Abstract
Despite the limited mobility of freshwater mussels, locomotion, especially burrowing, may be a critical part of their ecology. The effects of temperature on burrowing activities in freshwater mussels have not received much attention. In the laboratory, we studied the effects of three temperatures (ca. 10, 20, and 30°C) on mussel burrowing behaviors and performance. Behaviors assessed include latency to valve opening, latency until the foot becomes extended, and latency until burrowing. We also quantified burrowing performance by measuring burrowing duration. Mussels were significantly more likely to extend their foot and ultimately burrow at the highest experimental temperature. Burrowing performance was not significantly impacted, with burrowing duration being largely unaffected by temperature. This pattern suggests a hypothetical mechanism, whereby if some temperature threshold is reached that allows burrowing, the animal burrows normally. If that threshold is not attained, the mussel will not explore its environment nor burrow. The implications of this work are important to mussel biology and conservation because the thermal regimes of aquatic systems are changing with other global temperatures and smaller-scale effects are common, such as alteration of thermal regimes due to the outflow from dams. If mussels in these systems are affected they are likely, in turn, to affect community and ecosystem ecology in their native habitats.
Introduction
Temperature is arguably the most important factor influencing behavior and performance of ectothermic animals (Huey and Kingsolver Citation1989; Bennett Citation1990; Angilletta et al. Citation2002). In mobile ectotherms such as reptiles, decreases in locomotor performance resulting from low ambient temperatures can oftentimes be mitigated by moving toward and using warmer microhabitats to help maintain higher optimal body temperatures (i.e., behavioral thermoregulation). In aquatic ectotherms with relatively limited movement abilities, such as bivalves, seasonal and daily fluctuations in water temperature play a large role in limiting the physiological mechanisms that underlie both behavior and movement abilities which, in turn, directly impact bivalve ecology and natural history (Huey and Stevenson Citation1979).
The biomechanics of burrowing in bivalves have been well studied (e.g., Trueman Citation1983; Hull et al. Citation1998; Tallqvist Citation2001) and has been shown to be influenced by factors such as substrate grain size (Lewis and Reibel Citation1984; De La Huz et al. Citation2002; Candido and Romero Citation2007). Variation in burrowing behaviors in nature varies with season (Watters et al. Citation2001), flow (Da Maio and Corkum Citation1997), and disturbance (Lewis and Reibel Citation1984). To our knowledge, no study has empirically quantified the thermal influence on burrowing behavior and performance (i.e., latency and rate of burrowing) in freshwater mussels. Allen and Vaughn (Citation2009) suggested that differences in temperature could have played a role in the changes in activity and movement distances they observed in four species of freshwater mussels. As in most other ectotherms, metabolic rates increase with increasing temperature in mussels (Polhill and Dimock Citation1996). Since resting metabolic rates are usually tightly coupled with activity metabolism (Willmer et al. Citation2000), mussels should exhibit enhanced activity and burrowing performance at higher temperatures similar to those observed in other ectothermic animals. However, small increases in temperature (above 33°C) have been shown to decrease survival in many freshwater mussels because of the low thermal tolerance of multiple life stages (Pandolfo et al. Citation2010). Likewise, physiological functions typically increase with increasing temperature; however, some mussel species experience inhibition as ambient temperature approaches the upper limit of thermal tolerance (Spooner and Vaughn Citation2006). Therefore, mussels might display reduced burrowing capabilities at temperatures exceeding their thermal optimum.
We aim to quantify the effects of temperature on burrowing behaviors and performance of the Pink Heelsplitter (Potamilus alatus) below their upper thermal tolerance (< 34°C; Pandolfo et al. Citation2010) to provide insight into how the alteration of thermal regime due to anthropogenic factors (e.g., climate change, changes in land usage by industry) influences mussel behavior. We hypothesize that mussels will be more likely to burrow and burrow more quickly at higher experimental temperatures that are, presumably, closer to the species’ thermal optimum. In addition, we expect that the thermal dependencies of burrowing behaviors and performance will be similar to other ectothermic animals that exhibit larger initial increases in performance as temperature increases and a leveling off at higher temperatures.
Methods
Study animals
Pink Heelsplitters (P. alatus) were hand-collected from various beds of mussels (ranging from 0.5–4 m in depth) in Kentucky Lake near Hancock Biological Station throughout the summer and autumn (Murray, KY, USA). Animals were maintained in mesocosm enclosures at Hancock Biological Station after collection. Each individual collected was tagged by affixing a label containing a uniquely numbered flat Floy shellfish tag (Floy Tag & Mfg. Inc., Seattle, WA, USA) using Loctite® Super Glue Gel (Henkel Corporation, Rocky Hill, CT, USA) near the umbo on the right valve. We recorded mass, length, and width of each individual and placed them in holding tanks containing lake water until trials could be conducted. Thirteen individuals were used in a repeated-measures experimental design in an effort to minimize the number of animals used in the study.
Experimental setup
Burrowing behaviors and performance were assessed by videotaping mussels burrowing in 75.7 L glass aquaria filled with 70 mm of washed sand. The aforementioned aquarium, along with an additional 75.7 L aquarium filled with 70 mm of sand used for acclimating mussels to experimental temperatures, were placed inside one of three 719-L, circulating, artificial streams (Living Stream System®, Frigid Units Inc.) containing both a chiller and heater to control water temperature. The artificial streams, which were located in a greenhouse at Hancock Biological Station, contained a pump that circulated the water at approximately 150 L per minute. Placement of the smaller aquaria within, but not completely submerged in, the artificial stream allowed for a more precise maintenance of a stable water temperature throughout the duration of each trial. Both the experimental and acclimation aquaria were filled with water collected from Kentucky Lake and aerated using an air stone.
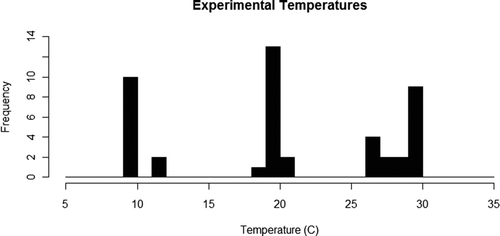
We used data collected as part of the long-term monitoring program on Kentucky Lake to determine an appropriate range of temperatures. Temperatures, monitored using a YSI® sonde (Yellow Springs Instruments, Inc., Yellow Springs, OH, USA) positioned within 1 m of the bottom, ranged between 3.1 and 31.7°C (data available at http://www.murraystate.edu/hbs/; accessed 20 July 2011). Temperatures were monitored at 16 sites that represented the habitat types in the lake and were relatively homogeneous for the last 10 years (unpublished data, T.D. Levine), which indicates that the reported range of temperatures should be similar at the sites from which mussels were collected. Therefore, we examined burrowing behaviors and performance at three temperature treatments. Temperatures within the experimental aquaria throughout the length of each trial varied slightly (± 1–3°C) from the average low (10.3°C), medium (20.1°C), and high (28.9°C) experimental temperatures (Figure ) which we refer to throughout the manuscript as nominally 10, 20, and 30°C.
Figure 1 Histogram showing experimental temperatures used in each trial to assess burrowing performance and behavior in Potamilus alatus
Each of the three experimental setups was used to examine burrowing at all three temperature treatments in different random sequences assigned to each mussel (1 – medium, high, low; 2 – high, low, medium; 3 – low, medium, high). Three setups were used to run trials simultaneously and each mussel was subjected to a randomly selected sequence of temperatures to account for the potential effect of the setup used and order effects. Mussels were placed into a separate acclimation tanks that shared the living stream and thermal conditions with the test aquarium tank for 24 h. After this acclimation period, mussels were moved into the test aquarium within the same artificial stream and the trial was initiated. Burrowing trials were conducted one individual at a time until all trials were complete in a given setup for that particular temperature. Once trials were complete, mussels were placed into an approximately 1200-L holding tub filled with lake water. After each mussel had undergone a trial at their first assigned temperature, we randomly reassigned the temperature of the tanks and ran the second trial for each mussel, after re-acclimating each mussel in each trial. This process was repeated for the third temperature in the sequence.
Burrowing trials
Following acclimation to experimental temperatures, mussels were placed individually in the experimental aquaria and videotaped (Sony® DCR-SX63 Handycam and Sony® Digital8 DCR-TRV460 cameras) for a minimum of 5 h. Video was later analyzed to determine burrowing success versus failure where a burrowing failure was scored if no burrowing behavior was exhibited during the entire 5-h trial. Burrowing behaviors quantified from video analyses included latency to valve opening, latency until the foot became extended, and latency until burrowing, which was defined as when the extended foot made contact with the substrate. We also quantified burrowing performance by measuring the duration of burrowing.
Statistical analyses
All statistical analyses were conducted with R software (version 2.12.1, R Core Development Team). Because a substantial number of individuals failed to complete several burrowing behaviors, we used a logistic regression to determine whether a significant relationship existed between temperature and the likelihood that a mussel would exhibit a burrowing behavior at all. We did this with several steps in the burrowing process, including valve opening, foot extension, and burrowing.
We used generalized linear mixed-effects models available in the lme4 package and used random effects to account for repeated measures on individuals and incorporated the effects of temperature in R (version 3.1-102), to test for changes in latency to burrowing behaviors. This model uses a Wald z-test to calculate z-scores, which are used to determine whether the model has a significant fit to the data. This method should be robust to the unbalanced number of samples in the dataset resulting largely from failures to burrow at some temperatures. We used the same method to test for altered latencies to valve opening, foot extension, and initiation of burrowing. We did the same for the duration of burrowing, a proxy for speed and a performance measure for the amount of time that it took for a mussel to complete a burrowing bout, once started. Events in the burrowing sequence can occur independently from one another; for example, a mussel may wait for a long time to begin burrowing, but may then complete a very rapid burrowing bout. Therefore, we analyzed the each event separately.
Results
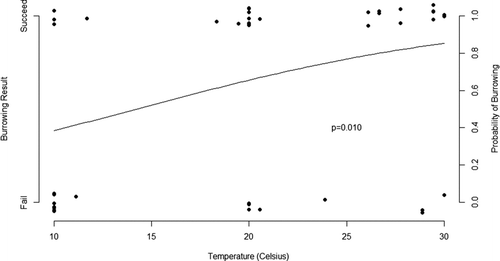
The proportion of individuals that successfully completed behaviors leading to burrowing varied among temperatures. In each case, successful completion of the behavior was more likely in warm water than in cool water. Individuals failed to open their valves 58.3% of the time at 10°C, but only failed to open their valves in 11.7% of trials at 30°C (46 observations, 13 groups, z = 2.621, p = 0.009). Likewise, mussels failed to burrow 66.6% and 23.5% of the time at 10 and 30°C, respectively (46 observations, 13 groups, z = 2.594, p = 0.010). Inversely, fitted probabilities of failure to burrow were greater than 60% at lower experimental temperatures and less than 20% at the highest experimental temperatures (Figure ).
Figure 2 Probability of burrowing (based on burrowing successes and failures) of Potamilus alatus at different temperatures are plotted at the top and bottom of the graph, respectively. Points have been jittered vertically (random variation added), so that overplotted points can be seen clearly. The fitted line represents the relationship between burrowing success and temperature using logistic regression
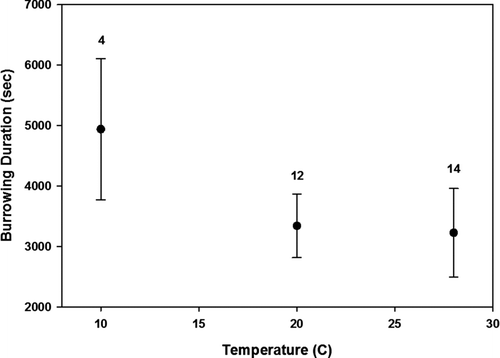
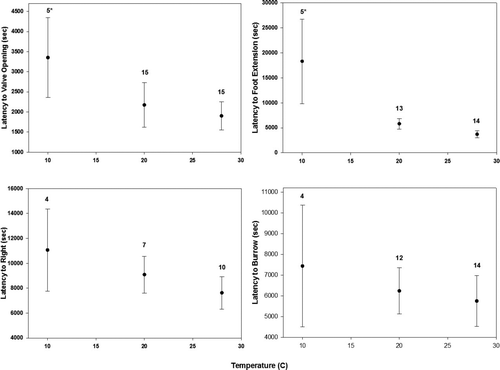
Early behaviors in the burrowing sequence were significantly affected by temperature, while later ones were not. Latency to valve opening was significantly affected by temperature (35 observations, 13 groups, z = −3.14, p < 0.001, Figure ). Similarly, latency to foot extension was significantly affected by temperature (32 observations, 12 groups, z = −3.305, p < 0.001, Figure ). For both of these aforementioned behaviors, latency times were highest at the lowest experimental temperature (p > 0.05) and no significant differences were detected between the medium and highest experimental temperatures. However, latency until mussels righted themselves was not affected by temperature (16 observations, 10 groups, z = −1.34, p = 0.18, Figure ) nor was latency until initiation of burrowing (30 observations, 12 groups, z = −0.961, p = 0.336, Figure ) or duration of the burrowing bout (30 observations, 12 groups, z = −0.429, p = 0.668, Figure ). In some cases, events in the burrowing sequence could not be seen, which resulted in artificially decreased sample sizes for some behaviors.
Figure 3 The influence of the three temperature treatments (nominally 10, 20, and 30°C) on (a) latency to valve opening, (b) latency to foot extension, (c) latency to right, and (d) latency to burrow in Potamilus alatus. Numbers above each temperature group represent the sample size based on the number of individuals that completed the behavior. * = significant differences following pair-wise comparisons at the α = 0.05 level
Discussion
Thermal effects of performance and behavior
We found that temperature strongly affected whether mussels burrowed or not but that rates of burrowing either exhibited a weaker relationship to temperature or none at all. We found that burrowing success, that is, whether a given mussel burrowed before the maximum time (5 h) had elapsed, was strongly dependent upon temperature. Burrowing is often initiated when the foot is extended from the shell and foot extension may constitute an exploratory phase in the sequence of events that lead to a successful burrowing bout. The significant effect of temperature on both foot extension and overall burrowing success suggests that there is something analogous to willingness to burrow that controls the exploratory first steps in burrowing. Thus, because exploration of the environment is a necessary precursor to burrowing, foot extension and burrowing success are closely linked.
Valve opening may be a requisite behavior, because it precedes passage of water over the gills, which is required for both respiration and filter feeding. We found that the time it took to open the valve was significantly higher at lower experimental temperatures. This finding is not surprising considering that both metabolic rate is lower and dissolved oxygen in the water is higher in colder water. Duration of the burrowing bout is a measure of performance, essentially related to speed of burrowing. This is the time that it takes for a mussel to initiate the burrowing process to the time that it settles into a stable and relatively long-term (hours to weeks) position in the substratum. The lack of a significant difference in burrowing duration at different temperatures is somewhat surprising due to the fact that most studies of temperature effects on locomotor performance in ectothermic animals have found a highly significant thermal dependence with performance increasing with temperature (Angilletta et al. Citation2002). This is due to the effects of temperature on the underlying physiological and biochemical mechanisms that determine performance and behavioral capabilities (Bennett Citation1990). Like other performance capacities in animals, burrowing in mussels is likely determined primarily by muscle contraction speed and efficiency and rate of oxygen consumption. Increasing temperature has been shown to cause an increase in heart rate, and hence, an increase in metabolic rate in a number of mollusc species (e.g., Polhill and Dimock Citation1996).
The lack of thermal dependency in burrowing duration could be due to the utilization of mostly glycolytic muscle that quickly transitions to anaerobic energy pathways to fuel bursts of burrowing activity or because the middle and upper temperatures used in this study were close to or within the species’ thermal optima where Q10 values are typically close to 1 (Bennett Citation1990). Most likely, the large amount of inter-individual variation in this study obscured significant relationships between burrowing and temperature. Waller et al. (Citation1999) noted that movements and righting of several unionid species increased in intensity by 10% and 8%, respectively, on average for every 1°C increase in temperature between 7°C and 21°C.
The observation that individuals were less likely to attempt to burrow at lower temperatures, which may be more ecologically relevant, suggests a significant physiological cost of burrowing at lower temperatures despite no relationship being observed between burrowing duration and temperature in the present study. Like other ectothermic animals, we found a decrease in locomotor behaviors expressed with decreasing temperatures that can be compared to an increase in stationary behaviors found in other animals at lower temperatures (e.g., Hertz et al. Citation1982; Gerald Citation2008). Exhibiting stationary behaviors rather than choosing to move at lower body temperatures is advantageous for many mobile animals because remaining motionless is a more effective anti-predator strategy than attempting to flee a predator with a temperature-induced depression in speed and endurance. Freshwater mussels may refrain from burrowing into the substrate following a dislodgement event at lower temperatures because of the reduced efficiency of the underlying physiological mechanisms of burrowing in conjunction with predation risk. However, non-buried mussels are highly vulnerable to high water flow (Imlay Citation1972). Consequently, an interaction between temperature and water velocity could influence burrowing behaviors and performance in complex ways. Further studies are needed to empirically test these hypotheses with freshwater mussels.
Ecological and conservation implications
Freshwater mussels (Unionidae) are filter-feeding bivalves that constitute up to 90% of benthic invertebrate biomass in some areas (Negus Citation1966). Mussels strongly affect invertebrate communities by increasing biomass (Vaughn and Spooner Citation2006) and affecting structural elements, including organic components of the substratum and periphyton (Spooner and Vaughn Citation2006). Consequently, alterations in mussel burrowing behaviors may have significant impacts on the entire freshwater community. Because significant differences in burrowing behaviors exist among temperatures, we expect that environmental changes at both the local and regional scale will affect the behavior of mussels. Feeding (Yeager et al. Citation1994), reproductive (Amyot and Downing Citation1998), and display behaviors (Waller et al. Citation1999) are influenced by an individual's orientation and burrowing ability, which can be altered by changes in water temperature. There are a number of ways the thermal regimes of water bodies can be altered so as to influence mussel burrowing. Anthropogenic causes such as industrial outflows, damming, and management of water can have substantial effects on their thermal regimes and, in turn, on metabolic and vital rates of the organisms in them (Poff and Hart Citation2002). Some of these practices already have documented effects on downstream mussel fauna (Galbraith and Vaughn Citation2011). Alterations of thermal regimes, due to either natural or anthropogenic reasons, are likely to be exacerbated by mechanisms such as mussel burrowing patterns (Caissie Citation2006). Results from the present and previous studies suggest that the ability to burrow into the substrate following displacement due to high water flow or predators can be modified by changes in the thermal regime. Since mussel burrowing results in a re-suspension of sediments which, in turn, re-releases nutrients back into the water column having positive effects on algal communities, burrowing and other life functions of mussels can have substantial impacts on both physical and biotic aspects of aquatic habitats (Spooner and Vaughn Citation2006, Vaughn and Spooner Citation2006). Therefore, altered burrowing patterns may exacerbate anthropogenic changes to these environments.
Conclusions
We found that temperature significantly influenced burrowing behaviors of P. alatus such as probability of burrowing and latency to extend the foot at the beginning of burrowing. Interestingly, we found that temperature had no significant effect on burrowing duration. Lower temperatures resulted in a mussel taking longer to burrow or not burrow at all, which likely results from the reduction in performance capabilities. Anthropogenic alteration of thermal regimes can modify the burrowing behaviors of freshwater mussels that can lead to an increased risk of mortality due to high flow rates or predation. Studies examining burrowing during changes in temperature and flow rate would be beneficial in determining the impact of environmental changes on freshwater mussels.
Acknowledgements
We thank S. Tyler Flynt and Gary Rice for assistance with fieldwork. We also thank Dr. Claire Fuller, Dr. Kerri Wrinn, and Lisette Torres for helpful comments in developing this article. Funding for this project was provided by Hancock Biological Station and Watershed Studies Institute at Murray State University.
References
- Allen , D C and Vaughn , C C . 2009 . Burrowing behavior of freshwater mussels in experimentally manipulated communities . Journal of the North American Benthological Society , 28 : 93 – 100 . doi: 10.1899/07-170.1
- Amyot , J and Downing , J A . 1998 . Locomotion in Elliptio complanata (Mollusca: Unionidae): a reproductive function? . Freshwater Biology , 37 : 345 – 354 .
- Angilletta , M J , Niewiarowski , P H and Navas , C A . 2002 . The evolution of thermal physiology in ectotherms . Journal of Thermal Biology , 27 : 249 – 268 . doi: 10.1016/S0306-4565(01)00094-8
- Bennett , A F . 1990 . Thermal dependence of locomotor capacity . American Journal of Physiology , 28 : R253 – R258 .
- Caissie , D . 2006 . Thermal regime of rivers: a review . Freshwater Biology , 51 ( 8 ) : 1389 – 1406 .
- Candido , L T and Romero , S MB . 2007 . A contribution to the knowledge of the behavior of Anaodontites trapesialis (Bivalvia: Mycetopodidae): the effect of sediment type on burrowing . Belgian Journal of Zoology , 137 : 11 – 16 .
- Da Maio , J and Corkum , L D . 1997 . Patterns of orientation in unionids as a function of rivers with differing hydrological variability . Journal of Molluscan Studies , 63 : 531 – 539 . doi: 10.1093/mollus/63.4.531
- De La Huz , R , Lastra , M and Lopez , J . 2002 . The influence of sediment grain size on burrowing, growth and metabolism of Donax trunculus L. (Bivalvia: Donacidae) . Journal of Sea Research , 47 : 85 – 95 . doi: 10.1016/S1385-1101(02)00108-9
- Galbraith , H S and Vaughn , C C . 2011 . Effects of reservoir management on abundance, condition, parasitism and reproductive traits of downstream mussels . River Research and Applications , 27 : 193 – 201 . doi: 10.1002/rra.1350
- Gerald , G W . 2008 . Feign versus flight: influences of temperature, body size and locomotor abilities on death feigning in neonate snakes . Animal Behavior , 75 : 647 – 654 .
- Hertz , P E , Huey , R B and Nevo , E . 1982 . Fight versus flight: body temperature influences defensive responses of lizards . Animal Behavior , 30 : 676 – 679 .
- Huey , R B and Kingsolver , J G . 1989 . Evolution of thermal sensitivity of ectotherm performance . Trends in Ecology and Evolution , 4 : 131 – 135 . doi: 10.1016/0169-5347(89)90211-5
- Huey , R B and Stevenson , R D . 1979 . Integrating thermal physiology and ecology of ectotherms: a discussionof approaches . American Naturalist , 19 : 357 – 366 .
- Hull , P J , Cole , R G , Creese , R G and Healy , T R . 1998 . An experimental investigation of the burrowing behavior of Paphies australis (Bivalvia: Mesodesmatidae) . Marine and Freshwater Behavior and Physiology , 31 : 167 – 183 . doi: 10.1080/10236249809387071
- Imlay , M J . 1972 . Greater adaptability of freshwater mussels to natural rather than artificial displacement . Nautilus , 86 : 76 – 79 .
- Lewis , J B and Riebel , P N . 1984 . The effect of substrate on burrowing in freshwater mussels (Unionidae) . Canadian Journal of Zoology , 62 : 2023 – 2025 .
- Negus , C L . 1966 . A quantitative study of growth and production of unionid mussels in the River Thames at Reading . Journal of Animal Ecology , 35 : 513 – 532 . doi: 10.2307/2489
- Pandolfo , T J , Cope , W G , Arellano , C , Bringolf , R B , Barnhart , M C and Hammer , E . 2010 . Upper thermal tolerances of early life stages of freshwater mussels . Journal of the North American Benthological Society , 29 : 959 – 969 . doi: 10.1899/09-128.1
- Poff , N L and Hart , D D . 2002 . How dams vary and why it matters for the emerging science of dam removal . BioScience , 52 ( 8 ) : 659 – 668 .
- Polhill , J B and Dimock , R V . 1996 . Effects of temperature and PO2 on the heart rate of juvenile and adult freshwater mussels (Bivalvia: Unionidae) . Comparative Biochemistry and Physiology , 114A : 135 – 141 .
- Spooner , D E and Vaughn , C C . 2006 . Context-dependent effects of freshwater mussels on stream benthic communities . Freshwater Biology , 51 : 1016 – 1024 .
- Tallqvist , M . 2001 . Burrowing behavior of the Baltic clam Macoma balthica: effects of sediment type, hypoxia and predator presence . Marine Ecology Progress Series , 212 : 183 – 191 .
- Trueman , E R , Saleuddin , A SM and Wilbur , K M . 1983 . “ Locomotion in mollusks ” . In Physiology, Part 1 (The Mollusca 4) , 155 – 199 . New York : Academic Press .
- Vaughn , C C and Spooner , D E . 2006 . Unionid mussels influence macroinvertebrate assemblage structure in streams . Journal of the North American Benthological Society , 25 ( 3 ) : 691 – 700 . doi: 10.1899/0887-3593(2006)25[691:UMIMAS]2.0.CO;2
- Waller , D L , Gutreuter , S and Rach , J J . 1999 . Behavioral responses to disturbance in freshwater mussels with implications for conservation and management . Journal of the North American Benthological Society , 18 ( 3 ) : 381 – 390 . doi: 10.2307/1468451
- Watters , G T , O'Dee , S H and Chordas , S . 2001 . Patterns of vertical migration in freshwater mussels (Bivalvia: Unionidae) . Journal of Freshwater Ecology , 16 : 541 – 549 .
- Willmer , P , Stone , G and Johnston , I . 2000 . Environmental physiology of animals , Oxford : Blackwell Science .
- Yeager , M M , Cherry , D S and Neves , R J . 1994 . Feeding and burrowing behaviors of juvenile rainbow mussels Villosa iris (Bivalvia: Unionidae) . Journal of the North American Benthological Society , 13 : 217 – 227 . doi: 10.2307/1467240