Abstract
Yellow perch Perca flavescens (Mitchill) are typically collected using multi-mesh gill nets; however, gear selectivity information for this species is sparse. Selectivity using experimental gill nets was determined for yellow perch using data collected from southern Lake Michigan from 2007 to 2012 using stretch mesh sizes of 12.5, 16, 20, 25, 32, 38, 46, 51, 64, and 76 mm. Female selectivity was best described by a model with a skewed (log-normal) distribution while males were best described by a normal distribution. When sex data were combined, the skewed (log-normal) distribution provided the best model. Differences in selectivity between sexes were minimal, but likely due to the variant size structure of the local population sampled, with females the larger sex. These findings suggest that bias in individual gill net selectivity can be used to target specific yellow perch size groups or to replace other gear types with unknown selectivity.
Introduction
Because gill nets are highly selective (Hamley Citation1975; Clarke & King Citation1986; Walsh Citation1992), identifying their selectivity biases is necessary when implementing sampling protocols to properly manage a recreational or commercial fishery (Millar Citation1992). Selection is defined by any process that causes the probability of capture to vary with the characteristics of the fish and differs from the term ‘selectivity,’ which is the quantitative expression of selection (Hamley Citation1975). However, this definition can be problematic because selectivity is dependent on several assumptions that underlie the capture process including: (1) the occurrence of fish coinciding in time and space with the use of the gear, (2) fish then encountering the sampling gear, and (3) fish being retained by the sampling gear (Holst et al. Citation1998). If the length–frequency distributions of the sampled fish population are known, selectivity can be calculated directly, typically by mark–recapture experiments (Mattson Citation1994; Millar & Holst Citation1997). However, accurate knowledge about length–frequency distributions is rare and selectivity is more commonly calculated indirectly, where estimates are obtained by comparing the observed catch frequencies caught by specific gear sizes.
The literature describing gill net selectivity of some percids is well established (Hamley & Regier Citation1973; Jensen Citation1986; Grant et al. Citation2004; Irwin et al. Citation2008; Vandergoot et al. Citation2011). For example, a study of walleye Sander vitreus suggests that incorporating selectivity functions into estimates of age composition would reduce bias (Vandergoot et al. Citation2011). Further, walleye have been shown to have positively skewed selectivity curves and up to 40% of the ideal-sized fish that encounter a given mesh size would escape (Grant et al. Citation2004). In contrast, little is known about the selectivity of yellow perch Perca flavescens (Mitchill) using multi-mesh gill nets, despite their typical use when sampling this species (Evans & Jude Citation1986; Shroyer & McComish Citation2000; Horns Citation2001). Lott and Willis Citation(1991) described the efficiency of gill nets in capturing yellow perch based on modal lengths in four different mesh sizes but did not estimate selectivity parameters. Kraft and Johnson Citation(1992) compared selectivities of fyke nets and gill nets; however, their study was limited to only 60 mm stretch mesh gill nets. Thus, there is a gap in the literature regarding information about gill net selectivity for yellow perch. Closing this gap would lead to more informed management of this valued sport and commercial fish.
Indirect model calculations of gill net selectivity are straightforward: parameters of a length distribution (e.g., mode, standard deviation [SD], and skewness) are input to a selectivity model which typically results in a uniform, bell-shaped curve. Mesh sizes are believed to be length-selective (Jensen Citation1986) and selectivity could be related to morphometric differences in the anterior portion of the fish. Yellow perch growth rates are also known to be sexually dimorphic (Wilberg et al. Citation2005) and if these differences extend to those portions of the fish typically entangled in a gill net, we believe it may influence selectivity curves.
The objective of this study was to estimate gill net selectivity of yellow perch using catch data from southern Lake Michigan collected from 2007 to 2012. We hypothesized that we should be able to link all post-larval sizes of yellow perch (>50 mm) with a defined mesh size. Although the distribution of fish around the mode hypothetically might be normally distributed, we also expected to find a positive skewed distribution based on selectivity shown in a related species (walleye; Grant et al. Citation2004).
Methods
Field sampling
Two sampling periods were used to collect yellow perch for this study. First, fish were sampled in September from two near-shore sample zones in the Indiana waters of Lake Michigan from 2007 to 2011. Sites M and K were located approximately 2 km west and east of Michigan City, Indiana, respectively (). Each net was 40 m long and 1.5 m deep, consisting of four monofilament panels with stretch mesh sizes 12.5, 16, 20, and 25 mm each 10 m in length. Four-hour sets were fished at the 5-m depth contours during the day and night for a total effort of eight gill net lifts per year. For purposes of this study, we will refer to these nets as the ‘micromesh’ nets.
Figure 1. Location of four sample sites (M, K, G, and EC) located in the Indiana waters of Lake Michigan from 2007 to 2012.
The second sampling effort collected yellow perch using larger monofilament and multifilament gill nets in June, July, and August from four near-shore sample zones in the Indiana waters of Lake Michigan from 2010 to 2012. Sites M and K were as above, site G was located 6 km north of Burns Harbor, IN, while site EC was located approximately 0.75 km southwest of East Chicago (). These gill nets consisted of six 45.6-m panels of 32, 38, 46, 51, 64, and 76 mm stretch mesh sizes, resulting in an overall gill net length of 274 m. Mesh sizes 32–46 mm were monofilament panels and mesh sizes 51–76 were multifilament panels. In this study, we refer to these nets as the ‘large’ gill nets. Nets were set at dusk along 5- and 10-m (2010 and 2011 during June and July only) depth contours near the center of each index zone and recovered after approximately 12 hours at sites M, K, G, and EC, resulting in a total effort of 32 gill net lifts in 2010 and 2011, and 12 gill net lifts in 2012.
Gill net selectivity analyses
Gill net selectivity was estimated using the Share Each LEngth's Catch Total (SELECT) method through R code developed by Russell B. Millar, available at http://www.stat.auckland.ac.nz/∼millar/selectware/code.html. The SELECT method is a generalized linear model approach of indirect estimation of selectivity (Millar Citation2000). Maximum likelihood is used to estimate parameters and the data are assumed to be Poisson-distributed where the variance of the data must be equal to the mean. If this distribution is violated, then the SELECT method can be viewed as a quasi-likelihood method (Millar & Fryer Citation1999). Four models to describe selection curves are available in the R code (normal fixed spread, normal proportional spread, gamma, and log-normal). All models have two parameters and are unimodal (Millar & Fryer Citation1999). The two normal models have symmetrical tails while the gamma and log-normal models are positively skewed. We evaluated all models and used the equal effort of mesh size assumption. Bin width of frequency distribution was set at 10-mm increments. Model selection was based on the deviance statistic (Millar & Fryer Citation1999) and the model with the lowest deviance statistics was considered the best. Assessment of model fit was evaluated by examination of the deviance residual plots. Higher deviance residuals indicate that more fish were caught than would be expected by the model and lower deviance residuals indicate that fewer fish were caught than would be expected by the model. Because yellow perch growth rates are sexually dimorphic (Wilberg et al. Citation2005), large mesh gill net selectivity was estimated separately for males and females as well as in a combined sex analysis. Selectivity in the micromesh was only fit to combined sex, as gender was difficult to determine with these smaller fish. Further, models were fit separately to the micromesh and large mesh gill nets because the model assumes equal effort among mesh sizes. Summary statistics (mean, median, etc.) represent all years combined.
Results
A total of 809 yellow perch were captured in the first sampling period (2007–2011) and used in the micromesh gill net selectivity analysis (). The mean total lengths (TLs), mm (gill net size, mm) captured were 73 (12.5), 84 (16), 111 (20), and 132 (25). The log-normal model () produced the best fit for the micromesh collected fish () and the deviance residuals indicated a random pattern of residuals (A). The residual finding indicated that the individual mesh sizes caught either more (higher residuals) or fewer (lower residuals) than would be expected by the model but it was not consistent or large.
Table 1. Summary statistics of yellow perch Perca flavescens collected in the micromesh gill nets. Mesh size, minimum, maximum, median, and averages in mm.
Table 2. Results of model selection with the SELECT method for micromesh gill net selectivity estimates of yellow perch Perca flavescens collected in southern Lake Michigan, 2007–2011, only for mesh sizes 12.5, 16, 20, and 25 mm. The model with the lowest deviance statistics was considered the best.
Figure 2. Gill net selectivity of combined sex yellow perch based on the log-normal distribution for 12.5, 16, 20, and 25 mm mesh sizes of micromesh gill nets (2007–2011) and 32, 38, 46, 51, 64, and 76 mm mesh sizes of large mesh gill nets (2010–2012) in southern Lake Michigan.
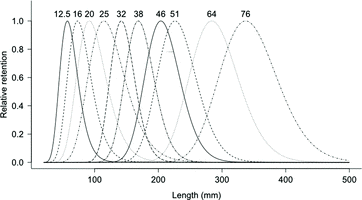
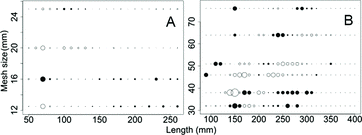
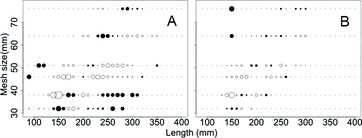
Figure 3. Deviance residual plot for the selection curves fit to combined sex yellow perch based on (A) the log-normal distribution for 12.5, 16, 20, and 25 mm mesh sizes of micromesh gill nets (2007–2011) and (B) 32, 38, 46, 51, 64, and 76 mm mesh sizes of large mesh gill nets (2010–2012) in southern Lake Michigan. Mesh sizes 12.5–46 mm were monofilament and 51–76 mm were multifilament. Circles represent residual values (open positive; closed negative), with the area of the circle proportional to the square of the residual.
In the second sampling period (2010–2012) using the large mesh gill nets, 2105 yellow perch were used in the analysis, consisting of 1994 females and 111 males (). This imbalance of fish by sex was not by design but rather represented the totality of fish caught by the sampling. The mean TLs, mm (gill net size, mm) of females captured were 151 (32), 180 (38), 201 (46), 218 (51), 264 (64), and 294 (76). Male mean TLs, mm (gill-net size, mm) captured were 150 (32), 175 (38), 205 (46), 206 (51), 228 (64), and 263 (76). With the large mesh, the log-normal model () was identified as the best model for combined sex of yellow perch (). There was no trend identified in the plot of deviance residuals ((B)). The log-normal model () also produced the best fit for females, while the normal with proportional spread model () produced the best fit for males based on model deviance (). The plot of deviance residuals did not exhibit a trend for either females or males ((A) and 5(B)). Modal lengths were longer for males than females in all size classes of gill net ().
Table 3. Summary statistics of yellow perch Perca flavescens collected in large mesh gill nets. Mesh size, minimum, maximum, median, and average are in mm.
Table 4. Results of model selection with the SELECT method for experimental gill net selectivity estimates of yellow perch Perca flavescens collected in southern Lake Michigan, 2010–2012, for mesh sizes 32, 38, 46, 51, 64, and 76 mm. The model with the lowest deviance statistics was considered the best. Parameter estimates are means and standard errors (SE).
Table 5. Female and male yellow perch Perca flavescens modal lengths (mm) from best fit selectivity models.
Figure 4. Gill net selectivity of female (A) and male (B) yellow perch based on the log-normal distribution (female) and normal with proportional spread (male) for 32, 38, 46, 51, 64, and 76 mm mesh sizes in southern Lake Michigan, 2010–2012.
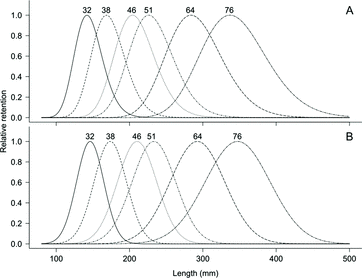
Figure 5. Deviance residual plot for the selection curves fit to female (A) and male (B) yellow perch based on the log-normal distribution (female) and normal with proportional spread (male) for 32, 38, 46, 51, 64, and 76 mm mesh sizes of large mesh gill nets (2010–2012) in southern Lake Michigan. Mesh sizes 32–46 mm were monofilament and 51–76 mm were multifilament. Circles represent residual values (open positive; closed negative), with the area of the circle proportional to the square of the residual.
Discussion
In our study of yellow perch, gill nets captured larger fish as mesh size increased. Conceptually, this relationship was expected, as all fishing gears are selective in the size and species they catch (Hayward et al. Citation1989; Hayes et al. Citation1996; Appleberg Citation2000). However, we have now established a metric for yellow perch that can relate specific gill net size with the size of fish captured. This relationship has broad and immediate implications for selective assessments by management or research scientists in the areas of, for example, broodstock analysis, recruitment, or year class strength. It has further implications for the specificity of catch and bycatch in the commercial fishing industry.
Gill nets are considered to be highly selective to fishes (Clarke & King Citation1986) and typically dependent on stretch mesh size. The gill net sizes used in this study captured yellow perch that ranged in size from 55 to 349 mm TL. This range included all fish that were of age 1 and older based upon known growth rates and maximum lengths in the Great Lakes (Hubbs & Lagler Citation2004; Headley & Lauer Citation2008). By using the entire battery of nets employed in this study, managers and researchers should be able to use this single collection gear to evaluate all yellow perch in a population, with the exception of young-of-the-year fish.
Our analyses further determined that selectivity was additionally dependent on the population size structure being fished. This impact of size structure was most evident when evaluating the difference between male and female selectivity. The maximum size of females captured was greater in every large mesh size, as well as the median and average female size for the 51–76-mm mesh. Females grow larger than males (Headley & Lauer Citation2008) in this portion of Lake Michigan and females are more abundant than males when fish exceed 200 mm (Lauer & Doll Citation2013). This historical uneven distribution of length by sex and our paucity of larger males (less than 20 each for the 38–76-mm mesh sizes) in the catch may have influenced the selectivity results. This size structure and selectivity hypothesis was also discussed by Albert Citation(2004) for the closely related European perch Perca fluviatilis, where he suggested that differences in length distributions (and hence selectivity) reflected the most abundant size classes in the study areas. Despite the differences in maximum, median, and average values between sexes in the larger mesh sizes, we are proposing that sexual differences in selectivity were not found or not biologically important. Male and female median and average lengths were the same or similar for mesh sizes 32, 38, and 46 where the numbers of both sexes appeared statistically large enough to discern sexual differences. Thus, it appears that, despite the known dimorphic differences in growth (Purchase et al. Citation2005; Uphoff & Schoenebeck Citation2013), any potential morphometric differences were not apparent in similar-sized fishes, regardless of sex.
In the selectivity models, modal lengths do not follow the trend in central tendency of yellow perch lengths by sex. Male modal lengths were larger than females for all mesh sizes; however, female median and mean lengths were larger for mesh sizes 46, 51, 64, and 76 mm. These results can also be explained by the abundance and size structure of fish available to be sampled. With limited sample size, the modal lengths could be sensitive to a few large fish. Further, females have been described as having faster growth rates than males and exhibit larger asymptotic lengths and make up a larger percentage of the population (Headley & Lauer Citation2008). Thus, more and larger females are available to be snagged, resulting in a skewed distribution with a slightly smaller mode and heavy right tail.
Snagging very large fish can also lead to biased parameter estimates if the fish are sufficiently large to be considered outliers. For example, the maximum size of the yellow perch sampled in the micromesh nets could be considered an outlier and would bias results under a model assuming normally distributed data. However, a skewed, log-normal distribution was generally the best fit model in this study.
Hamley Citation(1975) advocated that the physical attributes of the mesh, including thickness of twine, material, color, hanging scope, and method of fishing influenced selectivity. While we did not specifically test for these attributes, interpretation of the deviance residual plots does not support Hamley's Citation(1975) claim ((B) and . The large mesh gill nets were composed of monofilament and multifilament materials and if the different materials were to have a large influence on the model predicted catch, we would expect a consistent trend in residuals where one material would either catch more or fewer (i.e., higher or lower deviance residuals) than the other material. We did not see such a consistent pattern in our analysis.
Management implications
Size selectivity of sampling gear is a major obstacle in obtaining unbiased population length–frequency assessments (Ney Citation1999), which can be remedied with the use and comparison of multiple gears (Carline et al. Citation1984). In this study, using multiple mesh sizes and not multiple gears, we demonstrated that judicious sampling can effectively target specific sizes of fish. We further suggest that selectivity is closely related to mesh size and not the type of net material. This latter finding has implications for those individuals who are evaluating data collected using multiple net types and dimensions, despite having the same mesh size. Our findings also created model parameters (e.g., μ and σ) that can be used to calculate yellow perch selectivity for sizes not defined in this study. For example, if spawning stocks were of specific interest our models could be used to estimate the statistically most likely size range of a particular mesh, thus, eliminating the uncertainty associated with cursory knowledge of median or mean size captured per mesh size. Armed with this information, we can refine our sampling strategies, targeting and focusing only those fish that relate to study objectives.
Acknowledgements
We acknowledge the Indiana Department of Natural Resources (IDNR) for allowing us the use of their facilities over the period of study. We thank the many staff and students at Ball State University who have contributed to this project over the years.
Additional information
Funding
References
- Albert A. 2004. Selectivity of gillnet series in sampling of perch (Perca fluviatilis L.) and roach (Rutilus rutilis L.) in the coastal sea of Estonia. Final Project. Reykjavik: The United Nations University, Fisheries Training Programme.
- Appleberg M. 2000. Swedish standard methods for sampling freshwater fish with multimesh gillnets. Fiskeriverket Inf. 1:1–32.
- Carline RF, Johnson BL, Hall TJ. 1984. Estimation and interpretation of proportional stock density for fish populations in Ohio impoundments. North Am J Fish Manage. 4:193–154.
- Clarke DR, King PE. 1986. The estimation of gillnet selection curves for Atlantic herring (Clupea harengus L.) using length/girth relations. Journal du Conseil International pour l’Exploration de la Mer. 43:77–82.
- Evans MS, Jude DJ. 1986. Recent shifts in Daphnia community structure in southeastern Lake Michigan: a comparison of the inshore and offshore regions. Limnol Oceanogr. 31:56–67.
- Grant GC, Radomski P, Anderson CS. 2004. Using underwater video to directly estimate gear selectivity: the retention probability for walleye (Sander vitreus) in gill nets. Can J Fish Aquatic Sci. 61:168–174.
- Hamley JM. 1975. Review of gillnet selectivity. J Fish Res Board Can. 32:1943–1969.
- Hamley JM, Regier HA. 1973. Direct estimation of gillnets selectivity to walleye (Stizostedion vitreum vitreum). J Fish Res Board Can. 30:817–830.
- Hayes DB, Ferreri CP, Taylor WJ. 1996. Active capture methods. In: Murphy BE, Willis DW, editors. Fisheries techniques. 2nd ed. Bethesda, MD: American Fisheries Society; p. 193–219.
- Hayward RS, Margraf FJ, Knight CT, Glomski DJ. 1989. Gear bias in field estimation of the amount of food consumed by fish. Can J Fish Aquatic Sci. 46:959–966.
- Headley HC, Lauer TE. 2008. Density-dependent growth of yellow perch in southern Lake Michigan, 1984–2004. North Am J Fish Manag. 28:57–69.
- Holst R, Madsen N, Mouth-Poulsen T, Fonseca P, Campos A. 1998. Manual for gillnet selectivity. Hjorring: European Commission.
- Horns WH. 2001. Spatial and temporal variation in length at age and condition of yellow perch in southern Lake Michigan during 1986–1988. North Am J Fish Manag. 21:580–591.
- Hubbs CL, Lagler KF. 2004. Fishes of the Great Lakes region. Ann Arbor, MI: University of Michigan Press.
- Irwin BJ, Treska TJ, Rudstam LG, Sullivan PJ, Jackson JR, VanDeValk AJ, Forney JL. 2008. Estimating walleye (Sander vitreus) density, gear catchability, and mortality using three fishery-independent data sets for Oneida Lake, New York. Can J Fish Aquatic Sci. 65:1366–1378.
- Jensen JW. 1986. Gillnet selectivity and the efficiency of alternative combinations of mesh sizes for some freshwater fish. J Fish Biol. 28:637–646.
- Kraft CE, Johnson BL. 1992. Fyke-net and gill-net selectivities for yellow perch in Green Bay, Lake Michigan. North Am J Fish Manag. 12:230–236.
- Lauer TE, Doll JC. 2013. Dynamics and models of the yellow perch in Indiana waters of Lake Michigan and near-shore fish community characteristics. Final Report for 2012-2013, Federal Aid Project F-18-R. Indianapolis, IN: Indiana Department of Natural Resources.
- Lott JP, Willis DW. 1991. Gill net mesh size efficiency for yellow perch. Prairie Naturalist. 23:139–144.
- Mattson NS. 1994. Direct estimates of multi-mesh gillnet selectivity to Oreochromis shiranus chilwae. J Fish Biol. 45:997–1012.
- Millar RB. 1992. Estimating the size-selectivity of fishing gear by conditioning on the total catch. J Am Stat Assoc. 87:962–968.
- Millar RB. 2000. Untangling the confusion surrounding the estimation of gill selectivity. Can J Fish Aquatic Sci. 57:507–511.
- Millar RB, Fryer R. 1999. Estimating the size-selection curves of towed gears, traps, nets and hooks. Rev Fish Biol Fish. 9:89–116.
- Millar RB, Holst R. 1997. Estimation of gillnet and hook selectivity using log-linear models. ICES J Marine Sci. 54:471–477.
- Ney JJ. 1999. Practical use of biological statistics. In: Kohler CC, Hubert WA, editors. Inland fisheries management in North America. 2nd ed. Bethesda, MD: American Fisheries Society; p. 167–191.
- Purchase CF, Collins NC, Morgan GE, Shuter BJ. 2005. Sex-specific covariation among life-history traits of yellow perch (Perca flavescens). Evol Ecol Res. 7:549–566.
- Shroyer SM, McComish TS. 2000. Relationship between alewife abundance and yellow perch recruitment in southern Lake Michigan. North Am J of Fish Manag. 20:220–225.
- Uphoff CS, Schoenebeck CW. 2013. Quantifying inter-population variability in yellow perch sexual size dimorphism. J Freshwater Ecol. 27:507–516.
- Vandergoot CS, Kocovsky PM, Brenden TO, Liu W. 2011. Selectivity evaluation for two experimental gill-net configurations used to sample Lake Erie walleyes. North Am J Fish Manag. 31:832–842.
- Walsh SJ. 1992. Size-dependent selection at the footgear of a groundfish survey trawl. North Am J Fish Manag. 12:625–633.
- Wilberg MJ, Bence JR, Eggold BT, Makauskas D, Clapp DF. 2005. Yellow perch dynamics in southwestern Lake Michigan during 1986–2002. North Am J Fish Manag. 25:1130–1152.
