Abstract
Habitat use by smoky madtoms (Noturus baileyi) and yellowfin madtoms (Noturus flavipinnis) was quantified in lower Abrams Creek within the Great Smoky Mountains National Park during the summers of 2007–2010. Variables were measured at both macrohabitat and microhabitat spatial scales within locations where each species was present. Reach-level macrohabitat data were analyzed using principal component analysis to identify variables associated with each species’ presence within a reach. Classification trees were developed to describe microhabitat use for each species and to compare differences in microhabitat use between species. Distributions of both species were similar in lower Abrams Creek with each becoming more abundant within downstream reaches. Smoky madtoms predominantly used riffle and run microhabitats with gravel and cobble basal substrates, while yellowfin madtoms used pool microhabitats away from riffles with low velocity and cobble-sized cover rocks. Neither species was ever encountered within the same microhabitat, suggesting summer habitats were partitioned. Models developed in this study can be used to identify potential reintroduction zones or to assist in conservation efforts.
Introduction
More than 25% of the nearly 700 fish species found in southeastern United States waters are considered imperiled (Southeastern Fishes Council Citation2008). Madtom catfishes of the ictalurid genus Noturus are disproportionately represented among these imperiled fishes (Warren et al. Citation2000). These catfish species are at greater risk due to their specialization for lotic, benthic habitats (Angermeier Citation1995). Two madtom species that are federally listed and endemic to the southern Appalachian mountains are the smoky madtom (Noturus baileyi Taylor) and yellowfin madtom (Noturus flavipinnis Taylor, ).
Figure 1. Smoky madtom (top, photo by J. Miller) and yellowfin madtom (bottom, photo by K. Gibbs) in lower Abrams Creek, Great Smoky Mountains National Park, Tennessee.

The smoky madtom is a small species whose history and current status typifies the threats facing endemic freshwater fauna of the region (Dinkins & Shute Citation1996). This species was originally described by Taylor Citation(1969) from samples collected during the 1957 reclamation of Abrams Creek, Great Smoky Mountains National Park (GRSM). In an attempt to establish a trophy non-native rainbow trout (Onchorhyncus mykiss) fishery (Lennon & Parker Citation1959), application of rotenone, a piscicide, effectively removed all native fishes from the main stem of lower Abrams Creek (Lennon & Parker Citation1959). Abrams Creek was the most diverse stream in GRSM prior to rotenone application (Simbeck Citation1990). After application, smoky madtoms were presumed to be extinct until discovery of a population in nearby Citico Creek, Monroe County, TN in 1980 (Bauer et al. Citation1983). The Citico Creek population is the only known naturally occurring population for this species. The smoky madtom was added to the endangered species list in October 1984 (U.S. Fish and Wildlife Service [USFWS] Citation1984).
Smoky madtoms reach a maximum total length of 73 mm and have an average lifespan of 2 years (Dinkins & Shute Citation1996). From spring to late fall, Citico Creek smoky madtoms are often found associated with riffle habitat, particularly riffle crests, and are known to use palm-sized slab rocks (i.e., cobble) for concealment during daylight hours. During winter, they inhabit calm, deep pools (Dinkins & Shute Citation1996). The smoky madtom is primarily nocturnal with a diet consisting largely of benthic macroinvertebrates which is consistent with other Noturus species (Dinkins & Shute Citation1996).
Another madtom species similar in history and imperilment to the smoky madtom is the yellowfin madtom. Yellowfin madtoms were also described by Taylor Citation(1969) in a revision of the genus Noturus. This description was made from preserved samples collected in the late 1800s from several locations on the upper Tennessee River. Taylor surmised that impoundment of the Tennessee River and logging practices within the watershed led to this species’ extinction. However, individuals were collected in 1968 from the Powell River, Hancock County, TN (Dinkins & Shute Citation1996), which led to a redescription of the species (Taylor et al. Citation1971). At present, two additional yellowfin madtom populations are known to exist, including one population in Copper Creek, Scott and Russell counties, VA, and another in Citico Creek, Monroe County, TN, where it was discovered with the smoky madtom in 1980 (Bauer et al. Citation1983). Small population sizes and the fragmented distribution of the species resulted in federal listing as a threatened species in 1977 (USFWS Citation1977).
Yellowfin madtoms are nocturnal, spending daylight hours concealed beneath benthic substrate and undercut banks, or hidden in leaf litter. The species grows larger than smoky madtoms, reaching a maximum total length of 134 mm and living approximately 3–4 years (Dinkins & Shute Citation1996). In Citico Creek, yellowfin madtoms occur in low-velocity areas and are found in shallow pools less than 2 m deep (Dinkins & Shute Citation1996). Though no verified yellowfin madtom specimens were ever collected from Abrams Creek, madtom specimens collected and identified as brindled madtoms (Noturus miurus Jordan) during rotenone treatment were most likely misidentified based on native ranges of both species (Shute et al. Citation2005). Therefore, it is speculated that given the relative proximity to Citico Creek and the similar gradient, discharge, and substrate composition of the two streams, yellowfin madtoms probably occurred in Abrams Creek prior to reclamation (Dinkins & Shute Citation1996).
The USFWS recovery plans for both madtom species suggest reintroduction into formerly occupied ranges (USFWS Citation1983; Biggins Citation1985), with the goal of establishing viable reproductive populations. Abrams Creek was identified as suitable for reintroduction due to the acute nature of the original extirpation and continued federal protection of a majority of the watershed within GRSM boundaries (Shute et al. Citation2005). In 1986, Conservation Fisheries Incorporated (CFI) began reintroducing both smoky and yellowfin madtoms into Abrams Creek (Shute et al. Citation2005). Since reintroductions began, more than 3100 smoky madtoms and 1500 yellowfin madtoms have been propagated and released into Abrams Creek (Shute et al. Citation2005).
Abrams Creek flows through Blount County, TN and lies within the western section of GRSM. The Abrams Creek watershed (197.7 km2), mostly within the boundaries of GSRM, comprises approximately 348 km of streams and has a drainage area of approximately 23,000 hectares (Parker & Pipes Citation1990; Shute et al. Citation2005). Ordovician Jonesboro Limestone contributes to elevated conductivity, pH, and alkalinity levels, resulting in a highly productive aquatic ecosystem (Shaffer Citation2004). Abrams Creek is a moderate-sized (10–25 m width), coolwater stream with an average temperature not exceeding 23 °C during summer months (Shaffer Citation2004). At 23.5 river kilometer (rkm), upstream from the mouth at Chilhowee Reservoir, Abrams Falls plummets 7.6 m and divides the stream into upper Abrams Creek and lower Abrams Creek, isolating aquatic communities into two distinct segments (Simbeck Citation1990).
Upper Abrams Creek originates in Cades Cove, which is a historic heritage site with high visitor usage during summer and fall, with Abrams Falls as the downstream boundary. For historical preservation, GRSM allowed pastured cattle in Cades Cove until 1999, resulting in sediment loading and elevated nutrient concentrations (Matthews Citation1978). Improved water and habitat quality has been achieved by restoring riparian vegetation, fencing, and removing cattle from upper Abrams Creek and its tributaries (Fraley Citation1998).
Lower Abrams Creek extends 23.5 km downstream from Abrams Falls to the embayment created by Chilhowee Reservoir (Shute et al. Citation2005). The average width of Abrams Creek within this 23.5 km section is 18 m, with an average gradient of 3.5% (Shaffer Citation2004). Dominant substrates within this section include cobble, boulder, and bedrock. Reintroduction zones for smoky and yellowfin madtoms were located in lower Abrams Creek (Rakes & Shute Citation2007).
Currently, both species have established reproducing populations (Shute et al. Citation2005) and distinct distributions (Throneberry Citation2009) within Abrams Creek. Smoky and yellowfin madtoms are known to populate the lowest 17.4 km of Abrams Creek; however, macrohabitat (reach-level) and microhabitat use of both madtom species within Abrams Creek is not known. Habitat use should be studied at multiple spatial scales (Muhlfeld et al. Citation2001) to develop a complete understanding of habitat associations for each species. Microhabitat studies are important because they can identify specific stream locations that fish select based on a variety of factors (Muhlfeld et al. Citation2001). Data collected from microhabitats can be used to identify significant habitat variables and to build microhabitat models useful in management and conservation efforts (Schmidt Citation2007). Such data would be helpful to National Park Service personnel, tasked with restoring and conserving these species within Abrams Creek, by identifying precise locations within stream reaches used by each species, which may aid in future conservation efforts. Therefore, the objectives of this study were to (1) determine macrohabitat and microhabitat for both madtom species in Abrams Creek, (2) develop models that predict each species’ presence, and (3) determine if these species partition habitat within lower Abrams Creek.
Methods
Macrohabitat
Lower Abrams Creek, in its entirety, was divided into consecutive 200 m reaches. Ten consecutive 200 m reaches were considered a section (2000 m). Upstream and downstream boundaries of each 200 m reach were marked with forestry flagging and geographic coordinates were obtained using a handheld Global Positioning System (GPS) unit (GarminTM 76 CS; http://www.garmin.com). Three reaches were randomly selected from each 2000 m section. Habitat parameters and water quality were measured within all selected reaches.
Habitat units were classified as riffle, run, or pool as described by Overton et al. Citation(1997) and length of each habitat unit within a reach was measured. Wetted width (m), depth (cm), and dominant substrates (%) were determined at 10 m transects within each 200 m reach. Depth was measured at three equidistant points along each transect. Dominant substrates were visually determined within a 0.25 m radius of each depth measurement. Substrate was classified using the modified Wentworth scale (Wentworth Citation1922). Detritus was characterized as decaying organic matter accumulated on the streambed. Gradient (%) was determined within each delineated reach using a clinometer.
Water quality parameters were measured within a run habitat of each randomly selected reach. If run habitats were not present, water quality was measured within a flowing pool. Temperature (°C), dissolved oxygen (mg/L), and conductivity (μS/cm) were measured (Model 85 meter, YSI, Inc., http://www.ysi.com) as well as pH (Analytical Measurements, Inc., http://www.analyticalmeasurements.com/). Discharge (m3/s) was calculated with a modified velocity–area method, using a portable flow meter (Marsh-McBirney Flo-Mate 2000, Hach Co., http://www.hach.com), within a run habitat of the most downstream site surveyed during each sampling day (McMahon et al. Citation1996).
Habitats were identified using underwater observation (i.e., snorkeling) beginning in mid-July 2007 and 2008 to avoid disturbing spawning and nesting smoky and yellowfin madtoms (Etnier & Starnes Citation1993; Dinkins & Shute Citation1996). Each randomly selected 200 m reach was visually divided into equidistant lanes and snorkeled by no less than four observers after habitat was measured. Each observer thoroughly sampled their assigned lane unless current was too powerful or sufficient depth was lacking. Observer competency was assured by pre-survey training conducted by experienced observers (Thurow Citation1994). Whenever possible, observations were validated by a qualified observer. Lane size varied based upon number of observers and changes in stream width. If a lane became too shallow or current was too powerful, the observer exited the stream and returned to the same lane when depth or current allowed. All observers continued upstream, adjacent to one another, for the duration of the survey to ensure a thorough sample. Observers searched under each palm-sized or larger slabrock to determine presence or absence of madtom species. Rocks were lifted gently, with one edge of the rock still in contact with the streambed, and disturbed sediment was allowed to clear before gently replacing the rock. Species and number of each madtom observed and corresponding macrohabitat (i.e., habitat unit and cover rock size) were recorded for each sampled reach.
Microhabitat
Eight 200 m reaches that were identified as being occupied by either species during macrohabitat analysis were used to conduct microhabitat surveys. Microhabitat was defined as the habitat within a 0.25 m2 area of a location occupied by either species. Field work and data collection began in June 2009 and concluded in September 2010. Snorkel surveys were conducted as described for macrohabitat surveys. When a specimen was encountered, each cover rock was carefully replaced to its original location after species identification. Lead weights (85 g) marked with highly visible forestry flagging were placed directly on top or in front of the cover rock. Flagging was color-coded to represent each species and surveyors recorded microhabitat data for each marked location after sampling the entire 200 m reach (Schmidt Citation2007).
Depth (cm) was measured with a top-setting wading rod. Current velocity (m/s) and dissolved oxygen (D.O.) (mg/L) were measured at substrate and at 60% total depth. Temperature (°C) and conductivity (μS) were recorded at substrate. Cover rocks at occupied locations were measured (cm) along the two longest axes to calculate an approximate surface area (cm2). Substrate beneath the cover rock and dominant substrate within a 0.25 m2 area surrounding each present location were visually assessed and categorized using a modified Wentworth scale (Wentworth Citation1922). Distance to nearest bank (m) and distance to nearest riffle (m) were also measured. Habitat type (i.e., riffle, run, or pool) was visually assessed and recorded.
Unoccupied locations were randomly chosen from five equidistant points along seven transects used during macrohabitat delineation. Seven transects furthest from the greatest concentration of observed occupied locations within a reach were used, and three locations were selected along each transect (Schmidt Citation2007). Unoccupied locations were not used if they occurred within 5 m of an occupied location (Schmidt Citation2007). A maximum of 21 unoccupied locations were measured within each 200 m reach (Schmidt Citation2007). In some cases, fewer unoccupied locations were measured due to proximity of occupied locations. Locations designated as unoccupied were observed underwater to ensure that neither target species was present. All habitat variables measured at occupied locations were measured at unoccupied locations.
Statistical analysis
Principal component analysis (PCA) was used to identify influential macrohabitat variables within yellowfin and smoky madtom distributions. PCA is an ordination technique to graphically represent similarity among samples (Clarke & Warwick Citation2001). PCA reduces dimensionality of complex data-sets to ≤5 interpretable ordination axes based on eigenvectors of principal components that account for the greatest variation among samples (Clarke & Warwick Citation2001; Kwak & Peterson Citation2007). Small data-sets (n = 27) with multiple variables with high correlation, such as in-stream habitat data, can be effectively analyzed using this technique. Habitat type and substrate composition percentages were arcsine transformed to approximate normality (Zar Citation1999). Continuous variables (i.e., width, depth, and rkm) were not transformed. PCA was performed using Primer v6 software (PRIMER-E Citation2006).
Classification tree methods (Breiman et al. Citation1984) were used to describe yellowfin and smoky madtom microhabitat use. Large, complex ecological data-sets with categorical and continuous variables can be efficiently modeled and interpreted with classification and regression trees (CART) analysis (Breiman et al. Citation1984; De’ath & Fabricius Citation2000). Categorical response variables (i.e., occupied vs. unoccupied or yellowfin madtom vs. smoky madtom) were hierarchically partitioned into the most parsimonious dichotomous groupings based on the most influential explanatory variables within each subsequent grouping to produce explanatory and predictive classification tree models (De’ath & Fabricius Citation2000). Categorical explanatory variables (i.e., habitat type, cover rock type, basal substrate, and dominant substrate) were assigned numerical values (e.g., riffle = 1, run = 2, pool = 3), whereas continuous variables (i.e., temperature, conductivity, depth, D.O. at substrate, D.O. at 60% depth, velocity at substrate, velocity at 60% depth, cover rock area, distance to bank, and distance to riffle) were unchanged. Full tree growth resulted in terminal nodes with individuals from only one response category; however, this commonly results in over-fitting data (De’ath & Fabricius Citation2000). Therefore, cross-validation of 10 data subsets provided misclassification error rates for each intermediate and terminal node. The best model was determined to be the subtree with the lowest number of terminal nodes within one standard error of the subtree with the lowest error rate. Analyses and selection of classification trees were conducted using default settings and procedures implemented by Salford Systems CARTTM 6.0 software (Steinberg & Colla Citation1995).
These analyses will help identify suitable habitat outside of current yellowfin and smoky madtom distributions within Abrams Creek for monitoring of natural range extensions or as future reintroduction sites. Long-term monitoring data-sets should be used to validate these models and evaluate conservation efforts.
Results
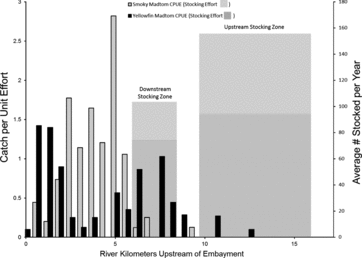
Throughout summer months of 2007 and 2008, 78 smoky madtoms were observed in 9 of 17.4 km of lower Abrams Creek that was surveyed. During the same time period, 89 yellowfin madtoms were observed in 77% (13.4 of 17.4 km) of lower Abrams Creek. Yellowfin madtoms had a larger range distribution compared to smoky madtoms and the entire yellowfin madtom distribution overlapped the smoky madtom distribution (). However, smoky madtoms had the most contiguous distribution of the two madtom species. The majority of both species were detected downstream of stocking locations in the lower portion of Abrams Creek (). Both species co-occupied 9 of the 27 reaches surveyed for macrohabitat analysis.
Figure 2. Distribution map of smoky and yellowfin madtoms determined during macrohabitat snorkeling surveys conducted during 2007 and 2008 in lower Abrams Creek, Blount County, TN.
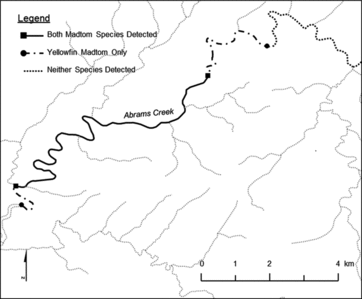
Figure 3. Comparison of catch per unit effort during macrohabitat snorkeling surveys to average stocking density per year for smoky and yellowfin madtoms in lower Abrams Creek.
Macrohabitat
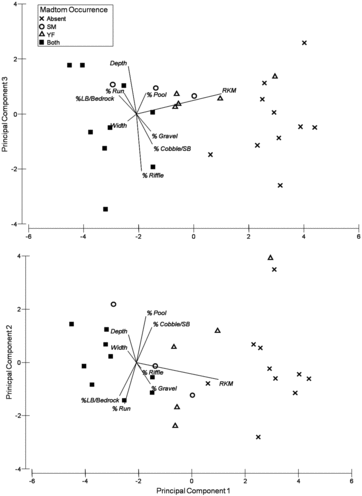
Most smoky madtoms (n = 58) were found in run habitats under cobble substrates during 2007–2008 macrohabitat surveys. Yellowfin madtoms were found in every habitat type, but were predominately associated (n = 87) with run and pool habitats. Cover rocks ranged in size from cobble to large boulder, with most individuals (n = 79) associated with cobble. Principal components (PC) 1–3 cumulatively accounted for 82.2% of the variation among sample sites with PC1 accounting for most of the variation (53.2%, ). Distance from embayment had the greatest influence in sites without either species present (). Both species co-occurred in many sites with little consistency among variable loadings for the 3 PCs (). This analysis not only reflects the reach-level habitat heterogeneity among sites, but also demonstrates that both species occurred most frequently in downstream locations ().
Table 1. Correlation coefficients of habitat variables with principle component vectors (PC1, PC2, and PC3). Values in parenthesis indicate the proportion of the total variability predicted by the PC variable.
Microhabitat
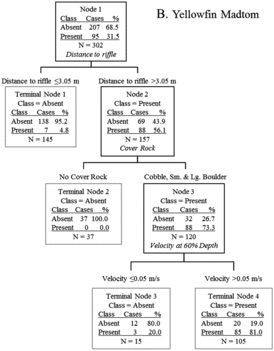
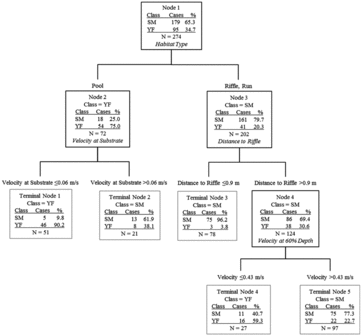
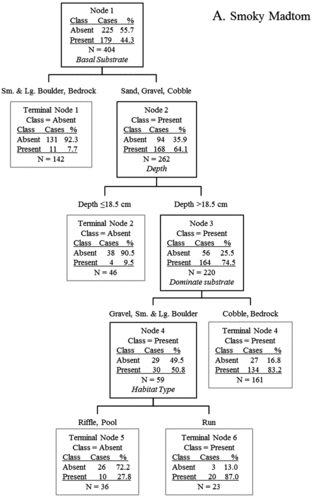
In total, microhabitat data were collected from 179 smoky madtom locations and 95 yellowfin madtom locations in lower Abrams Creek during 2009 and 2010. Although distribution of both species overlapped extensively and both species co-occurred in most sampled reaches within the overlapping distribution, microhabitat use was different for each. Most (∼74.9%) of smoky madtoms were observed in microhabitats dominated by cobble and bedrock substrates with sand, gravel, or cobble basal substrates in depths >18.5 cm (). The majority (∼89.5%) of yellowfin madtoms were observed >3 m from riffles under cobble or boulder cover rocks (). When occupied locations were compared, it was apparent that smoky madtoms more frequently inhabited riffles and runs, whereas yellowfin madtoms inhabited low-velocity pools (). Overall, smoky madtoms used a greater variety of microhabitats compared to yellowfin madtoms and partitioning of microhabitat within co-occurring reaches was evident.
Figure 5. Classification tree analysis for microhabitat data of occupied (present) and unoccupied (absent) locations of smoky madtoms in lower Abrams Creek. Predictor variables in italics resulted in the most parsimonious split of parent nodes.
Discussion
Habitat use by madtoms varies greatly; however, most species are confined to lotic environments (Burr & Stoeckel Citation1999; Banks & DiStefano Citation2002). Habitat surveys of the stonecat (Noturus flavus; Walsh & Burr Citation1985) and Neosho madtom (Noturus placidus; Fuselier & Edds Citation1994) observed riffle use in summer months. Midway Citation(2008) also observed the use of cobble cover rocks in swift-moving water by the Carolina madtom (Noturus furiosus).
The smoky madtom displays habitat use typical of many lotic madtoms, including association with increased current velocities, close proximity to riffle habitat, and utilization of cobble or boulder substrates (Taylor Citation1969). Smoky madtom habitat use in Citico Creek has been described as consisting of palm-sized substrate (i.e., cobble) in riffles, runs, and adjacent areas (Etnier & Starnes Citation1993; Dinkins & Shute Citation1996). Dinkins & Shute Citation(1996) most often observed the Citico Creek source population using cobble substrates in close proximity to riffle habitat. Results of this study confirmed that most smoky madtoms in lower Abrams Creek used habitats dominated by cobble and bedrock substrate with relatively high current velocities. However, individuals were observed in all habitat types (i.e., riffles, runs, and pools) under a wide range of current velocities.
The range in current velocities and habitat types used by smoky madtoms reflects that the species is more of a habitat generalist compared to yellowfin madtoms. Both species occupied microhabitats dominated by cobble and used cobble cover rocks; however, smoky madtoms avoided low-velocity pools occupied by yellowfin madtoms and instead inhabited microhabitats with increased current velocities.
Unlike most Noturus spp., yellowfin madtoms are most often associated with low current velocities and deep areas available in pool habitats. Habitation of deeper, slow-moving water with lower dissolved oxygen levels is well documented among other larger catfishes such as channel catfish (Ictalurus punctatus), and various bullhead (Ameiurus sp.) species, but is less common among madtoms during summer months (Burr & Stoeckel Citation1999). Increased depth results in more available space in the water column and the larger body size of yellowfin madtoms may allow for better exploitation of pool habitats when compared to smoky madtoms. Grossman and Freeman Citation(1987) documented size-dependent variation in habitat use by several stream species, in which larger individuals occupied deeper microhabitats.
Historical collection records of yellowfin madtoms demonstrate the species’ ability to use a variety of habitat types. The earliest specimens described by David Starr Jordan in 1890 were taken from weedy areas on the North Fork of the Holston River, VA (Taylor Citation1969) and the species occurs in large, highly silted pools on the Powell River, TN (Etnier & Starnes Citation1993). Lower Abrams Creek is essentially devoid of such weedy habitat and is fairly swift, clear, and silt-free.
The yellowfin madtom has been found to use cover rocks of various sizes (i.e., cobble, small boulder, and large boulder, Dinkins & Shute Citation1996). In lower Abrams Creek, cobbles were used as cover rocks by almost 46% of observed specimens, whereas small boulder accounted for 44% of cover rocks used, and large boulder comprised the remaining 10%. Perhaps the larger size of yellowfin madtoms, as compared to smoky madtoms, resulted in their use of larger cover rocks (i.e., small and large boulder).
Size of cover rock played a role in the ability of some reaches to be effectively sampled. Many large flat slab rocks (i.e., large boulder) could not be effectively sampled. Yellowfin madtoms may have used interstitial spaces beneath these large rocks when present, thereby remaining undetected if boulders were too large to be lifted. Specimens were also observed using bedrock overhangs and cracks for shelter. Only one individual was observed near the mouth of Abrams Creek in a reach with increased depth and dominated by large boulders and bedrock.
Use of pool microhabitats, less than 2 m deep with relatively low current velocities, by yellowfin madtoms in lower Abrams Creek is consistent with life history descriptions from the Citico Creek population (Dinkins & Shute Citation1996). Yellowfin madtom microhabitat in lower Abrams Creek is characterized by moderately deep long pools, with decreased dissolved oxygen concentrations and cobble cover rocks on top of gravel substrate. Individuals were rarely observed less than 10 m from the nearest riffle. In addition, past studies have observed yellowfin madtoms seeking cover in leaves or other detrital material that accumulates in pools (Etnier & Starnes Citation1993), but such habitats were uncommon in Abrams Creek during summer sampling.
Although distributions of smoky and yellowfin madtoms overlap within lower Abrams Creek, neither species was observed beneath the same cover rock or within the same microhabitat during sampling. This suggests some level of competitive interaction may exist between the species, especially when considering the similarity in dietary requirements and life history strategies. Dinkins and Shute Citation(1996) postulated that the two species are able to exist sympatrically in Citico Creek because of differences in habitat use during warmer months when they are most active. These differences were documented during this study, with most yellowfin madtoms inhabiting long, deep pools and smoky madtoms occupying habitats with increased current velocities and moderate depths in close proximity to riffles. However, both species were frequently observed under cobble cover rocks with gravel basal substrate in the different habitat types. Smoky madtoms displayed a far more generalist niche breadth with regard to habitat use than did yellowfin madtoms.
Diverse ecosystems are often more stable than those with reduced diversity (Groom et al. Citation2006) and these madtom species were likely a small portion of a previously diverse benthic, insectivore guild that formerly occupied Abrams Creek prior to reclamation (Simbeck Citation1990). No population or count data exist for either madtom species prior to extirpation in 1957. Banded sculpin (Cottus carolinae), stonecat (N. flavus), and various other species would undoubtedly have competed with the currently imperiled fishes for available habitat and resources within Abrams Creek. The feasibility and risk of re-establishing other extirpated species within Abrams Creek should be explored (Pearsons & Hopely Citation1999).
Finally, young-of-the-year of both species were observed throughout their respective distributions within lower Abrams Creek during this study. Past surveys by Rakes and Shute Citation(2007) have also documented reproduction, suggesting that these populations are currently self-supporting. However, work remains as future conservation efforts will likely play a large role in determining the fate of both species. Seasonal and ontogenic shifts in habitat requirements should also be assessed to determine long-term sustainability of these populations (Rosenfeld Citation2003). Such efforts should include long-term monitoring of population trends, investigation of the genetic viability of both populations, and continued reintroductions, if warranted by results of population and genetic analysis. Knowledge of habitat use, as well as the habitat models and other suggestions presented here, should be incorporated in future recovery efforts.
Acknowledgements
We thank numerous individuals who supported this project including Steve Moore of the Great Smoky Mountains National Park and J.R. Shute and Patrick Rakes of Conservation Fisheries, Inc., without whose efforts neither madtom species would be present in Abrams Creek. Numerous individuals were instrumental in the completion of this project including Shane Billings, Johnathan Davis, Ben Hutton, Cody McPeake, Austin Robertson, and numerous National Park Service personnel.
Additional information
Funding
References
- Angermeier PL. 1995. Ecological attributes of extinction-prone species: loss of freshwater fishes of Virginia. Conservation Biol. 9:143–158.
- Banks SM, Distefano RJ. 2002. Diurnal habitat associations of the Madtoms Noturus albater, N. exilis, and N. flavus in Missouri Ozark streams. Am Midland Nat. 148:138–145.
- Bauer BH, Dinkins GR, Etnier DA. 1983. Discovery of Noturus baileyi and N. flavippinnis in Citico Creek, Little Tennessee River system. Copeia. 1983:558–560.
- Biggins RG. 1985. Recovery plan for smoky madtom (Noturus baileyi) Asheville Endangered Species Field Station. Atlanta (GA): US Fish and Wildlife Service.
- Breiman L, Friedman JH, Olshen RA, Stone CG. 1984. Classification and regression trees. Belmont (CA): Wadsworth International Group.
- Burr BM, Stoeckel JN. 1999. The natural history of madtoms (genus Noturus) North America's diminutive catfishes. In: Irwin ER, WA Hubert, CF Rabeni, Schramm HL Jr., T. Coon, editors. Catfish 2000: Proceedings of International Ictalurid Symposium. Bethesda (MD): American Fisheries Society; p. 51–87.
- Clarke KR, Warwick RM. 2001. Change in marine communities: an approach to statistical analysis and interpretation. 2nd ed. Plymouth (UK): PRIMER-E.
- De’ath G, Fabricius KE. 2000. Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology. 81:3178–3192.
- Dinkins GR, Shute PW. 1996. Life histories of Noturus baileyi and N. flavipinnis (Pisces: Ictaluridae), two rare madtom catfishes in Citico Creek, Monroe County, Tennessee. Bull Ala Museum Nat Hist.18:43–69.
- Etnier DA, Starnes WC. 1993. Fishes of Tennessee. Knoxville (TN): University of Tennessee Press.
- Fraley SJ. 1998. A baseline survey of aquatic macroinvertebrate communities at seven sites in the Abrams Creek system, Great Smoky Mountains National Park, Blount County, Tennessee [ dissertation]. Knoxville (TN): University of Tennessee; 114 p.
- Fuselier L, Edds D. 1994. Seasonal variation in habitat use by the Neosho madtom (Teleosti: Ictaluridae: Noturus placidus). Southwest Nat. 39: 217–223.
- Groom MJ, Meffe GK, Carroll CR. 2006. Principles of conservation biology. 3rd ed. Sunderland (MA): Sinauer Associates Incorporated.
- Grossman GD, Freeman MC. 1987. Microhabitat use in a stream fish assemblage. J Zool Soc London. 212:151–176.
- Kwak TJ, Peterson JT. 2007. Community indices, parameters, and comparisons. In: Guy CS, Brown ML, editors. Analysis and interpretation of freshwater fisheries data. Bethesda (MD): American Fisheries Society; p. 677–763.
- Lennon RE, Parker PS. 1959. The reclamation of Indian and Abrams Creeks, Great Smoky Mountains National Park. Special Scientific Report – Fisheries 306. Washington (DC): United States National Park Service.
- Matthews RC, Jr. 1978. Ecological survey of Abrams Creek in the Great Smoky Mountains National Park. Management Report No. 28. Gatlinburg (TN): National Park Service, Southeast Region, Uplands Field Research Laboratory. 195 p.
- McMahon TE, Zale AV, Orth DJ. 1996. Aquatic habitat measurements. In: Murphy BR, Willis DW, editors. Fisheries techniques. 2nd ed. Bethesda (MD): American Fisheries Society; p. 83–115.
- Midway SR. 2008. Habitat ecology of the Carolina madtom Noturus furiosus, an imperiled endemic stream fish [ unpublished master's thesis]. Raleigh (NC): North Carolina State University.
- Muhlfeld CC, Bennett DH, Martoz B. 2001. Summer habitat use by Columbia River redband trout in the Kootenai River drainage, Montana. North Am J Fish Manag. 21:223–235.
- Overton CK, Wollrab SP, Roberts BC, Radko MA. 1997. Fish and fish habitat standard inventory procedures handbook. General Technical Report INT-GTR-346. Washington (DC): United States Department of Agriculture, Forest Service Intermountain Research Station.
- Parker CR, Pipes DW. 1990. Watersheds of Great Smoky Mountains National Park: a geographical information system analysis. Research/Resources Management Report SER-91/01. Southeast Regional Office. Atlanta (GA): United States Department of the Interior, National Park Service; 126 p.
- Pearsons TN, Hopely CW. 1999. A practical approach for assessing ecological risks associated with fish stocking programs. Fisheries. 9:16–23.
- PRIMER-E. 2006. PRIMER-E Ltd, Plymouth Marine Laboratory, Plymouth, UK.
- Rakes PL, Shute JR. 2007. Captive propagation and monitoring of rare southeastern fishes: Unpublished report to the Tennessee wildlife resources agency. Contract No. FA-99-13085-00.
- Rosenfeld J. 2003. Assessing the habitat requirements of stream fishes: an overview and evaluation of different approaches. Trans Am Fish Soc. 132:953–968.
- Schmidt, CU. 2007. Seasonal Microhabitat use of the threatened spotfin chub Erimonax monachus in the emory river watershed [ unpublished master's thesis]. Cookeville (TN): Tennessee Technological University.
- Shaffer GP. 2004. Evaluation of smallmouth bass in Abrams Creek and Little River within Great Smoky Mountains National Park [ unpublished master's thesis]. Cookeville (TN): Tennessee Technological University.
- Shute JR, Rakes PL, Shute PW. 2005. Reintroduction of four imperiled fishes in Abrams Creek, Tennessee. Southeast Nat. 4:93–110.
- Simbeck DJ. 1990. Distribution of the fishes of Great Smoky Mountains National Park [ unpublished master's thesis]. Knoxville (TN): University of Tennessee.
- Southeastern Fishes Council. 2008. Desperate dozen scientific report. Available from: http://ichthyology.usm.edu/sfc/DesDoz_report.pdf
- Steinberg D, Colla P. 1995. CART: tree-structured non-parametric data analysis. San Diego (CA): Salford Systems.
- Taylor WR. 1969. A revision of the catfish genus Noturus Rafinesque with an analysis of higher groups in the Ictaluridae. Bull Am Museum Nat Hist. 282:1–315.
- Taylor WR, Jenkins RE, Lachner EA. 1971. Rediscovery and description of the ictalurid catfish Noturus flavipinnis. Proc Biol Soc Wash. 83:469–476.
- Thurow RF. 1994. Underwater observation. In: Murphy ML, Willis DW, editors. Fisheries techniques. 2nd ed. Bethesda (MD): American Fisheries Society; p. 533–554.
- Throneberry JK. 2009. Reintroduction success of smoky madtom Noturus baileyi and yellowfin madtom Noturus flavipinnis in Abrams Creek, Great Smoky Mountains National Park [master's thesis]. Cookeville (TN): Tennessee Technological University.
- [USFWS] U.S. Fish and Wildlife Service. 1977. Threatened and endangered plants and animals. Fed Regist. 42:45527–45529.
- [USFWS] U.S. Fish and Wildlife Service. 1983. Recovery plan for yellowfin madtom (Noturus flavipinnis). Atlanta (GA): U.S. Fish and Wildlife Service.
- [USFWS] U.S. Fish and Wildlife Service. 1984. Determination of endangered status and designation of critical habitat for the smoky madtom (Noturus baileyi). Atlanta (GA): U.S. Fish and Wildlife Service.
- Walsh SJ, Burr BM. 1985. Biology of the stonecat, Noturus flavus (Siluriformes: Ictaluridae), in central Illinois and Missouri streams, and comparisons with Great Lakes populations and congeners. Ohio J Sci.85:85–96.
- Warren ML, Burr BM, Walsh SJ. 2000. Diversity, distribution and conservation status of the native freshwater fishes of the southeastern United States. Fisheries. 25:7–28.
- Wentworth CK. 1922. A scale of grade of grade and class for clastic sediment. J Geol. 30: 377–392.
- Zar JH. 1999. Biostatistical analysis. 4th ed. Upper Saddle River (NJ): Prentice Hall, Inc.