Abstract
Variations in hydrolytic activity of six extracellular enzymes were measured in subsurface waters in eutrophic Lake Chełmżyńskie. The ranking of potential activity rates of the assayed enzymes was: lipase > aminopeptidase > phosphatase > α-D-glucosidase > chitinase > β-D-glucosidase. The selected extracellular enzymatic activities were all characterized by a distinct seasonal variability and depended on the location of the research site. In spring and in autumn, the waters of Lake Chełmżyńskie were more eutrophic than in summer. Significant differences in enzyme activity between different parts of the studied lake were demonstrated. In autumn, stations near the town of Chełmża (I–II) were more eutrophic than the stations far from the town; this was supported by the activity of aminopeptidase being higher in the urban area of the lake than in the zone away from town. On the other hand, in spring the activity of aminopeptidase near town (stations II–IV) was lower than far from town (stations VI, VII, X and XI). The enzyme chitinase exhibited higher activity far from town both in summer and autumn.
Introduction
Heterotrophic bacteria play a key role in the cycling and mineralization of organic matter in freshwater ecosystems (Overbeck and Chróst Citation1990; Mudryk and Skórczewski Citation2004). The bulk of organic substances in aquatic ecosystems are macromolecular and, thus, not able to be assimilated by bacterial cells. Prior to being taken up by microbial cells and serving as a source of carbon, nitrogen, and energy, high molecular weight biomolecules must be decomposed by extracellular enzymes into simple compounds (Patel et al. Citation2000; Kuznetsova and Lee Citation2001). Many heterotrophic bacteria are known to synthesize and regulate extracellular enzymes, which can degrade a wide variety of natural polymers to monomers or oligomers (Unanue et al. Citation1999; Kiersztyn et al. Citation2002; Zhang et al. Citation2007). According to the definition given by Priest (Citation1984), extracellular enzymes are generally located outside the cytoplasmic membrane. Chróst (Citation1991) differentiated between enzymes that are still attached to their producers (ectoenzymes) and those that are dissolved in the water or are adsorbed to particles (extracellular). Extracellular enzymes occurring in water basins are produced mainly by bacteria. To a minor extent, they may also originate from autolytic processes and from other taxa like fungi or phytoplankton (Rosenstock and Simon Citation1993; Martinez et al. Citation1996). A variety of ecto- and extracellular enzymes participate in the decomposition of organic matter including peptidase, endo- and exonucleases, 5′- nucleotidase, lipase, α- and β-glucosidases and alkaline phosphatase (Siuda and Chróst Citation2001; Kiersztyn et al. Citation2002; Mudryk and Skórczewski Citation2004). Most of them are inducible enzymes and their synthesis and activity in lake water is regulated by induction/repression mechanisms inside the cells (Chróst and Siuda Citation2002). If the substrate is present in the environment, then low-molecular-weight products accumulate to a certain level, enter the cell and serve as inducers. The ectoenzyme may have several inducing compounds (Hollibaugh and Azam Citation1983). It is well documented that synthesis of many ectoenzymes produced by aquatic microorganisms is repressed by the end product that accumulates in the cell or in the surrounding environment (Kiersztyn et al. Citation2002). Their activity is also strongly affected by various physico-chemical factors in the environment. Over the past three decades, several artificial substrates that release fluorescent compounds after enzymatic hydrolysis have been used in aquatic microbial ecology to estimate extracellular enzyme activities (Hoppe Citation1983; Chróst and Gajewski Citation1995; Vrba et al. Citation1997; Mudryk and Podgórska Citation2006). They allow for the measurement of in situ microbial extracellular enzymatic activities in freshwater and marine ecosystems in a direct, simple and rapid way. For each class of hydrolytic enzyme, artificial substrates are available in which a monomer is covalently linked to a fluorescent molecule such as 4-methylumbelliferone (MUF) or 7-amino-4-methylcoumarin (MCA). Enzyme activity generates a fluorescent product from the nonfluorescent substrate. The ecological significance of these methods in aquatic environments has been under discussion (Chróst Citation1991; Vrba Citation1992). However, knowledge of the mechanisms of organic matter colonization and enzymatic degradation by bacteria is still fragmentary and inadequate.
The main purpose of this experiment was to analyze enzyme activity in the water column of eutrophic Chełmżyńskie Lake and to examine the role of ecto- and extracellular enzymes in organic matter decomposition and utilization. The lake is situated near the town of Chełmża and arable lands represent a major part of its catchment area. We measured and compared activities of the six ecto- and extracellular enzymes at five stations near the town and five stations far from town.
Methods
Study area
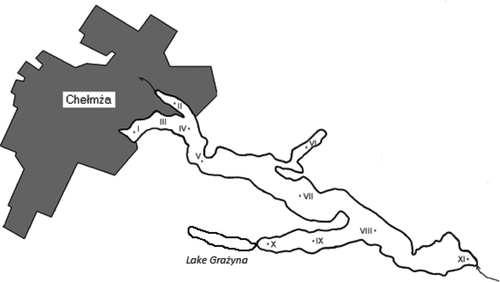
Chełmżyńskie Lake is a eutrophic water body situated in central Poland and belongs to the Fryba-Vistula River basin (Figure ). Table presents the most important morphometric and trophic data pertaining to the lake. The watershed of the lake primarily includes arable lands, which constitute 72% of the immediate watershed. The urbanized areas of Chełmża are located along the northwest side of the lake. Lake Chełmżyńskie is eutrophic mainly because of terrestrial runoff from its agrarian surroundings and from the town of Chełmża, whose runoff is discharged to the lake through a sewage system. There are five resorts, two campgrounds, and one agrotourism farm located on the lakeshore. Summer housing development is undergoing strong growth. Both the proximity of the town and the watershed land use have had negative impacts on the water quality of the lake.
Table 1 Morphometric and trophic characteristics of Chełmżyńskie Lake (Regional Inspectorate for Environmental Protection, 2001)
Sampling
At each of the 11 sampling sites, water samples (from the euphotic zone, depth of 15–20 cm) were taken three times in 2005: spring (2 May), summer (15 July) and autumn (18 October). Phytoplankton production and bacterial utilization of organic matter occur simultaneously and both processes are tightly coupled in the euphotic zone of lakes (Chróst and Siuda Citation2002). Five of the sampling sites were located near the town of Chełmża and six were located far from the town and upstream of it (Figure ). The water was collected using sterile pipettes and poured into sterile glass bottles. The samples were transported to the laboratory in an icebox, whose temperature did not exceed 4°C, until they were used for analysis. The time between sample collection and enzymatic analyses usually did not exceed 2–3 h.
Estimation of enzyme activity
Qualitative measurements of microbial extracellular potential enzyme activities in lake water samples were carried out using fluorescently labeled model substrates (Hoppe Citation1983). In order to determine the activity spectrum of the following hydrolytic enzymes (α-D-glucosidase (EC 3.2.1.20), β-D-glucosidase (EC 3.2.2.21), chitinase (EC 3.2.1.30), phosphatase (EC 3.1.3.1-2) lipase (EC 3.1.1.3)), the following methyl-umbelliferyl (MUF) substrates were used: MUF-α-D-glucoside, MUF-β-D-glucoside, MUF-N-acetyl- β-D-glucosaminide, MUF-phosphate and MUF-butyrate. In addition, the activity of aminopeptidase (EC 3.4.1.1) was determined using an L-amino-4-methylcoumarin substrate, MCA-leucine (L-leucine-4 methylcoumarinyl-7 amide). All MUF and MCA substrates were purchased from Sigma Aldrich Company, LLC (USA). Stock solutions of MUF and MCA substrates were dissolved in methylcellosolve™ (ethyleneglycomonomethylether, EGME, Sigma Aldrich Co., USA, http://www.sigmaaldrich.com) to a concentration of 2 mmol L−1 and then stored at −20°C.
Prior to carrying out the experiment, stock solutions were thawed and diluted in sterile deionized water. The enzyme activity was measured in duplicate 4 mL samples with 0.5 mL of a model fluorogenic substrate solution supplemented with 0.5 mL of Tris-HCl buffer solution (10 mmol L−1 final concentration, pH 7.6) to control the pH. The final concentration of substrate was 20 μmol L−1. The final concentration of added substrate was based on the saturation curve. Samples were mixed and incubated for 3–5 h at the in situ temperature in the dark. Blanks were inactivated with HgCl2 (100 μL) prior to the start of the assay and processed in parallel. Incubations were stopped by adding 100 μL of HgCl2. Before the measurement of fluorescence, 100 μL of alkaline solution (2 M NaOH + 0.4 M EDTA) was added to the tubes to convert MUF to its more fluorescent anionic form (Rulík and Spáčil Citation1999). The fluorescence was measured using the Hitachi F 2500 spectrofluorometer (F-2500, Hitachi High Technologies America, Inc., USA; http://hitachi-hta.com/). Excitation/emission wavelengths were centered on 318/445 nm for MUF and 345/425 for MCA, the optimum wavelengths according to Skórczewski et al. (Citation1999). The fluorescence units were converted into moles of substrate released per L per unit time using an internal standard curve (Martinez and Azam Citation1992; Mallet and Debroas Citation1999). Before each experiment the procedure was calibrated by fluorescence reading of MUF and MCA standard solutions (0.1–20 μmol).
Statistical analyses
Statistical analyses were conducted using STATISTICA 6.0. The main analytical method was analyses of variance (ANOVA), in which we compared the two independent factors influencing enzymatic activities in lake water (season of the year and site location) followed by Tukey's honestly significant difference (HSD) test.
Results
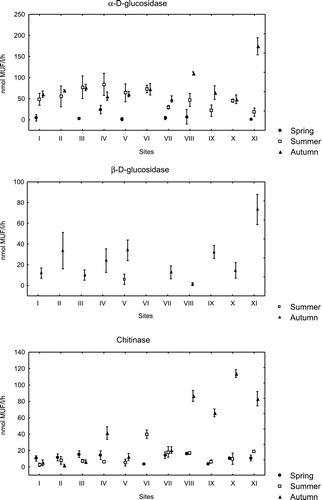
The microbial hydrolytic potential activities of six enzymes measured in Lake Chełmżyńskie are summarized in Table . It follows from the tests carried out that the enzyme lipase had the highest levels of potential activity. Aminopeptidase and phosphatase were also fairly active, although their activity was about four to ten times lower than the activity of lipase. Chitinase and β-D-glucosidase had the lowest levels of potential activity. In spring and in summer (except site V), α-D-glucosidase had values that were below detection. The ranking of the activity rates of the assayed enzymes was usually: lipase > aminopeptidase > phosphatase > α-D-glucosidase > chitinase > β-D-glucosidase.
Table 2 Range, mean and standard deviation (SD) of selected extracellular enzymatic activities (nmol L−1 h−1) in the water of Lake Chełmżyńskie during spring, summer and autumn at 11 stations
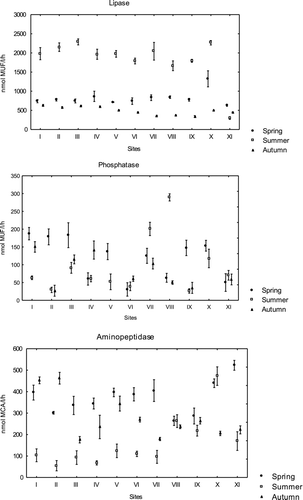
The selected extracellular enzymatic activities were all characterized by a distinct seasonal variability and depended on the location of the research site (two-way ANOVAs, all p < 0.001, Table ). The enzymes lipase and aminopeptidase showed the highest activity in spring and in summer at site X situated far from town (Figure ). This site differed statistically from the rest of the sites (p < 0.001). In autumn, the activities of lipase and aminopeptidase were highest at the sites I, II and IV (near town), which differed significantly from other sites. The enzyme phosphatase showed the highest activity near the town at sites I–III (p < 0.001) in spring and in autumn (Figure ). In autumn at the sites V and X, the enzyme phosphatase was not measurable and sites V and X, as well as sites II and IX (with low phosphatase activity) differed statistically from the rest of the sites (p < 0.001). In summer, the activity of phosphatase was higher far from town than near town and sites VII and VIII differed from the rest of the sites (p < 0.001).
Table 3 Results of analysis of variance (two-way ANOVA) of extracellular enzymatic activities according to the season of the year and location of the sampling sites
Figure 2 Mean activity of lipase, phosphatase (nmol MUF L−1 h−1) and aminopeptidase (nmol MCA L−1 h−1) in water of Lake Chełmżyńskie in three seasons at 11 different sampling sites (I–XI). Error bars represent 1 SD above and below the mean
The enzyme chitinase exhibited higher activity far from town both in summer and in autumn (Figure ). Sites VI, with the highest chitinase activity in summer, and X, with the highest chitinase activity in autumn, differed from the rest of the sites (p < 0.001). In autumn, the activity of chitinase was relatively high at the sites VII, IX and XI and these sites also differed significantly from the rest of the sites. In summer, α-D-glucosidase had the higher activity near the town at sites III and IV, which differed from sites VII–XI (Figure ). In autumn, the activity of α-D-glucosidase was highest at the sites VIII and XI far from the town and in spring there were no statistically important differences among the sites of the lake. Activity of β-D-glucosidase in spring and summer (except site V) was not measurable and in autumn the highest level of activity of β-D-glucosidase was detected at the site XI that differed from the rest of the sites (p < 0.001). In autumn, relatively high level of β-D-glucosidase activity was measured at site II near town and at sites V and IX far from the town, which differed from the rest of the sites (p < 0.001).
Discussion
The rate of decomposition of organic macromolecules in surface waters is usually determined not only by the number of bacteria capable of carrying out those processes but also by the level of activity of their extracellular hydrolytic enzymes (Chróst and Siuda Citation2002; Hoppe et al. Citation2002; Hakulinen et al. Citation2005) and the quantity and quality of organic compounds (Yiyong et al. Citation2002; Mudryk and Podgórska Citation2006). Lipids are products of photosynthesis of many species of diatoms and other microalgae and are widely distributed in aquatic environments (Reemtsma et al. Citation1990). Freshwater invertebrates are another important source of fatty acids and lipids in lake water. The content of lipids in zooplankton is strictly dependent on the concentration and composition of edible phytoplankton. Cyanobacteria (Anabaena spp., Oscillatoria spp.) contain markedly lower lipid concentrations than diatoms. Among extracellular enzyme activities assayed in Lake Chełmżyńskie, lipase had the highest potential activity level. Lipolytic enzymes are actively exported by living microorganisms or are released as free enzymes after the lysis of their cells (Chróst Citation1991). Many researchers (Mudryk Citation1998; Mudryk and Skórczewski Citation2004) have drawn attention to the high activity of lipolytic enzymes in surface water. In Lake Chełmżyńskie, we found the highest values of lipase activity in summer. According to Wainman and Lean (Citation1990), the content of lipids in zooplankton increases in late summer and autumn when edible algae (diatoms, green algae and chrysophytes) dominate the phytoplankton community of lake ecosystems. Particularly high concentrations of lipids in water are observed shortly after the decay of phytoplankton blooms (Nagata and Kirchman Citation1992). Algal blooms in Lake Chełmżyńskie last from spring to late summer; thus, the high activity of lipolytic enzymes observed in summer is understandable.
Proteins (aminopeptidase substrates) are one of the most common nitrogen-rich constituents of organic matter. They are also the most important and easily utilized nitrogen, carbon and energy sources for aquatic microheterotrophs (Rosenstock and Simon Citation1993; Kroer et al. Citation1994). They are hydrolyzed by extracellular peptidases into monomers or oligomers, namely, amino acids and peptides (Ainsworth and Goulder Citation2000). In this form, they can be actively assimilated by bacteria and used in respiratory processes or in biosynthesis of cellular structures.
Aminopeptidase is produced constitutively by bacteria (Laurent and Servais Citation1995) and its activity is tightly related to bacterial number and to some extent is proportional to bacterial biomass (Laurent and Servais Citation1995; Siuda and Chróst Citation2002). Aminopeptidase activity in water basins depends to a great extent on the concentration of proteins and increases with the degree of eutrophication of the lake. Many researchers (Thompson and Sisabaugh Citation2000; Siuda and Chróst Citation2002; Mudryk and Skórczewski Citation2004) have reported high activities of aminopeptidase in highly eutrophic water basins. This is probably the reason why a high potential activity of aminopeptidase was also noted in Lake Chełmżyńskie. The high activity of the enzyme in spring and in autumn is closely connected with algal blooms and the high content of proteins in the lake in May and October.
In Lake Chełmżyńskie, in addition to the activities of lipases and proteases, the potential activity of phosphatases was also high. Extracellular phosphatases could be important for the supply of phosphorus to heterotrophic and autotrophic microorganisms. Phytoplankton and bacteria can release significant quantities of phosphatases into the water. These enzymes occur in two forms: alkaline and acid (Münster and Chróst Citation1990; Hoppe et al. Citation2002). Since the pH of lakes is often alkaline (pH 7.2–9.5), phosphatases that exhibit their maximal activities in acid water probably are of only minor importance. Phosphate is the most important regulator of the synthesis and activity of bacterial phosphatases; high phosphatase activities in surface waters coincide with high phosphate concentrations (Hoppe et al. Citation2002). As the phosphate concentration in Lake Chełmżyskie is fairly high (0.030–0.099 mg P L−1, Regional Inspectorate for Environmental Protection in Bydgoszcz Citation2001), high potential phosphatase activity was also noted (the highest value of phosphatase activity in the lake was noted in summer far from town). Other researchers have reported phosphatase decreases with increasing phosphorus availability, depending on the nitrogen:phosphorus ratio (Chróst and Siuda Citation2002; Mackey et al. Citation2007).
The enzymes α-D-glucosidase, chitinase and, in particular, β-D-glucosidase exhibited low levels of potential activity as compared with other enzymes tested in the lake. This is surprising in view of high concentrations of polysaccharides in aquatic ecosystems (Benner et al. Citation1992). According to some researchers (Tholosan et al. Citation1999; Unanue et al. Citation1999; Arrieta and Herndl Citation2002), kinetic studies of enzyme activity in aquatic systems often reveal biphasic or multiphasic enzyme kinetics, suggesting the co-existence of different β-glucosidases. Thus, the fluorogenic substrates used to measure the activities of those enzymes are probably inappropriate, and the activities are actually much higher than those measured. Such a possibility was indicated by Martinez et al. (Citation1996). On the other hand, results of many other studies (Brown and Goulder 1996; Mudryk and Skórczewski Citation2004) also indicate low activity of these enzymes in water basins. Rulík and Spáčil (Citation1999) examined activity of α-glucosidase and β-glucosidase in surface water, interstitial water, and bed sediments of a small lowland stream. They observed a very low level of hydrolytic activity in the water column and interstitial water, as compared with the gravel-sand sediment.
Several reports point out that microbial enzymatic activities are closely related to the indices of water eutrophication and/or the trophic status of aquatic ecosystems (Laurent and Servais Citation1995; Siuda and Chróst Citation2002; Chróst and Siuda Citation2002). According to Siuda and Chróst (Citation2002), selected enzymatic microbial activities (i.e. activity of alkaline phosphatase, esterase and especially aminopeptidase) are very practical for rapid recognition of the current trophic status of lakes. Activities of these enzymes increase exponentially along a trophic gradient and correlate significantly with the trophic state index of lakes. Based on this assumption, we can conclude that in spring and in autumn the waters of Lake Chełmżyńskie were more eutrophic than in summer. In autumn, stations near the town of Chełmża (I–II) were more eutrophic than the stations far from town since the activity of aminopeptidase was higher in the urban area of the lake than in the zone away from the town. On the other hand, in spring the activity of aminopeptidase near town (stations II–IV) were lower than far from town (stations VI, VII, X and XI). Cauchie (Citation2002) reported that arthropods are one of the main chitin producers in the hydrosphere. Similarly, the surface layer of the body of terrestrial insects contains significant amounts of chitin (Tellam et al. Citation2000). These organisms provide significant amounts of chitin to the soil that can get into the lake through runoff. This study shows that the highest activity of the enzymes lipase and aminopeptidase in spring and in summer and the enzyme chitinase in autumn was detected at site X. This station is supplied with eutrophic waters from small Lake Grażyna connected directly to Lake Chełmżyńskie.
Notes
bd = Below detection.
F = among-groups variance/within-groups variance; p = significance.
References
- Ainsworth , A M and Goulder , R . 2000 . The effects of sewage-works effluent on riverine extracellular aminopeptidase activity and microbial leucine assimilation . Water Research , 34 : 2551 – 2562 . doi: 10.1016/S0043-1354(99)00415-7
- Arrieta , J M and Herndl , G J . 2002 . Changes in bacterial β-glucosidase diversity during a coastal phytoplankton bloom . Limnology and Oceanography , 47 : 594 – 599 . doi: 10.4319/lo.2002.47.2.0594
- Benner , R , Pakulski , J D and McCarty , M . 1992 . Bulk chemical characteristics of dissolved organic matter in the ocean . Science , 255 : 1561 – 1564 . doi: 10.1126/science.255.5051.1561
- Cauchie , H M . 2002 . Chitin production by arthropods in the hydrosphere . Hydrobiology , 470 : 63 – 95 . doi: 10.1023/A:1015615819301
- Chróst , R J . 1991 . “ Environmental control of the synthesis and activity of aquatic microbial ectoenzymes ” . In Microbial enzymes in aquatic environments , Edited by: Chróst , R J . 29 – 50 . New York : Springer-Verlag .
- Chróst , R J and Gajewski , A . 1995 . Microbial utilization of lipids in lake water . FEMS Microbiology Ecology , 18 : 45 – 50 . doi: 10.1016/0168-6496(95)00039-D
- Chróst , R J and Siuda , W . 2002 . “ Ecology of microbial enzymes in lake ecosystems ” . In Enzymes in the environment: activity, ecology, and applications , Edited by: Burns , R G and Dick , R P . 39 – 85 . New York : Marcel Dekker .
- Hakulinen , R , Kähkönen , M and Salkinoja-Salonen , M . 2005 . Vertical distribution of sediment enzyme activities involved in the cycling of carbon, nitrogen, phosphorus and sulphur in three boreal rural lakes . Water Research , 39 : 2319 – 2326 . doi: 10.1016/j.watres.2005.04.037
- Hollibaugh , J T and Azam , F . 1983 . Microbial degradation of dissolved proteins in seawater . Limnology and Oceanography , 28 : 1106 – 1116 . doi: 10.4319/lo.1983.28.6.1104
- Hoppe , H G . 1983 . Significance of exoenzymatic activities in the ecology of brackish water: measurements by means of methylumbelliferyl-substrates . Marine Ecology-Progress Series , 11 : 299 – 311 . doi: 10.3354/meps011299
- Hoppe , H G , Arnosti , C and Herndel , G F . 2002 . “ Ecological significance of bacterial enzymes in marine environment ” . In Microbial enzymes in the environment. Activity, ecology, and applications , Edited by: Burns , R C and Dick , R P . 73 – 107 . New York: : Marcel Dekker .
- Kiersztyn , B , Siuda , W and Chróst , R J . 2002 . Microbial ectoenzyme activity: useful parameters for characterizing the trophic conditions of lakes . Polish Journal of Environmental Studies , 11 : 367 – 373 .
- Kroer , N , Jørgensen , N OG and Coffin , R B . 1994 . Utilization of dissolved nitrogen by heterotrophic bacterioplankton: a comparison of three ecosystems . Applied and Environmental Microbiology , 60 : 4116 – 4123 .
- Kuznetsova , M R and Lee , C . 2001 . Enhanced extracellular enzymatic peptide hydrolysis in the sea-surface microlayer . Marine Chemical , 73 : 319 – 322 . doi: 10.1016/S0304-4203(00)00116-X
- Laurent , P and Servais , P . 1995 . Fixed bacterial biomass estimated by potential exoproteolytic activity . Canadian Journal of Microbiology , 41 : 749 – 756 . doi: 10.1139/m95-103
- Mackey , K RM , Labiosa , R G , Calhoun , M , Street , J H and Paytan , A. 2007 . Phosphorus availability, phytoplankton community dynamics, and taxon-specific phosphorus status in the Gulf Aqaba Red Sea. . Limnology and Oceanography , 52 : 875 – 885 . doi: 10.4319/lo.2007.52.2.0873
- Mallet , C and Debroas , D . 1999 . Relations between organic matter and bacterial proteolytic activity in sediment surface layers of an eutrophic lake (Lake Aydat, Puy de Dôme, France) . Archiv Fur Hydrobiologie , 145 : 39 – 56 .
- Martinez , J and Azam , F . 1992 . Periplasmic aminopeptidase and alkaline phosphatase activities in a marine bacterium: Implications for substrate processing in the sea . Marine Ecology-Progress Series , 92 : 89 – 97 . doi: 10.3354/meps092089
- Martinez , J , Smith , D C , Steward , D F and Azam , F . 1996 . Variability in ectohydrolytic enzyme activities of pelagic marine bacteria and its significance for substrate processing in the sea . Aquatic Microbial Ecology , 10 : 223 – 230 . doi: 10.3354/ame010223
- Mudryk , Z. 1998 . Generic composition and metabolic activity of bacteria inhabiting surface seawater layers . Oceanologica Studies , 3 : 57 – 70 .
- Mudryk , Z and Podgórska , B . 2006 . Enzymatic activity of bacterial strains isolated from marine beach sediments . Polish Journal of Environmental Studies , 15 : 441 – 448 .
- Mudryk , Z and Skórczewski , P . 2004 . Extracellular enzyme activity at the air-water interface of an estuarine lake . Estuarine, Coastal and Shelf Science , 59 : 59 – 67 . doi: 10.1016/j.ecss.2003.08.001
- Münster , U and Chróst , R J . 1990 . “ Organic composition and microbial utilization of dissolved organic matter. ” . In Aquatic microbial ecology , Edited by: Overbeck , J and Chróst , R J . 28 – 36 . New York : Springer-Verlag .
- Nagata , T and Kirchman , D L . 1992 . Release of macromolecular organic complexes by heterotrophic marine flagellates . Marine Ecology-Progress Series , 83 : 233 – 240 . doi: 10.3354/meps083233
- Overbeck , J and Chróst , R J . 1990 . Aquatic microbial ecology: Biochemical and molecular approaches , New York : Springer-Verlag .
- Patel , A B , Fukami , K and Nishijama , T. 2000 . Regulation of seasonal variability of aminopeptidase activities in surface and bottom waters of Uranouchi Inlet, Japan. . Aquatic Microbial Ecology , 21 : 139 – 149 . doi: 10.3354/ame021139
- Priest , F G . 1984 . Extracellular enzymes , 1 – 79 . Wokingham : Van Nostrand Reinhold .
- Reemtsma , T , Haake , B , Ittekkot , V , Nai , R R and Brockman , U H . 1990 . Downward flux of particulate fatty acids in the central Arabian Sea . Marine Chemical , 29 : 277 – 299 . doi: 10.1016/0304-4203(90)90018-8
- Regional Inspectorate for Environment Protection in Bydgoszcz . 2001 . Water quality statement of lakes Grażyna and Chełmżyńskie , 1 – 35 . Bydgoszcz : Regional Inspectorate for Environmental Protection .
- Rosenstock , B and Simon , M . 1993 . Utilization of dissolved combined and free amino acids by planktonic bacteria in Lake Constance . Limnology And Oceanography , 38 : 1521 – 1531 . doi: 10.4319/lo.1993.38.7.1521
- Rulík , M and Spáčil , R . 1999 . Assessment of extracellular enzyme activities of α - and β-glucosidase in sediments of a small lowland stream (Sitka Stream, Czech Republik) . Universitatis Palackianae Olomucensis. Facultas Rerum Naturalium. Biologica. , 37 : 99 – 105 .
- Siuda , W and Chróst , R J . 2001 . Utilization of selected dissolved organic phosphorus compounds by bacteria in lake water under non-limiting orthophosphate conditions . Polish Journal of Environmental Studies , 10 : 475 – 483 .
- Siuda , W and Chróst , R J . 2002 . Decomposition and utilization of particulate organic matter by bacteria in lakes of different trophic status . Polish Journal of Environmental Studies , 11 : 53 – 65 .
- Skórczewski , P , Mudryk , Z and Kukliński , B . 1999 . Optimization of measurement enzyme activity using fluorogenic substrates in water . Balt Coast Zone , 3 : 41 – 52 .
- Tellam , R L , Vuocolo , T , Johnson , S E , Jarmey , J and Pearson , R D . 2000 . Insect chitin synthase cDNA sequence, gene organization and expression . European Journal of Biochemistry , 267 : 6025 – 6043 . doi: 10.1046/j.1432-1327.2000.01679.x
- Tholosan , O , Lamy , F , Garcin , J , Polychronaki , T and Bianchi , A . 1999 . Biphasic extracellular proteolytic enzyme activity in benthic water and sediment in the northwestern Mediterranean Sea . Applied and Environmental Microbiology , 65 : 1619 – 1626 .
- Thompson , A J and Sisabaugh , R L . 2000 . Matric and particulate phosphatase and aminopeptidase activity in limnetic biofilms . Aquatic Microbial Ecology , 21 : 151 – 159 . doi: 10.3354/ame021151
- Unanue , M , Ayo , B , Agis , M , Slezak , D , Herndl , G J and Iriberri , J . 1999 . Ectoenzymatic activity and uptake of monomers in marine bacterioplankton described by a biphasic kinetic model . Microbial Ecology , 37 : 36 – 48 . doi: 10.1007/s002489900128
- Vrba , J. 1992 . Seasonal extracellular enzymes activities in decomposition of polymeric organic matter in a reservoir . Archives Limnology , 37 : 33 – 42 .
- Vrba , J , Kofroňová-Bobková , J , Pernthaler , J , Šimek , K , Macek , M and Psenner , R . 1997 . Extracellular, low-affinity b-N-acetylglucosaminidase linked to the dynamics of diatoms and crustaceans in freshwater systems of different trophic degree . Internationale Revue Der Gesamten Hydrobiologie , 82 : 277 – 286 . doi: 10.1002/iroh.19970820213
- Wainman , B C and Lean , R S . 1990 . Seasonal trends in planktonic lipid content and lipid class . Verhandlungen der Internationalen Vereinigung fur Limnologie , 24 : 416 – 419 .
- Yiyong , Z , Jianqiu , L and Min , Z. 2002 . Temporal and spatial variations in kinetics of alkaline phosphatase in sediments of a shallow Chinese eutrophic lake (Lake Donghu) . Water Research , 36 : 2084 – 2090 . doi: 10.1016/S0043-1354(01)00405-5
- Zhang , B , He , P-J , Lü , F , Shao , L M and Wang , P . 2007 . Extracellular enzyme activities during regulated hydrolysis of high-solid organic wastes . Water Research , 41 : 4468 – 4478 . doi: 10.1016/j.watres.2007.06.061