Abstract
Ponds represent discrete sites surrounded by a terrestrial landscape that is inhospitable for aquatic macroinvertebrates. Such ecosystems are appropriate for testing the influence of area, isolation, and correlated variables such as habitat diversity and hydroperiod, on aquatic macroinvertebrate communities. We tested three hypotheses in natural and non-impacted ponds located in a matrix of coastal dunes without human interference in southern Brazil: (1) area, habitat diversity, and hydroperiod increase the total macroinvertebrate richness and density, while isolation among ponds decreases the total macroinvertebrate richness and density; (2) the influence of environmental factors depends on the dispersal strategies of macroinvertebrates; and (3) macroinvertebrate composition is determined by the interaction of area, habitat diversity, hydroperiod, and isolation and differs among macroinvertebrates with different dispersal strategies. Fourteen ponds were sampled four times from October 2007 to August 2008 in the Lagoa do Peixe National Park coastline. Our results indicated that the pond area influenced only flying macroinvertebrate richness. The isolation and habitat diversity did not determine the richness, density, and composition of macroinvertebrates (total, flying, and non-flying). The richness, density, and composition of pond macroinvertebrates were determined mainly by hydroperiod. The mosaic created by the variation in pond hydroperiod would provide for a greater number of taxa with distinct dispersal capacities within the landscape. However, temporary ponds have disappeared at an alarming rate and there is a need to promote conservation of wetlands with different hydroperiods in southern Brazil.
Introduction
The distribution of species in insular habitats, such as wetland systems, has been an important topic in modern ecology and conservation biology. Wetlands are important sites for biological conservation because they support rich biodiversity and have high productivity (Mitsch & Gosselink Citation2000). However, biodiversity in wetlands has been reduced throughout most of the world by agricultural, urban, and industrial development (Shine & Klemm Citation1999). The rapid degradation of wetlands and the insufficient status of scientific knowledge on patterns of species richness in these systems have led to the urgent need for ecological studies. However, most of these studies were developed in modified and fragmented wetlands inserted in a landscape changed by human activities. In this sense, the understanding of species composition and richness patterns in natural and non-impacted wetlands located in landscapes without direct human interference is just as important for wetland ecology and conservation planning.
Island biogeography and metapopulation theories have been used to characterize the spatial dynamics of communities in continental ecosystems such as forests and wetlands (Harris Citation1984; Hall et al. Citation2004). The theory of island biogeography (MacArthur & Wilson Citation1967) predicts that larger islands closer to the continent have higher species richness than smaller and more isolated ones. Increased richness with increasing area may be related to a reduced extinction rate (Tracy & George Citation1992). Another explanation is related to the greater capacity of colonization by individuals – an ecosystem's colonization potential increases with area (Ricklefs & Lovette Citation1999). On the other hand, the habitat diversity hypothesis states that the effect of area on species richness is solely through habitat diversity, and larger areas are more diverse because they contain more habitat types (Ricklefs & Lovette Citation1999). Moreover, increased isolation among ecosystems reduces immigration rate and increases the chance of species extinction (MacArthur & Wilson Citation1967). However, the influence of isolation on biological communities is directly associated with the organism's ability to reach colonizable habitats (Hanski Citation1999). In isolated environments, species with low dispersal ability will likely have lower chances of colonization and higher chances of extinction than species with high dispersal ability.
Freshwater habitats can be thought of as islands of water surrounded by land, and several studies have applied the equilibrium theory to freshwater organisms (Nilsson & Nilsson Citation1978; Oertli et al. Citation2002; Brose Citation2003). However, not all groups of organisms respond similarly to habitat size and isolation (Scheffer & van Geest Citation2006). In wetlands, there is a positive relationship between macroinvertebrate richness and area for specific taxonomic groups and for macroinvertebrates in general (Oertli et al. Citation2002; Sanderson et al. Citation2005; Stenert & Maltchik Citation2007; Studinski & Grubbs Citation2007). However, other studies have shown that this relationship was not significant for either Sphaeriidae and Coleoptera or the macroinvertebrate community (Oertli et al. Citation2002; Brose Citation2003; Hall et al. Citation2004; Batzer et al. Citation2004). These conflicting results show that there is not a consensus on the species-area relationship in wetland macroinvertebrates.
The effect of isolation on macroinvertebrate communities has received less attention. Wetland macroinvertebrates occur in habitats that represent discrete sites surrounded by an inhospitable terrestrial landscape. The impact of isolation on a macroinvertebrate community depends on the dispersal ability of the organisms (Morris Citation2012). Freshwater invertebrates achieve dispersal through a variety of mechanisms that can be broadly categorized as active, especially through aerial dispersion (adult insects), or passive, which may occur through transport by animal vectors or wind and often involves a specific desiccation-resistant stage in the life cycle (Bilton et al. Citation2001). Some studies found no significant relationship between aquatic and terrestrial invertebrate richness and habitat isolation (Brose Citation2003; Scheffer & van Geest Citation2006; Jonsson et al. Citation2009). However, other studies have shown that isolation is an important parameter for snail distribution (Brönmark Citation1985) and colonization pattern of predatory aquatic insects (Wilcox Citation2001).
The most often cited factor that directly and indirectly affects pond macroinvertebrate communities is hydroperiod (duration, timing, and predictability), because it influences other abiotic (dissolved oxygen, pH, nutrients, salinity) and biotic (primary production, detritus breakdown, predation, competition) factors that affect invertebrates (Wissinger Citation1999). Larger, permanent wetlands supported a higher richness than smaller, intermittent ones because many macroinvertebrate species are not adapted to tolerating or escaping the dry phase (Williams Citation1996; Wissinger Citation1999). Moreover, macroinvertebrate communities in intermittent wetlands are distinct from those in permanent ones, because they need to have morphological, life history or dispersal mechanisms to enable survival during the dry phase (Wiggins et al. Citation1980; Williams Citation1996; Tarr et al. Citation2005). On the other hand, Wissinger et al. Citation(2009) did not find temporary-habitat specialists and the macroinvertebrate species found in temporary communities were a nested subset of those in permanent communities.
Wetlands are considered to be ecological islands in a terrestrial matrix, and such systems are appropriate to test the influence of area, isolation, and correlated variables such as habitat diversity and hydroperiod on species richness, density, and composition (Heino Citation2002; Oertli et al. Citation2002; Jones et al. Citation2003; Stenert & Maltchik Citation2007; Stenert et al. Citation2008). Most of these studies were motivated by an increase in wetland fragmentation resulting from human activities (Hall et al. Citation2004; Guadagnin & Maltchik Citation2007). However, studies analyzing the species distribution patterns in non-impacted wetlands located in landscapes without direct human interference are still rare. Since landscape factors may affect aquatic macroinvertebrate richness, density, and composition in fragmented wetlands (Stenert & Maltchik Citation2007; Stenert et al. Citation2008), and macroinvertebrates comprise a heterogeneous group of organisms with different dispersal strategies (Bilton et al. Citation2001), we tested three hypotheses in natural and non-impacted ponds in a coastal dune landscape in southern Brazil: (1) higher area, habitat diversity, and longer hydroperiod increase the total macroinvertebrate richness and density while isolation among ponds decreases the total macroinvertebrate richness and density; (2) the influence of environmental factors depends on the dispersal strategies of macroinvertebrates; and (3) macroinvertebrate composition is determined by the interaction of area, habitat diversity, hydroperiod and isolation and differs among macroinvertebrates with different dispersal strategies.
Methods
Study area
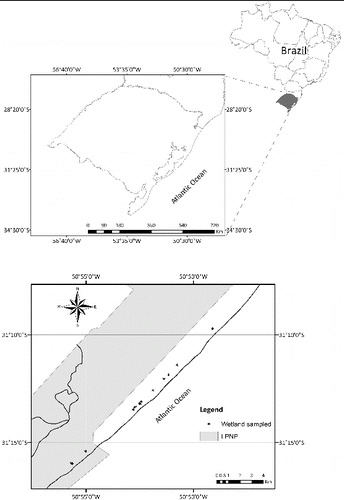
The coastal plain in southern Brazil, which covers approximately 37,000 km2 along a stretch of 640 km of the Atlantic seacoast, is one of the regions with the highest concentration of wetlands in southern Brazil (Maltchik Citation2003). The study area is located in the Lagoa do Peixe National Park (LPNP), which is the only conservation unit in southern Brazil included in the Ramsar Convention (). The conservation unit has an area of 344 km2 (31° 02′ – 31° 48′ S; 50° 77′ – 51° 15′ W) with a maximum length of 62 km and a mean width of 6 km.
Figure 1. Wetlands sampled in the coastline of Lagoa do Peixe National Park (LPNP), Rio Grande do Sul State, Brazil.
The region has a flat topography and moist subtropical climate. The absence of hills and the low altitude (<20 m above sea level) of the study area make the climatic conditions (precipitation and temperature) similar throughout the study region (Rambo Citation2000). The annual precipitation ranges between 1150 and 1450 mm with an annual mean of 1250 mm (Tagliani Citation1995). The mean temperatures range between 14.6 °C in winter and 22.2 °C in summer, and the mean annual temperature is 17.5 °C (Klein Citation1998).
Sample collection
This study was performed at the LPNP coastline (), which is characterized by small ponds distributed along a sand dune landscape with typical dune herbaceous vegetation. The ponds were identified through satellite images. Fourteen ponds were randomly selected from a total of 35 in the LPNP coastal dune region (distance less than 200 m from the sea). Four samplings were carried out from October 2007 to August 2008, and each sampling location was recorded with a global position system (GPS) receiver (GPS III Plus, Garmin, Inc., http://garmin.com). The variation of physical and chemical water parameters of each pond (salinity, conductivity, TDS, pH, temperature, dissolved oxygen, turbidity, nitrate, soluble reactive phosphorus, oxidation reduction potential) during the studied period was measured by a portable meter (U-22, Horiba, http://www.horiba.com). The water depth of each pond was measured with a graduated staff at each sampling occasion.
The area of each pond was measured during the largest flood period (winter) with a Quickbird image extracted from GIS software (Google Earth ProTM 4.2.1). Habitat diversity was quantified by counting the number of distinct habitats of dominant vegetation. Six habitat types were identified: (1) submerged vegetation (e.g., Myriophyllum aquaticum, Potamogeton spp.); (2) floating-leaved plants (e.g., Ludwigia peploides, Salvinia spp.); (3) stands of Azolla; (4) rush-like plants (emergent plants taller than 30 cm with stem-like leaves, such as Androtrichum trigynum and Juncus effusus); (5) leafy emergent plants (emergent plants taller than 30 cm with branched stems and leaves, such as grasses and sedges); and (6) low emergent plants (herbaceous plants shorter than 30 cm such as decumbent grasses, Hydrocotyle bonariensis, Xyris jupicai, and Eriocaulon modestum). The habitat diversity was the cumulative number of vegetation habitats in the pond systems. The minimum size to consider a habitat was approximately 10% of the total area of each wetland. The pond isolation was measured by the minimum distance (edge–edge) to the nearest pond in the landscape (permanent or intermittent). The hydroperiod length of each pond corresponded to the number of sampling events with surface water (1–4). Permanent ponds maintained surface water in all sampling periods and intermittent ones did not have surface water for at least one sampling period.
Macroinvertebrate sampling
At each sampling occasion, the macroinvertebrates were collected with a dip-net (30 cm wide, 250-μm mesh) by kicking up the substrate and then sweeping above the disturbed area to capture dislodged or escaping macroinvertebrates (Rosenberg et al. Citation1997). Three random sweeps of 1 m each were carried out in each identified habitat, encompassing approximately 1 m2 area sampled per habitat. The total number of sweeps per pond system ranged from 3 to 18, depending on the number of habitats (from 1 to 6). The sampling effort varied to ensure a representative measure of total richness and composition of each pond (Gotelli & Colwell Citation2001). Sweeps were pooled into 0.5-L plastic buckets and preserved in situ with 10% formaldehyde. In the laboratory, the samples were washed through a 250-μm sieve to remove leaves, stems, and other debris and the resulting material was preserved with 80% ethanol. Macroinvertebrates were identified to the level of family, according to Lopretto and Tell Citation(1995), Merritt and Cummins Citation(1996) and Fernández and Domínguez Citation(2001). Macroinvertebrates were classified into two groups according to dispersal form: (1) flying macroinvertebrates, which included only insect families and (2) non-flying macroinvertebrates, which included crustaceans, molluscs, worms, and mites.
Data analyses
The richness and abundance of macroinvertebrates per pond were determined as the total number of families and mean number of individuals collected over the year (four samplings) at each pond, respectively. The statistical analyses were performed using rarefied richness and density of macroinvertebrates. Macroinvertebrate richness was rarefied to the smallest number of individuals from a pond. Rarefaction analyses were performed separately for total macroinvertebrate, flying and non-flying richness using 1000 interactions by the vegan package on the program RTM statistical program version 2.14.2 (RDCT Citation2012). The macroinvertebrate density was calculated as the ratio between macroinvertebrate abundance and number of habitats in each pond. In other words, the total abundance found in each pond was divided by the number of habitats present in the pond to minimize the variation in the sampling effort among sites (one to six habitats). This correction was performed for total, flying, and non-flying abundance.
The correlation between environmental variables was tested using the Pearson's correlation (area, habitat diversity, isolation, and hydroperiod). First, simple linear regressions were performed to manually select which environmental variables (area, habitat diversity, isolation, and hydroperiod) should be incorporated into the multiple regression model (p < 0.25). Then, the influence of environmental variables selected on rarefied total richness and total density was analyzed by multiple linear regression with backward elimination (cut-off point at p = 0.10). The influence of environmental factors was also assessed separately for the two macroinvertebrate groups (flying and non-flying). Pond area and density were square-root transformed before statistical analyses to ensure the normality of the data set and to give less weight to the few dominant macroinvertebrate families. The analyses were performed on program R version 2.14.2, using the vegan package (RDCT Citation2012). These analyses were used to test the first and second hypotheses of our study.
Non-metric multidimensional scaling (NMDS) analysis was used to evaluate the variation of the total macroinvertebrate, flying, and non-flying composition among the coastal dune ponds. The analysis was performed with the Bray–Curtis dissimilarity index using two axes by the vegan package in program R version 2.14.2 (RDCT Citation2012). Three ordination models were performed to represent the total, flying, and non-flying macroinvertebrates. The NMDS was used to graphically represent the similarity of macroinvertebrate composition in two dimensions, but this analysis was not constrained by environmental variables. Thus, environmental variables (area, habitat diversity, isolation, and hydroperiod) were adjusted to the ordination model by the ‘envfit’ function of the vegan package (Oksanen et al. Citation2009). The correlation significance between variables and ordination axes (p < 0.05) was obtained by 1000 permutations. The data standardization ‘Wisconsin double standardization’ was performed before the NMDS analysis. Although this standardization is not equivalent to the rarefied richness for univariate data, it allowed us to reduce the influence of high values of abundance. It is indicated as a previous transformation in NMDS analysis when there are high levels of abundance in certain sites compared to others. Subsequently, we performed an indicator species analysis to verify the variation of each taxa between permanent and intermittent ponds, using the labdsv package in program R version 2.14.2 (RDCT Citation2012). The significance of the observed maximum indicator value (INDVAL) for taxa was derived from 1000 permutations. These analyses were used to test the third hypothesis of our study.
Results
The environmental variables of permanent and intermittent ponds are presented in . All ponds were considered freshwater systems since water salinity ranged from 0% to 0.1% over the study period. A total of 35,060 individuals distributed among 56 macroinvertebrate taxa were collected during the study period (). While flying macroinvertebrates (aquatic insects) were represented by 14,657 individuals and 42 families, non-flying macroinvertebrates were represented by 20,403 individuals from 14 taxa (). The most abundant insect family was Chironomidae, which comprised 53.1% of the flying macroinvertebrates collected. The majority of the insect families comprised less than 1% of the flying macroinvertebrates collected (). Dogielinotidae and Hydrobiidae were the most abundant non-flying macroinvertebrate families and represented 59.7% and 19.4% of the individuals sampled in this group, respectively (). However, Hydrobiidae occurred only in one pond over the study period ().
Table 1. Physical and chemical variables (mean ± standard deviation) of 14 ponds studied during the four seasons in southern Brazil. The pond 13 was measured only in spring.
Table 2. Total abundance, relative abundance (%), and frequency of occurrence (%) of macroinvertebrate taxa in ponds sampled in the coastline of Lagoa do Peixe National Park (LPNP), Rio Grande do Sul State, Brazil.
Table 3. Pond area, habitat diversity, isolation, hydroperiod, and macroinvertebrate (total, flying and non-flying) richness and abundance in permanent and intermittent ponds sampled in the coastline of Lagoa do Peixe National Park (LPNP), Rio Grande do Sul State, Brazil, over the annual seasons.
The values for pond area, habitat diversity, isolation, and hydroperiod in permanent and intermittent ponds are presented in . The total richness ranged from 7 to 38 families per wetland over the study period (). While flying macroinvertebrate richness ranged from 2 to 32 families, non-flying richness ranged from 2 to 12 families in the ponds (). The total macroinvertebrate density varied from 147 to 8518 individuals per pond. While the flying density ranged from 85 to 3958 individuals per pond, non-flying density ranged from 4 to 6806 individuals per pond (). None of the environmental variables studied were correlated: area and habitat diversity (r = 0.271, p = 0.349), area and hydroperiod (r = −0.227, p = 0.436), area and isolation (r = −0.361, p = 0.205), habitat diversity and hydroperiod (r = 0.209, p = 0.473), habitat diversity and isolation (r = −0.211, p = 0.470), and hydroperiod and isolation (r = −0.036, p = 0.902).
The total macroinvertebrate richness was positively related to hydroperiod (r2adj = 0.317, F1,12 = 7.043, p = 0.021). The flying macroinvertebrate richness was positively related to the area and hydroperiod (r2adj = 0.674, F2,11 = 14.5, p < 0.001). However, non-flying macroinvertebrate richness was not related to any variable (all models with p > 0.05). Wetland isolation and habitat diversity were not associated with total, flying, and non-flying macroinvertebrate richness (p > 0.05).
Total and non-flying macroinvertebrate densities were positively related to hydroperiod (r2adj = 0.317, F1,12 = 5.957, p = 0.031 and r2adj = 0.354, F1,12 = 8.131, p = 0.014, respectively). Flying macroinvertebrate density was not associated with the environmental variables (all models with p > 0.05). Pond area, isolation, and habitat diversity were not associated with total, flying, and non-flying macroinvertebrate densities (p > 0.05). These results are related to the first and second hypotheses of our study. While the first hypothesis was partially corroborated, the second was accepted since the influence of environmental factors on richness and density varied between the distinct dispersal groups of macroinvertebrates.
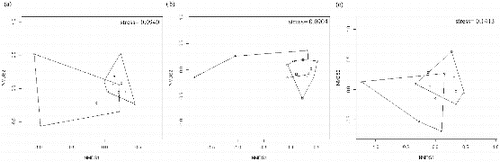
The dissimilarity of the macroinvertebrate composition ((a)), flying macroinvertebrate community ((b)), and non-flying macroinvertebrate community ((c)) were each represented by two axes in the multidimensional scaling. The gradient represented by the first NMDS axis separated permanent ponds from intermittent ones and the total, flying, and non-flying macroinvertebrate composition variations were related to hydroperiod (r2 = 0.764, p = 0.004; r2 = 0.822, p = 0.001; r2 = 0.489, p = 0.037, respectively) (). Wetland area, isolation and habitat diversity were not associated with total, flying, and non-flying macroinvertebrate composition variations. According to the species indicator analysis, Glossiphoniidae (INDVAL = 0.9507, p = 0.003), Acarina (INDVAL = 0.9225, p = 0.003), Corixidae (INDVAL = 0.9011, p = 0.047), Libellulidae (INDVAL = 0.8647, p = 0.005), Dytiscidae (INDVAL = 0.8515, p = 0.036), Hydrophilidae (INDVAL = 0.8122, p = 0.048), and Coenagrionidae (INDVAL = 0.7534, p = 0.022) were associated with permanent ponds. No macroinvertebrate taxa were characteristic of intermittent ponds (p > 0.05). These results are related to the third hypothesis of our study, showing that the macroinvertebrate composition of total, flying, and non-flying macroinvertebrates was determined by pond hydroperiod.
Figure 2. (a) Total macroinvertebrate dissimilarity of permanent (squares) and intermittent (circles) ponds, (b) flying macroinvertebrate dissimilarity of permanent (squares) and intermittent (circles) ponds, and (c) non-flying macroinvertebrate dissimilarity of permanent (squares) and intermittent (circles) ponds.
Discussion
Hydroperiod was positively related to total and flying macroinvertebrate richness and total and non-flying densities. Several studies suggest that species richness declines as hydroperiod decreases (Schneider & Frost Citation1996; Williams Citation1996, Citation2006; Wissinger Citation1999; Whiles & Goldowitz Citation2005). The surface water reduction may be one of the main causes of lower richness and abundance of wetland macroinvertebrates because the dry phase imposes such rigorous environmental conditions that only a limited number of species can survive in them (Wiggins et al. Citation1980; Williams Citation1996; Wissinger Citation1999). However, the lack of relationship between non-flying macroinvertebrate richness and hydroperiod could be explained by their strategies for enduring or avoiding periods of desiccation since non-flying macroinvertebrates are resident organisms that, for the most part, have drought-resistant life stages that usually have no autonomous means of dispersal (Wiggins et al. Citation1980; Hall et al. Citation2004).
Macroinvertebrate composition also differed between permanent and intermittent ponds. Tarr et al. Citation(2005), Collinson et al. Citation(1995), and Sanderson et al. Citation(2005) showed that macroinvertebrate taxa differed markedly along the hydroperiod gradient, with distinct genera dominating wetlands with varying hydroperiods. Furthermore, the species indicator analysis showed that some predator macroinvertebrate taxa such as Libellulidae, Coenagrionidae, Dytiscidae, Acarina, and Glossiphoniidae were observed mainly in ponds with longer hydroperiods in our study. Whiles and Goldowitz Citation(2005) stated that larger predatory macroinvertebrate taxa become abundant in wetlands with longer hydroperiods. This group consists mainly of aquatic insects that have limited or no drought-resistant life stages and are capable of active movement between wetlands during their life cycle, usually as flying adults (Wiggins et al. Citation1980). Another study found a distinct macroinvertebrate fauna composition between permanent and intermittent wetlands in southern Brazil, and predator insect families such as Gyrinidae, Belostomatidae, Naucoridae, Notonectidae, Lestidae, Aeshnidae, Tabanidae, and Ceratopogonidae were associated with permanent wetlands (Stenert & Maltchik Citation2007).
However, no macroinvertebrate taxa were characteristic of studied intermittent ponds, indicating the absence of a macroinvertebrate composition typical for this type of hydroperiod. This lack of unique taxa in the intermittent ponds goes against the classical literature of temporary waters (Wiggins et al. Citation1980; Williams Citation1996, Citation2006). On the other hand, a recent study developed in New Zealand showed that invertebrate species in temporary communities are a nested subset of those in permanent communities with the absence of temporary-habitat specialists (Wissinger et al. Citation2009). Some explanations for this result were that the hydroperiod of the studied temporary habitats in New Zealand is seasonally unpredictable and that phylogenetic constraints on the fauna have favored generalists or restricted the evolution of specialist invertebrate taxa. Stenert and Maltchik Citation(2007) also did not find typical macroinvertebrate taxa associated with intermittent wetlands. In the current study, the absence of a macroinvertebrate composition typical for intermittent ponds suggests that the taxa inhabiting intermittent ponds are a subgroup of those in permanent ones, reflecting a nested pattern of community composition. Moreover, this result may be partly interpreted in the light of the inherent biases associated with the taxonomic resolution limited to higher level such as family or order. However, family level is often adequate for revealing important characteristics of the natural ecosystems and community patterns (Furse et al. Citation1984; Heino & Soininen Citation2007; Melo & Hepp Citation2008).
Our study showed that pond area influenced only the richness of flying macroinvertebrates. Some studies also documented a significant relationship between insect communities and habitat size (Roth & Jackson Citation1987; Larson & House Citation1990). This result confirms that pond size may influence macroinvertebrate groups in different ways, and it may be associated with strategies for enduring or avoiding periods of desiccation (Wiggins et al. Citation1980). Insects are organisms that have limited or no drought-resistant life stages but they are capable of active dispersal between wetlands during their life cycle, usually as flying adults (Wiggins et al. Citation1980; Hall et al. Citation2004). Dispersal can be triggered by environmental conditions. For example, increased temperatures and falling water levels trigger dispersal in Heteroptera and Coleoptera (Bilton et al. Citation2001). Nevertheless, invertebrate species from most of the major groups, including insects, occur in small and temporary freshwater systems (Williams Citation2006). The intermittent ponds of North America are characterized by both crustaceans and insects (e.g., coleopterans, hemipterans, midges, mosquitoes, caddisflies) (Wiggins et al. Citation1980). In this sense, certain insect species specialized on small temporary wetlands because of increased food resources and lack of competition in these temporary systems.
The lack of relationship between pond area and total and non-flying macroinvertebrate richness may be related to the reduced size of the study ponds, narrow pond area range (0.01–0.24 ha) and short hydroperiod of some ponds. Species richness varies independent of area in small fragments (Lomolino & Weiser Citation2001) and several studies, including a study in southern Brazil (Stenert & Maltchik Citation2007), have not supported the relationship between richness and area in intermittent wetlands (Lake et al. Citation1989; Schneider & Frost Citation1996; Batzer et al. Citation2004). The ephemeral nature of intermittent wetlands may hinder the equilibrium between colonization and extinction (Wiggins et al. Citation1980; Lake et al. Citation1989; Hall et al. Citation2004).
Isolation is another biogeographic attribute that is associated with species richness. Isolation (distance to nearest pond) did not influence the richness, density, and composition of total, flying, and non-flying macroinvertebrate communities. The lack of relationship between richness and isolation was also verified in other studies (e.g., Brose Citation2003; Scheffer & van Geest Citation2006; Jonsson et al. Citation2009). This discrepancy may be related to the distinct dispersal capacities of macroinvertebrates and the scale of active dispersal. Active dispersal is mostly limited to insects, and wingless taxa disperse when small dormant eggs or cysts are carried by wind, water, or animal vectors (Bilton et al. Citation2001; Morris Citation2012). The capacity for flight is highly variable. While some insect taxa, such as beetles, dragonflies, and true bugs, are good flyers and may travel hundreds or thousands of kilometers (Williams Citation1957; Feng et al. Citation2006; Wikelski et al. Citation2006), shorter dispersal distances (less than 1 km) have been reported in adult mosquitoes (Service Citation1993; Bilton et al. Citation2001). In our study, isolation varied from 15 to 423 m, and this distance gradient may have not been enough to recognize the limiting effects of isolation on macroinvertebrate communities, since flying macroinvertebrates represented the majority of macroinvertebrate taxa observed. Moreover, although winged taxa are able to disperse through active flight, dispersal is often facilitated by wind (Dingle Citation1972). On the other hand, passively dispersed taxa, such as molluscs, have dormant life stages with adaptations to facilitate vector mediated dispersal (Morris Citation2012). Also, an aspect that should be considered is that the nearby ponds may be playing a role as potential source populations.
The peculiar result about the distribution pattern of Hydrobiidae in our study, where 3964 individuals occurred in only one pond, can be related to the recruitment failure of the species in the studied enclosed ponds. Some studies with a Hydrobia species (H. ulvae) show that its rare populations in small enclosed lagoons appear to suffer prolonged recruitment failure; the hypothesis that it cannot be successful in these habitats because of heavy larval mortality seems plausible. This recruitment failure would place it at a disadvantage in lagoons and prevent it from dominating mixed Hydrobia populations, and its life history strategy would restrict it to open systems. Some apparently suitable lagoon habitats lack hydrobiids or possess only declining populations of H. ulvae (Fenchel & Kofoed Citation1976; Barnes & Gandolfi Citation1998; Barnes Citation1999). The species found in this pond could be suffering the same effect (recruitment failure) in the environment studied.
Pond area only influenced flying macroinvertebrate richness. Isolation and habitat diversity did not determine macroinvertebrate richness, density, and composition (total, flying and non-flying). Our study indicated that the richness, density, and composition of pond macroinvertebrates were determined mainly by hydroperiod. The surface water reduction in these ponds decreased macroinvertebrate richness and density, modifying their composition. The mosaic created by the variation in pond hydroperiod would provide for a greater number of taxa with distinct dispersal capacities within the landscape. In this sense, our results increased the knowledge of the macroinvertebrate community patterns in southern Brazil ponds, particularly located in protected and unmodified landscapes. However, temporary ponds have disappeared at an alarming rate because these shallow wetlands are extremely sensitive to pressure from human activities (Wissinger Citation1999). Therefore, it is necessary to promote the conservation of wetlands with different hydroperiods in southern Brazil, where more than 90% of wetland systems have already been lost and the remaining ones are still at high risk due to agricultural expansion.
Additional information
Funding
References
- Barnes RSK. 1999. What determines the distribution of coastal hydrobiid mudsnails within northwestern Europe? P.S.Z.N.I.: Mar Ecol. 20:97–110.
- Barnes RSK, Gandolfi SM. 1998. Is the lagoonal mudsnail Hydrobia neglecta rare because of competitively-induced reproductive depression and, if so, what are the implications for its conservation? Aquat Conserv. 8:737–744.
- Batzer DP, Palik BJ, Buech R. 2004. Relationships between environmental characteristics and macroinvertebrate communities in seasonal woodland ponds of Minnesota. J North Am Benthol Soc. 23:50–68.
- Bilton DT, Freeland JR, Okamura B. 2001. Dispersal in freshwater invertebrates. Annu Rev Ecol Syst. 32:159–181.
- Brönmark C. 1985. Freshwater snail diversity: effects of pond area, habitat heterogeneity and isolation. Oecologia 67:127–131.
- Brose U. 2003. Island biogeography of temporary wetland carabid beetle communities. J Biogeogr. 30:879–888.
- Collinson NH, Biggs J, Corfield A, Hodson MJ, Walker D, Whitfield M, Williams PJ. 1995. Temporary and permanent ponds: an assessment of the effects of drying out on the conservation value of aquatic macroinvertebrate communities. Biol Conserv. 74:125–133.
- Dingle H. 1972. Migration strategies of insects. Science. 175:1327–1335.
- Fenchel T, Kofoed LH. 1976. Evidence for exploitative interspecific competition in mud snails (Hydrobiidae). Oikos 27:367–376.
- Feng HQ, Wu KM, Ni YX, Cheng DF, Guo YY. 2006. Nocturnal migration of dragonflies over the Bohai Sea in northern China. Ecol Entomol. 31:511–520.
- Fernández HR, Domínguez E. 2001. Guía para la Determinación de los Artrópodos Bentónicos Sudamericanos [Guide for Determining South American Benthic Arthropods]. Argentina: Universidad Nacional de Tucumán.
- Furse MT, Moss D, Wright JF, Armitage PD. 1984. The influence of seasonal and taxonomic factors on the ordination and classification of running-water sites in Great Britain and on the prediction of their macro-invertebrate communities. Freshwat Biol. 14:257–280.
- Gotelli NJ, Colwell RK. 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett. 4:379–391.
- Guadagnin DL, Maltchik L. 2007. Habitat and landscape factors associated with neotropical waterbird occurrence and richness in wetland fragments. Biodiversity and Conservation. 16:1231–1244.
- Hall DL, Willig MR, Moorhead DL, Sites RW, Fish EB, Mollhagen TR. 2004. Aquatic macroinvertebrate diversity of playa wetlands: the role of landscape and island biogeographic characteristics. Wetlands. 24:77–91.
- Hanski I. 1999. Habitat connectivity, habitat continuity, and metapopulations in dynamics landscapes. Oikos. 87:209–219.
- Harris LD. 1984. The fragmented forest. Chicago (IL): University of Chicago Press.
- Heino J. 2002. Concordance of species richness patterns among multiple freshwater taxa: a regional perspective. Biodiversity and Conservation. 11:137–147.
- Heino J, Soininen J. 2007. Are higher taxa adequate surrogates for species-level assemblage patterns and species richness in stream organisms? Biol Cons. 137:78–89.
- Jones JI, Li W, Maberly SC. 2003. Area, altitude and aquatic plant diversity. Ecography. 26:411–420.
- Jonsson M, Yeates GW, Wardle DA. 2009. Patterns of invertebrate density and taxonomic richness across gradients of area, isolation, and vegetation diversity in a lake-island system. Ecography. 32:963–972.
- Klein AHF. 1998. Regional climate. In: Seeliger U, Odebrecht C, Castello JP, editors. Os ecossistemas costeiro e marinho do extremo sul do Brasil [The costal and marine ecosystems of extreme southern Brazil]. Rio Grande: Ecoscentia; p. 5–7.
- Lake PS, Bayly IAE, Morton DW. 1989. The phenology of a temporary pond in western Victoria, Australia, with special reference to invertebrate succession. Archiv für Hydrobiologie. 115:171–202.
- Larson DJ, House NL. 1990. Insect communities of Newfoundland bog pools with emphasis on the Odonata. Canadian Entomol. 122:469–501.
- Lomolino MV, Weiser MD. 2001. Towards a more general species–area relationship: diversity on all islands, great and small. J Biogeography. 28:431–445.
- Lopretto EC, Tell G. 1995. Ecosistemas de Aguas Continentales: Metodologías para su Estudio [Continental water ecosystem: Methods for its study]. La Prata: Ediciones Sur.
- MacArthur RH, Wilson EO. 1967. The theory of island biogeography. Princeton (NJ): Princeton University Press.
- Maltchik L. 2003. Three new wetlands inventories in Brazil. Interciencia. 28:421–423.
- Melo AS, Hepp LU. 2008. Statistical tools for data analysis from biomonitoring. Oecol Bras. 12:463–486.
- Merritt RW, Cummins KW. 1996. An introduction to the aquatic insects of North America. Dubuque (IA): Kendall/Hunt Publishing Company.
- Mitsch WJ, Gosselink JG. 2000. Wetlands. New York: Wiley.
- Morris K. 2012. Wetland connectivity: understanding the dispersal of organisms that occur in Victoria's wetlands. Heidelberg: Arthur Rylah Institute for Environmental Research, Department of Sustainability and Environment.
- Nilsson SG, Nilsson IN. 1978. Species richness and dispersal of vascular plants to islands in Lake Möckeln, southern Sweden. Ecology. 59:473–480.
- Oertli B, Joey DA, Castella E, Juge R, Cambin D, Lachavanne JB. 2002. Does size matter? The relationship between pond area and biodiversity. Biol Conservation. 104:59–70.
- Oksanen J, Kindt R, Legendre P, O’Hara B, Simpson GL, Solymos P, Stevens MHH, Wagner H. 2009. Vegan: community ecology package. R package version 1.15–2; [cited 2010 Sep 25]. Available from: http://CRAN.Rproject.org/package=vegan.
- [RDCT] R Development Core Team. 2012. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; [cited 2012 Dec 13]. Available from: http://www.R-project.org.
- Rambo B. 2000. The Physiognomy of Rio Grande do Sul: Natural Monograph Assay. São Leopoldo (RS): Unisinos.
- Ricklefs RE, Lovette IJ. 1999. The roles of island area per se and habitat diversity in the species–area relationships of four Lesser Antillean faunal groups. J Anim Ecol. 68:1142–1160.
- Rosenberg DM, Davies IJ, Cobb DG, Wiens AP. 1997. Ecological monitoring and assessment network (EMAN – environment Canada) – protocols for measuring biodiversity: benthic macroinvertebrates in freshwaters. Winnipeg: Department of Fisheries & Oceans, Freshwater Institute.
- Roth AH, Jackson JF. 1987. The effect of pool size on recruitment of predatory insects and on mortality in a larval anuran. Herpetologica. 43:224–232.
- Sanderson RA, Eyre MD, Rushton SP. 2005. Distribution of selected macroinvertebrates in a mosaic of temporary and permanent freshwater ponds as explained by autologistic models. Ecography. 28:355–362.
- Scheffer M, van Geest GJ. 2006. Small habitat size and isolation can promote species richness: second-order effects on biodiversity in shallow lakes and ponds. Oikos. 112:227–231.
- Schneider DW, Frost TM. 1996. Habitat duration and community structure in temporary ponds. J North Am Benthol Soc. 15:64–86.
- Service MW. 1993. Mosquito ecology field sampling methods. London: Chapman & Hall.
- Shine C, Klemm C. 1999. Wetlands, water and the law: using law to advance wetland conservation and wise use. Gland (CH), Cambridge (UK) and Bonn: International Union for Conservation of Nature.
- Stenert C, Bacca RC, Mostardeiro CC, Maltchik L. 2008. Environmental predictors of macroinvertebrates communities in coastal wetlands of southern Brazil. Marine Freshwater Res. 59:540–548.
- Stenert C, Maltchik L. 2007. Influence of area, altitude and hydroperiod on macroinvertebrate communities in southern Brazil wetlands. Marine Freshwater Res. 58:993–1001.
- Studinski JM, Grubbs SA. 2007. Environmental factors affecting the distribution of aquatic invertebrates in temporary ponds in Mammoth Cave National Park, Kentucky, USA. Hydrobiologia. 575:211–220.
- Tagliani PRA. 1995. Estratégia de Planificação Ambiental para o Sistema Ecológico da Restinga da Lagoa dos Patos – Planície Costeira do Rio Grande do Sul [ Environmental planning strategy for Restinga ecological system of Patos Lagoon – Coastal Plain of Rio Grande do Sul (dissertation)]. São Carlos (SP): Universidade Federal de São Carlos.
- Tarr TL, Baber MJ, Babbitt KJ. 2005. Macroinvertebrate community structure across a wetland hydroperiod gradient in southern NewHampshire, USA. Wetlands Ecol Manag. 13:321–334.
- Tracy CR, George TL. 1992. On the determinants of extinction. Am Nat. 139:102–122.
- Whiles MR, Goldowitz BS. 2005. Macroinvertebrate communities in central Platte River wetlands: patterns across a hydrologic gradient. Wetlands. 25:462–472.
- Wiggins GB, Mackay RJ, Smith IM. 1980. Evolutionary and ecological strategies of animals in annual temporary pools. Arch Hydrobiol. 58:97–206.
- Wikelski M, Moskowitz D, Adelman JS, Cochran J, Wilcove DS, May ML. 2006. Simple rules guide dragonfly migration. Biol Lett. 2:325–329.
- Wilcox C. 2001. Habitat size and isolation affect colonization of seasonal wetlands by predatory aquatic insects. Isr J Zool. 47:459–476.
- Williams CB. 1957. Insect migration. Annu Rev. Entomol. 2:163–180.
- Williams DD. 1996. Environmental constraints in temporary fresh waters and their consequences for the insect fauna. J North Am Benthol Soc. 15:634–650.
- Williams DD. 2006. The biology of temporary waters. New York: Oxford University Press.
- Wissinger SA. 1999. Ecology of wetland invertebrates. In: Batzer DP, Rader RB, Wissinger SA, editors. Invertebrates in freshwater wetlands of North America: ecology and management. NewYork: Wiley; p. 1043–1053.
- Wissinger SA, Greig H, McIntosh A. 2009. Absence of species replacements between permanent and temporary lentic communities in New Zealand. J North Am Benthol Soc. 28:12–23.
