maximum emergent macrophyte height (cm) was recorded within the emergent macrophyte patch nearest to the colony. Submersed macrophyte coverage at each nest site was visually assessed by two independent readers using a 1 m2 quadrat placed in the middle of each colony and given a vegetation coverage score (VC-score, similar to the Braun-Blanquet scale; Murry & Farrell Citation2007) where 0–5% coverage = 1, 5–25% = 2, 25–50% = 3, 50–75% = 4, and 75–100% = 5. In the case where readers did not reach an agreement (< 5% of measurements), scoring was discussed and re-examined until a consensus was reached. Emergent macrophyte coverage was scored using the same method but at the nearest emergent vegetation patch instead of at the middle of the colony.
A substrate core sample was collected near the center of each colony, but not directly in the center of a nest, using a hand corer sampler (5.1 cm diameter × 50.8 cm long; Rickly Hydrological Company, http://www.rickly.com). Core depth for each sample was 8 cm (total volume of substrate per sample = 163 cm3). An 8-cm sampling depth was chosen because bluegill nests (i.e., bowls) have previously been reported to have this depth (Richardson Citation1913; Coggeshall Citation1924). Substrate core samples were brought back to the South Dakota State University campus where they were processed using the methods outlined in Skroch et al. (Citation2006). Substrate types were classified according to particle size: clay (<0.002 mm), silt (0.002 to 0.05 mm), sand (0.05 to 2 mm), and rock (>2 mm). Percent composition of particle size (i.e., inorganic) and organic material was estimated for each sample.
Seventy-five unused (i.e., sites without any evidence of nesting activity) sites were systematically placed (40 m apart) around the perimeter of the lake using ArcGIS® software (ESRI Citation2008) at a depth of 66 cm (average depth at the center of bluegill colonies located in this study). A random number generator selected 25 of the 75 unused sites which were then sampled (on the same day as nest sites) using the same methods described for the nest sites (excluding depth). Differences between random and nest sites were evaluated using a Kolmogorov-Smirnov test (cumulative frequency distributions) or a Kruskal-Wallis test (substrate composition) for continuous variables and a Fisher's exact test for categorical variables. An alpha of 0.10 was used for all statistical comparisons to guard against a Type II error.
Results
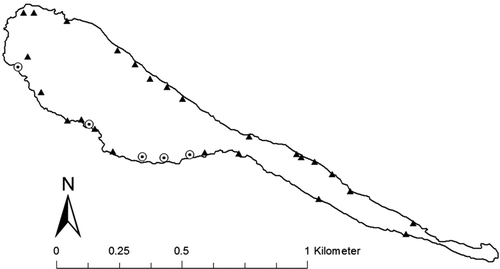
Five bluegill nesting colonies were found in West Long Lake along the southern shoreline (Figure ). Mean depth (±SE) of nest sites was 0.66 m (±0.04) and ranged from 0.52 to 0.75 m. Emergent macrophytes sampled within West Long Lake included arrowhead (Sagittaria spp.), cattail, softstem bulrush, and common reed. Submersed macrophytes sampled included Fries’ or flat-stalk pondweed (Potamogeton friesii), coontail, and star duckweed.
Figure 1. Bluegill nest colonies (dotted circles) and random sites (triangles) sampled within West Long Lake, Nebraska, USA in 2011.
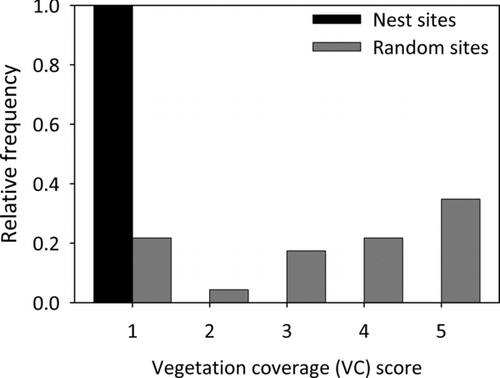
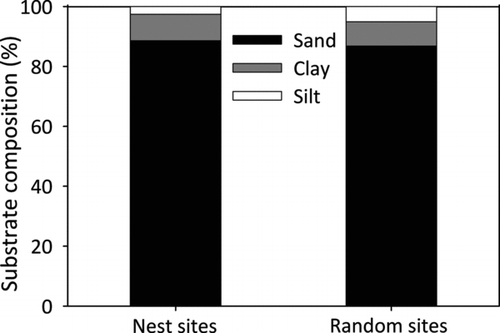
Three of the eight measured abiotic factors significantly differed between random and nest sites (Table ). Water temperature was higher at random sites compared to the nest sites and nest sites were further from shore than random sites. Southern fetch distances were lower at nest sites than at random sites (Table , Figure ). Dissolved oxygen, conductivity, maximum fetch, and substrate firmness were not significantly different between nest and random sites. Nest sites contained 89% sand, 8% clay, 3% silt, and 0% rock; while substrate at random sites was comprised of 86% sand, 10% clay, 4% silt, and 0% rock (Figure ). Organic composition was 2% for nest sites compared to 3% for random sites. Percent substrate composition between nest and random sites was not significantly different across all three primary substrate types: sand (χ 2 = 0.28, df = 1, p = 0.59; Kruskal-Wallis), clay (χ 2 = 0.72, df = 1, p = 0.40; Kruskal-Wallis), and silt (χ 2 = 0.08, df = 1, p = 0.77; Kruskal-Wallis). In addition, organic composition was similar between nest and random sites (χ 2 = 0.17, df = 1, p = 0.68; Kruskal-Wallis).
Continuous variables (mean, standard error, median, test statistic, and p-value) measured at 5 bluegill nest sites and 25 random sites in West Long Lake, Nebraska, USA in June 2011
Figure 2. Percent (%) substrate composition of bluegill nest sites and random sites sampled within West Long Lake, Nebraska, USA in June 2011.
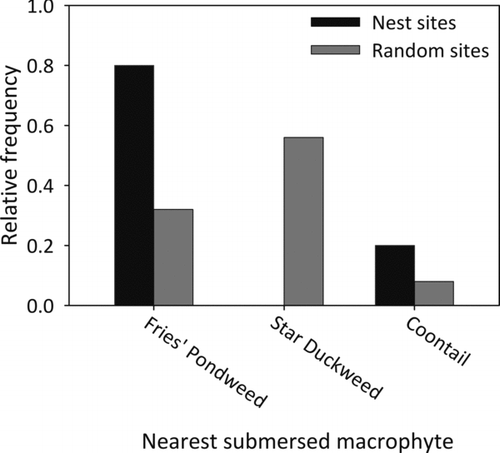
Three of the seven vegetative factors differed significantly between nest and random sites. Nest sites were located further from submersed macrophytes compared to random sites (Table ; Figure ). Submersed macrophyte coverage was greatest at random sites (p = 0.03, Fisher's; Figure ). Most nest sites were located near Fries’ pondweed, whereas random sites were primarily found near star duckweed (p = 0.04, Fisher's; Figure ). Emergent macrophyte height, distance, species (p = 0.60; Fisher's), and coverage (p = 0.81; Fisher's) were not different between nest and random sites (Table ).
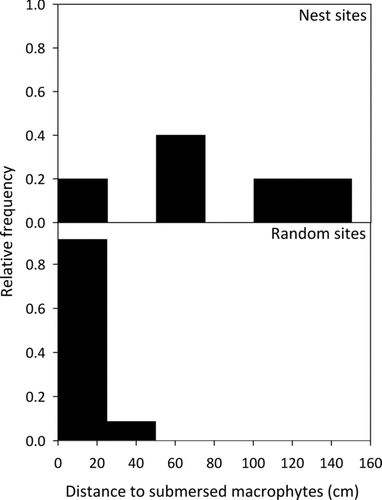
Figure 3. Relative frequency of the distance to nearest submersed macrophytes (cm) from nest sites and random sites within West Long Lake, Nebraska, USA in June 2011.
Discussion
Based on previous studies, we hypothesized that substrate composition would differ between nest and random sites. However, we found no difference between sites because substrate composition was relatively uniform (>80% sand) within West Long Lake. Other studies indicated that bluegills prefer sand or gravel substrate when selecting nest sites (Stevenson et al. Citation1969; Avila Citation1976; Bain and Helfrich Citation1983). Bluegills within Lake Cochrane selected areas characterized by a hard-bottom and gravel substrate (Gosch et al. Citation2006). Bain and Helfrich (Citation1983) also noted that suitable nesting substrate is a primary factor in determining recruitment success of Centrarchids. Therefore, nesting substrate appears to be a primary factor in the nest selection process for bluegills. Because West Long Lake substrate is predominately sand (an optimal substrate) bluegill may not be restricted to certain patches of adequate spawning locations, indicating that other factors may be more important within this lake.
One factor that appeared to be important in the nest selection process for bluegill in West Long Lake was submersed macrophytes. Nest sites were located further from submersed macrophytes and contained less submersed macrophyte coverage than random sites. All random sites (100%) were located less than 0.5 m from submersed macrophytes compared to just 20% of nest sites. Gosch et al. (Citation2006) also found that bluegill nest sites were located in areas with less macrophyte coverage. In addition, Avila (Citation1976) found that bluegills typically nest in areas void of macrophytes. Almost all bluegill nest sites were located near Fries’ pondweed rather than coontail or star duckweed. Star duckweed was found near 60% of the random sites but absent from all nesting sites. One explanation for the difference in macrophyte composition between sites could be related to structural differences between star duckweed and Fries’ pondweed. Star duckweed is typically considered a floating macrophyte species but can also be found submersed (Larson Citation1993), whereas Fries’ pondweed is always submersed. Star duckweed was submersed and found on the bottom of West Long Lake as opposed to floating. Furthermore, star duckweed is not deeply rooted in the substrate and may be easier for bluegills to dislodge compared to rooted macrophytes (i.e., Fries’ pondweed) if bluegill mechanically remove vegetation during nest building. Centrarchids typically construct nests by removing debris and forming a depression using a sweeping motion (Spotte Citation2007) but it is unclear whether bluegills simply selected sites with lower macrophyte coverage or that macrophytes were mechanically removed during nest building. Although submersed macrophytes differed significantly between nest and random sites, variables associated with emergent macrophytes did not significantly differ between nest and random sites. Thus, submersed macrophytes may play a larger role than emergent macrophytes in the nest selection process. Future research is warranted to separate the effects of bluegill nesting behavior and physical characteristics (i.e., submersed macrophytes) of nesting sites.
All bluegill nesting colonies were located on the south shoreline of West Long Lake. Prevailing winds in this region originate from the south (Kaemingk et al. Citation2011b), suggesting that wind protection may be related to bluegill nest site selection. Wind and wave action have been found to negatively affect nests and decrease nesting success of other Centrarchids such as largemouth bass (Kramer and Smith Citation1962) and smallmouth bass Micropterus dolomieu (Goff Citation1986; Steinhart et al. Citation2005). Pope and Willis (Citation1997) also found that black crappie Pomoxis nigromaculatus in South Dakota lakes selected for locations protected from wind and waves. In contrast, Gosch et al. (Citation2006) concluded that wind protection was not influential in the nest selection process for bluegill in Lake Cochrane. West Long Lake is much more shallow than Lake Cochrane (mean depth = 4.0 m) and wind and wave action may have more of an effect within shallower lakes. West Long Lake and Lake Cochrane differ substantially in morphometric and shoreline characteristics, with Lake Cochrane found at a lower elevation compared to the surrounding area and protected by trees, which was not the case at West Long Lake.
Surface water temperature was significantly cooler in nest sites than in random sites. Surface temperature may differ between sites because nest sites were further from submersed aquatic vegetation or because of temporal differences in measurements. However, random and nest sites differed by less than 1.5°C and therefore this difference may not be biologically important. In contrast to the Gosch et al. (Citation2006) study, nest sites in West Long Lake were located further from shore compared to random sites. The south side of West Long Lake contains greater emergent macrophyte coverage (M. Kaemingk, South Dakota State University, unpublished data), extending much further from shore than other areas of the lake, and thus bluegills may need to nest further from shore to avoid nesting in these emergent vegetation patches. However, dissolved oxygen, conductivity, substrate firmness, and maximum fetch were not different between nest and random sites in West Long Lake. Gosch et al. (Citation2006) found differences in dissolved oxygen levels and substrate firmness but no difference in maximum fetch between nesting and random sites.
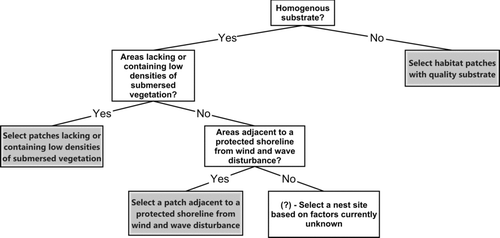
Our results and previous studies suggest that three primary habitat variables may play a pivotal role in nest site selection for bluegills: suitable nesting substrate, reduced levels of submersed macrophytes, and protection from wind and waves. However, some habitat variables may be more or less influential depending on lake characteristics and geographic location. Rejwan et al. (Citation1999) hypothesized that adult smallmouth bass face a hierarchical choice between factors when selecting nest sites. Our results indicate that bluegill may also prioritize among factors when selecting a suitable nest site (Figure ). Most studies indicate the influence of sand or gravel substrate on nest site selection (Stevenson et al. Citation1962; Avila Citation1976; Bain and Helfrich Citation1983; Gosch et al. Citation2006) followed by reduced submersed macrophyte coverage (Avila Citation1976; Gosch et al. Citation2006; this study) and protection from wind and waves (this study). Differences among water bodies may help to explain why some factors appear more important than others in the bluegill nest selection process. For example, we found suitable spawning substrate throughout West Long Lake and all nests were located along the south shoreline (wind and wave protection) whereas Gosch et al. (Citation2006) found nest sites along multiple shorelines in areas with gravel and hard bottom substrate, which were not found throughout the lake. Therefore, bluegills in Lake Cochrane (Gosch et al. Citation2006) appeared to select sites primarily based on substrate with less influence from wind and wave protection. Alternatively, bluegill nesting substrate was similar throughout West Long Lake, which allowed bluegill to nest in areas that provided the most protection from wind and waves. Future studies examining nest site selection for bluegill should test this hierarchical hypothesis (Figure ), which may lead to a more comprehensive understanding of this important process.
Figure 6. A conceptual hierarchical decision tree relating to bluegill nest site selection using results from this study and previous studies. Further information is needed on factors other than substrate, vegetation coverage, and shoreline protection that receive lower priority in the nest selection process.
This study and others suggest that efforts and resources should be directed toward improving or modifying substrate composition, submersed macrophyte dynamics, and protection from wind and waves (potentially in that order). Understanding the factors that affect bluegill nest site selection may lead to enhanced management processes and ultimately a more comprehensive understanding of the recruitment process of bluegill. Our results provide information on bluegill nest site selection in a natural lake with minimal anthropogenic disturbance and ultimately adds to the existing information on the bluegill recruitment process that is necessary for fisheries management.
Acknowledgements
We would like to thank D. Abler, Z. Brashears, M. Lindvall, J. Augspurger, the Nebraska Game and Parks Commission, and the U.S. Fish and Wildlife Service for field data collection assistance. We would like to thank the South Dakota State University Comprehensive Ecology and Fisheries Discussion Group (C.A. Hayer, D. Dembkowski, T. Rapp, J. Breeggemann, and D. Deslauriers), C. Miller and D. Shoup, and two anonymous reviewers for helpful comments during the manuscript review process. We would also like to thank the Jesse West Fisheries Research Endowment associated with Pond Boss magazine for providing funding for this project.
References
- Avila , V L . 1976 . A field study of nesting behavior of male bluegill sunfish (Lepomis macrochirus Rafinesque) . The American Midland Naturalist , 96 ( 1 ) : 195 – 206 . doi: 10.2307/2424577
- Bain , M B and Helfrich , L A . 1983 . Role of male parental care in survival of larval bluegills . Transactions of the American Fisheries Society , 112 ( 1 ) : 47 – 52 . doi: 10.1577/1548-8659(1983)112<47:ROMPCI>2.0.CO;2
- Bleed , A and Flowerday , C . 1989 . “ Introduction ” . In An Atlas of the Sand Hills , Edited by: Bleed , A and Flowerday , C . 1 – 15 . Lincoln , NE : University of Nebraska-Lincoln .
- Bremigan , M T and Stein , R A . 1994 . Gape-dependent larval foraging and zooplankton size: implications for fish recruitment across systems . Canadian Journal of Fisheries and Aquatic Sciences , 51 ( 4 ) : 913 – 967 . doi: 10.1139/f94-090
- Cargnelli , L M and Gross , M R . 1996 . The temporal dimension of fish recruitment: birth date, body size, and size-dependent survival in a sunfish (bluegill: Lepomis macrochirus) . Canadian Journal of Fisheries and Aquatic Sciences , 53 ( 2 ) : 360 – 367 . doi: 10.1139/f95-193
- Coggeshall , L T . 1924 . A study of the productivity and breeding habits of the bluegill, Lepomis pallidus (Mitch) . Proceedings of the Indiana Academy of Science , 33 : 315 – 320 .
- ESRI . 2008 . ArcMap Version 9.3. , Redlands , CA : Environmental Systems Research Institute, Inc. .
- Garvey , J E , Herra , T P and Leggett , W C . 2002 . Protracted reproduction in sunfish: the temporal dimension in fish recruitment revisited . Ecological Applications , 12 ( 1 ) : 194 – 205 . doi: 10.1890/1051-0761(2002)012[0194:PRISTT]2.0.CO;2
- Goff , G P . 1986 . Reproductive success of smallmouth bass in Long Point Bay, Lake Erie . Transactions of the American Fisheries Society , 115 ( 3 ) : 415 – 423 . doi: 10.1577/1548-8659(1986)115<415:RSOMSB>2.0.CO;2
- Gosch , N J , Phelps , Q E and Willis , D W . 2006 . Habitat characteristics at bluegill spawning colonies in a South Dakota glacial lake . Ecology of Freshwater Fish , 15 ( 4 ) : 464 – 469 . doi: 10.1111/j.1600-0633.2006.00178.x
- Kaemingk , M A , Clem , A and Galarowicz , T L . 2011a . The influence of habitat and environment on smallmouth bass (Micropterus dolomieu) nest sites and nest success in northern Lake Michigan . Journal of Great Lakes Research , 37 ( 2 ) : 380 – 385 . doi: 10.1016/j.jglr.2011.03.002
- Kaemingk , M A , Jolley , J C , Willis , D W and Graeb , B DS . 2011b . Exploring spatial distributions of larval yellow perch Perca flavescens, bluegill Lepomis macrochirus and their prey in relation to wind . Journal of Fish Biology , 78 ( 4 ) : 1132 – 1151 . doi: 10.1111/j.1095-8649.2011.02924.x
- Kaemingk , M A , Jolley , J C , Willis , D W and Chipps , S R . 2012 . Priority effects among young-of-the-year fish: reduced growth of bluegill sunfish (Lepomis macrochirus) caused by yellow perch (Perca flavescens) . Freshwater Biology , 57 ( 4 ) : 654 – 665 . doi: 10.1111/j.1365-2427.2011.02728.x
- Kramer , R H and Smith , Jr. LL . 1962 . Formation of year classes in largemouth bass . Transactions of the American Fisheries Society , 91 ( 1 ) : 29 – 41 . doi: 10.1577/1548-8659(1962)91[29:FOYCIL]2.0.CO;2
- Larson , G E . 1993 . Aquatic and wetland vascular plants of the northern Great Plains , Fort Collins (CO): U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. General Technical Report RM-238. .
- Miner , J G and Stein , R A . 1993 . Interactive influence of turbidity and light on larval bluegill (Lepomis macrochirus) foraging . Canadian Journal of Fisheries and Aquatic Sciences , 50 ( 4 ) : 781 – 788 . doi: 10.1139/f93-090
- Mitzner , L R . 1987 . Classification of crappie spawning habitat in Rathbun Lake, Iowa, with reference to temperature, turbidity, substrate and wind , Des Moines (IA) : Iowa Department of Natural Resources. Technical Report 1 .
- Murry , B A and Farrell , J M . 2007 . Quantification of native muskellunge nursery habitat: influence of body size, fish community composition, and vegetation structure . Environmental Biology of Fishes , 79 ( 1-2 ) : 37 – 47 . doi: 10.1007/s10641-006-9133-1
- Olson , M H , Colip , M R , Gerlach , J S and Mitchell , D L . 2006 . Quantifying ultraviolet light mortality risk in bluegill larvae: effects of nest location . Ecological Applications , 16 ( 1 ) : 328 – 338 . doi: 10.1890/05-0287
- Partridge , D G and DeVries , D R . 1999 . Regulation of growth and mortality in larval bluegill: implications for juvenile recruitment . Transactions of the American Fisheries Society , 128 ( 4 ) : 625 – 638 . doi: 10.1577/1548-8659(1999)128<0625:ROGAMI>2.0.CO;2
- Paukert , C P , Willis , D W and Holland , R S . 2002 . Sample size requirements for in situ vegetation and substrate classifications in shallow natural Nebraska lakes . North American Journal of Fisheries Management , 22 ( 4 ) : 1329 – 1333 . doi: 10.1577/1548-8675(2002)022<1329:SSRFIS>2.0.CO;2
- Phelps , Q E , Lohmeyer , A M , Wahl , N C , Zeigler , J M and Whitledge , G W . 2009 . Habitat characteristics of black crappie nest sites in an Illinois impoundment . North American Journal of Fisheries Management , 29 ( 1 ) : 189 – 195 . doi: 10.1577/M07-110
- Pope , K L and Willis , D W . 1997 . Environmental characteristics of black crappie (Pomoxis nigromaculatus) nesting sites in two South Dakota waters . Ecology of Freshwater Fish , 6 ( 4 ) : 183 – 189 . doi: 10.1111/j.1600-0633.1997.tb00161.x
- Rejwan , C , Collins , N C , Brenner , L J , Shuter , B J and Ridgway , M S . 1999 . Tree regression analysis on the nesting habitat of smallmouth bass . Ecology , 80 ( 1 ) : 341 – 348 . doi: 10.1890/0012-9658(1999)080[0341:TRAOTN]2.0.CO;2
- Richardson , R E . 1913 . Observations on the breeding habits of fishes at Havana, Illinois, 1910 and 1911 . Bulletin of the Illinois State Laboratory of Natural History , 9 ( 2 ) : 405 – 416 .
- Santucci , V J and Wahl , D H . 2003 . The effects of growth, predation, and first-winter mortality on recruitment of bluegill cohorts . Transactions of the American Fisheries Society , 132 ( 2 ) : 346 – 360 . doi: 10.1577/1548-8659(2003)132<0346:TEOGPA>2.0.CO;2
- Shoup , D E and Wahl , D H . 2008 . The effect of largemouth bass predation on overwinter survival of two size-classes of age-0 bluegills . Transactions of the American Fisheries Society , 137 ( 4 ) : 1063 – 1071 . doi: 10.1577/T07-038.1
- Shoup , D E and Wahl , D H . 2011 . Body size, food and temperature affect overwinter survival of age-0 bluegills . Transactions of the American Fisheries Society , 140 ( 5 ) : 1298 – 1304 . doi: 10.1080/00028487.2011.621812
- Skroch , K , Hoffman , C , Morris , C , Ulvestad , L and Gelderman , R . 2006 . Soil testing procedures in use at South Dakota State Soil Testing and Plant Analysis Laboratory , Brookings (SD) : South Dakota State University, Plant Science Department. Plant Science Pamphlet 26 .
- Spotte , S. 2007 . Bluegills: biology and behavior , Bethesda, MD : American Fisheries Society .
- Steinhart , G B , Leonard , N J , Stein , R A and Marschall , E A . 2005 . Effects of storms, angling, and nest predation during angling on smallmouth bass (Micropterus dolomieu) nest success . Canadian Journal of Fisheries and Aquatic Sciences , 62 ( 11 ) : 2649 – 2660 . doi: 10.1139/f05-171
- Stevenson , F , Momot , W T and Savoda , I FJ II . 1969 . Nesting success of the bluegill Lepomis macrochirus Rafinesque, in a small Ohio farm pond . The Ohio Journal of Science , 69 ( 6 ) : 347 – 355 .
- Stohr , L and Redlin , E . 2005 . Watershed project final report: Lake Cochrane/Lake Oliver watershed improvement project , Pierre (SD): South Dakota Department of Environment and Natural Resources, Section 319, Nonpoint Source Pollution Control Program. .