Abstract
Urban areas contribute disproportional nitrogen (N) loads to downstream aquatic ecosystems resulting in potential hypoxic ‘dead’ zones. Riparian areas along streams and rivers reduce inorganic N concentrations through denitrification, an anaerobic microbial process. Our study objective was to investigate the denitrification potential of riparian areas with differing land cover composition along the Licking River in Kentucky, USA – a tributary of the Ohio River. For one year we collected monthly samples from four sites along a 60 km reach of the Licking River. We experienced substantial drought conditions in the first half of our study period followed by record precipitation and flooding in the second half of our study period. Land cover surrounding the sample sites was classified into distinct classes hypothesized to contribute to N loads. We found flooding increased denitrification potential, water nitrate concentration, and soil organic matter, while drought conditions increased soil ammonium concentrations. Our site with the greatest impervious surface had the highest denitrification potential, and soil and water ammonium concentrations. However, we determined that denitrification potential was mostly driven by soil organic matter content and only slightly by soil nitrate among all study sites. Our data demonstrate that riparian zones of mid-sized rivers in urban areas can be integral in removing excess N during flood events and can do so year-round provided sufficient N and carbon resources are present.
Introduction
Excess nutrients from runoff continue to facilitate the formation of hypoxic zones in downstream water bodies every summer (Cooper & Brush Citation1991; Kemp et al. Citation2005); therefore, land managers and policy regulators have implemented a variety of best management practices (e.g., riparian buffers). Nitrogen (N) loads to aquatic systems can vary depending on the surrounding land use, which in turn affect N process rates. Agricultural, suburban, and urban areas contribute disproportionate amounts of carbon, suspended sediments, and nitrogen to river systems compared to more natural or undisturbed ecosystems (Meybeck Citation1982; Howarth et al. Citation1991; Vitousek et al. Citation1997; Cole et al. Citation2004). Nitrogen enrichment of large rivers occurs via point and non-point sources of organic and mineral N fertilizers from these human-induced land uses (Vitousek et al. Citation1997; Alexander et al. Citation2000; Naiman et al. Citation2002).
In urban areas, there is a substantial increase of N entering aquatic ecosystems from human activities (Howarth et al. Citation1996; Vitousek et al. Citation1997; Bernot & Dodds Citation2005). Urban watersheds are characterized by vast impervious surface, which increases hydrologic connectivity and transport of materials (i.e., organic matter and nutrients) beyond that of natural systems (Paul & Meyer Citation2001; Elmore & Kaushal Citation2008; Roy et al. Citation2009; Kaushal & Belt Citation2012). The high impervious surface and relative lack of vegetative cover in urban watersheds reduce the area available for absorption and infiltration of rainwater, increasing the amount and velocity of runoff, which has been formally called the ‘urban stream syndrome’ (Paul & Meyer Citation2001; Kohler et al. Citation2004; Walsh et al. Citation2005). Therefore, the excess N coupled with increased runoff in urban areas can result in fixed N inputs greatly exceeding those of surrounding natural areas (Jordan et al. Citation2003; Groffman et al. Citation2004; Wollheim et al. Citation2005; Kaushal et al. Citation2008).
Riparian zones, located at the interface of terrestrial and aquatic systems, can regulate the extent that terrestrial inputs modify river water chemistry (Hedin et al. Citation1998; Baker et al. Citation2007) by increasing nutrient retention and decreasing aquatic N enrichment (Brinson Citation1993; Zedler Citation2003). One way the riparian areas do this is through the natural process of denitrification, the microbial conversion of water soluble nitrate to forms of N gas (Peterjohn & Correll Citation1984; Hedin et al. Citation1998; McClain et al. Citation2003). Most types of denitrifying bacteria are found in low oxygen conditions where they utilize nitrate as the final electron acceptor to yield maximum energy (Morel & Hering Citation1993; Hedin et al. Citation1998; Boyer et al. Citation2006). The rate of denitrification is highly dependent on the availability of organic carbon, which is used as an electron donor to fuel metabolic processes, and on the concentration of nitrate in the overlying water (Hedin et al. Citation1998).
The availability of factors that drive the process of denitrification – soil moisture, soil oxygen, organic carbon, and nitrate – can vary between urban and natural ecosystems. For example, the increased rate of runoff during flooding events resulting from urban hydrology can cause soil patterns to diverge from natural systems by creating greater variability in soil moisture conditions and, therefore, soil oxygen (Pouyat & Effland Citation1998; DeKimpe & Morel Citation2000; Groffman et al. Citation2002, Citation2003). This oscillation between aerobic and anaerobic conditions can enhance both nitrification and denitrification rates (Groffman et al. Citation2002, Citation2003). Urban runoff is also known to provide a more variable supply of carbon than runoff from natural systems (Harrison et al. Citation2011; Paul & Meyer Citation2001; Kaushal & Belt Citation2012), which could lead to increased denitrification rates. Lastly, the high concentration of organic and inorganic N in urban runoff (Galloway et al. Citation2003; Mayer et al. Citation2007) can also lead to greater denitrification rates (Groffman et al. Citation2002, Citation2004; Wollheim et al. Citation2005; Harrison et al. Citation2011). Therefore, while rain events and flooding increase inputs of nutrients and organic matter into our waterways, drought conditions could result in a decreased amount of these important regulators.
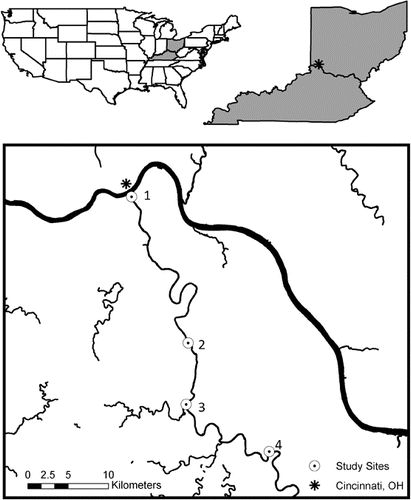
Researchers have begun to investigate differences in denitrification rates between urban and natural riparian zones with conflicting results. In studies comparing urban and forested riparian denitrification, some have found that N enrichment in the urban riparian areas led to high-potential denitrification rates compared to forested riparian areas (Groffman et al. Citation2002; Newcomer et al. Citation2012). No significant differences were found in other studies in potential denitrification between the urban and forested riparian areas due to high variability of denitrification rates and related factors, such as soil moisture and organic matter, in the urban riparian areas (Groffman & Crawford Citation2003) and due to low nitrate availability in the forested sites (Gift et al. Citation2010). The majority of published studies on riparian denitrification in urban areas have been conducted along small headwater streams, but little is known about denitrification rates in riparian areas of larger rivers. We investigated the difference in denitrification potential and regulators of denitrification in riparian areas with differing land cover composition along a mid-sized river (i.e., sixth and seventh orders). The Licking River flows northwest through eastern Kentucky towards its confluence with the Ohio River adjacent to downtown Cincinnati, OH. Specifically, we hypothesized that seasonal variation will drive higher denitrification potential rates during wet periods and lower rates during dry periods. In addition, we hypothesized that urban areas or areas with greater impervious surface would have higher denitrification potential rates due to increased N and organic matter availability and higher variability in soil moisture compared to more rural areas with less impervious surface.
Methods
Study sites
The Licking River has its headwaters in the mountains of southeastern Kentucky and flows for 488 km through rural hilly terrain, pocketed with agriculture, on its way towards its confluence with the Ohio River. The confluence divides the cities of Newport and Covington, KY, which is across the Ohio River from downtown Cincinnati, OH. The Licking River is a seventh-order river with a catchment area of 9310 km2 and an average discharge of 119.5 m3/s (USGS Citation2011). Climate in the region is within a transition zone between the northern limit of the humid subtropical climate and the southern limit of the humid continental climate. Average annual precipitation is approximately 112 cm.
Four study sites were located in the lower reach of the Licking River, beginning 0.5 km upstream of the confluence with the Ohio River (site 1), site 2 was 30.7 km upstream from the confluence in Visalia, KY, site 3 was 39.5 km upstream from the confluence south of Morning View, KY, and site 4 extended 59.3 km upstream in Butler, KY (). Geographic Information Systems (GIS) were used to determine the percent land cover. Using ArcGIS 10, a polygon representing a 1000-m river segment was created for each sampling site. The river segment was upstream from the sampling site. A 100-m buffer polygon was placed around the river segment polygon – extending 100 m upland from the river's edge on both sides of the river. Land cover within each buffer polygon was classified using a heads-up digitizing technique. Land cover describes the physical features on the landscape, while land use addresses functionality. Land cover classifications can advance our understanding of the important links between urban structure and function (Cadenasso et al. Citation2007). Land cover was defined using the following classes: coarse vegetation (trees and shrubs), fine vegetation (grasses), mixed vegetation (a relatively homogenous mix of coarse and fine vegetation), impervious surfaces (buildings and pavement), and bare soil. The area of each land cover class was summarized using the tabulate area tool in ArcGIS 10.0. Data were exported for statistical analyses.
Sample collection and analyses
Soil samples were collected monthly from each of the four sampling locations from June 2010 through May 2011. Six replicate soil cores (2.54 cm diameter by 10 cm depth) were extracted from the riparian area along the Licking River at each location during each sampling event. In the field, soil cores were placed on ice and then returned to the laboratory where they were stored in a 4 °C cooler until processing. In the laboratory, large roots and rhizomes were removed from each soil core using a 2-mm mesh sieve. A portion of each homogenized soil sample was dried at 70 °C to a constant weight and pulverized using a ball mill grinder. Organic matter content of each soil sample was obtained using the loss-on-ignition technique (Nelson & Sommers Citation1996).
Soil nitrate–N (NO3−–N) and ammonium–N (NH4+–N) were extracted from each sample with 2 M potassium chloride (KCl). NH4+–N was measured colorimetrically using a microplate reader and a method that replaces phenol with the sodium salt of 2-phenylphenol (PPS) as the substrate for the Berthelot reaction (Sims et al. Citation1995; Rhine et al. Citation1998; Sims Citation2006). NO3−–N was also measured colorimetrically on a microplate reader using the methods of Campbell et al. (Citation2006) and Ringuet et al. (Citation2011). Briefly, AtNaR2 (acquired from NECi, Lake Linden, MI) quantitatively reduced nitrate to nitrite in a phosphate buffer. All nitrite then diazotize with sulfanilamide and then react with N-(1-napthyl)ethylenediamine to form a pink color absorption that can be read at 540 nm (Patton & Kryskall Citation2011). Nitrite was negligible in our samples; therefore, for the rest of the manuscript we will refer to this measurement as NO3−–N.
Potential denitrification rates were measured using the denitrification enzyme assay (DEA, Smith & Tiedje Citation1979). All samples were amended with potassium nitrate (KNO3−), glucose, chloramphenicol, and acetylene and incubated under anaerobic conditions for 90 min (similar to Groffman et al. (Citation1999) and Hopfensperger et al. (Citation2009)). Gas samples were collected at 30 and 90 min, stored in evacuated glass vials, and analyzed for nitrous oxide (N2O) by electron capture gas chromatography. Rates were calculated from the linear rate of change in gas concentration (Groffman et al. Citation1999). During the DEA procedure, soil moisture was measured for each sample as gravimetric water content (Jarrell et al. Citation1999).
During each sampling event (Jun 2010–May 2011), two replicate water samples were taken from the Licking River at each sample location. The water samples were filtered with 0.45-μm membrane filters and analyzed for inorganic N concentration (same as above).
Statistical tests
To compare and contrast seasonal trends among the four study sites, we performed several repeated measures analysis of variance (RMANOVA) tests using a mixed model analysis with a compound symmetry covariance structure in SAS systems for Windows (SAS Institute Citation1985). For each RMANOVA, site was the independent variable and a measured quantitative variable, including denitrification potential, soil organic matter, soil water content, soil and water NH4+–N, and soil and water NO3–N, was the dependent variable (n = 96). The time factor for the RMANOVAs was separated into the drought season that occurred from June through November 2010 and the flood conditions that occurred from December 2010 through May 2011. In addition, we calculated the range, mean, and standard error for each measured variable during each season with summer represented by June–August 2010, fall was September–November 2010, winter encompassed December 2010–February 2011, and spring was March–May 2011.
ANCOVA was performed to demonstrate differences between denitrification potential and measured regulator variables among study sites. For the ANCOVAs, denitrification potential was the dependent variable, study site was the independent variable, and the co-variables were the measured regulators including soil and water NO3−–N and NH4+–N concentrations, soil organic matter, and soil water content. The assumption of homogeneity was checked and Tukey's honestly significant difference (HSD) post hoc test was performed for all ANCOVAs. To conform to assumptions of normality, denitrification potential and soil NO3−–N were log10 transformed, soil and water NH4+–N were natural log transformed, and water NO3−–N was square root transformed. Soil organic matter and soil water content did not need transformation. All statistical analyses were performed in SPSS version 19 (SPSS Statistics, Chicago, IL). Significance was determined at α = 0.05.
Results
The period of our sampling (June 2010–May 2011) included two extreme weather years for the region, which led to differences in our measured variables. The area experienced severe drought conditions in 2010 and was the 12th warmest summer in 138 years of records, with annual precipitation 20.3 cm below normal (NOAA Citation2010). In contrast, 2011 was the wettest year on record for the Cincinnati region with 186 cm of precipitation (Binau Citation2011). This included the wettest spring on record with 63 cm of rain (Binau Citation2011), which occurred during the last few months of sampling.
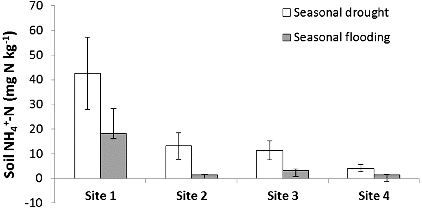
The high variability in precipitation during our study year – drought during June through November 2010 and flood conditions from December 2010 through May 2011 – led to variation in denitrification, N concentrations, and soil organic matter (). Denitrification potential was greater during flood conditions and lower during drought (F = 2.97, p < 0.01; ) and corresponded with the change in river discharge of the Licking River for the duration of our study (). Similar to denitrification, soil organic matter content of the riparian areas (F = 2.07, p = 0.04; ) and water NO3−–N concentration of the river (F = 191, p < 0.01; ) were also greater during flooding than during drought. Interestingly, as denitrification potential rates increased with flooding, soil NH4+–N decreased with flooding and increased with drought (F = 2.75, p < 0.01; ). Over the duration of the study, denitrification potential ranged from 0.01 to 760.5 ng N g dry soil−1 hr−1 and averaged 107.5 ± 15.51(SE) ng N g dry soil−1 hr−1. Site 1, with the greatest impervious surface, had the highest denitrification potential rates (F = 5.65, p < 0.01); ) and soil NH4+–N (F = 6.38, p < 0.01) during the flooded season, while site 4, the farthest site from the confluence with the Ohio River, had significantly lower soil organic matter content than the other sites during flooding (F = 15.28, p < 0.01). Riparian soil NO3−–N and river water NH4+–N showed more complex relationships with the seasonal changes in precipitation and site variation where some sites had higher concentrations during flooding while other sites had higher concentrations during drought.
Table 1. All sites averaged by season ± standard error for denitrification potential (DNF) and regulating factors. Minimum and maximum values in parentheses.
Figure 2. Average seasonal denitrification potential and standard error among study sites. Site 1 had the greatest impervious surface.
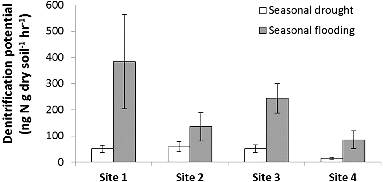
Figure 3. Average monthly riparian potential denitrification rate for all sites along the Licking River corresponds with river discharge. River discharge data were collected from USGS gage #03254520 (USGS Citation2011).
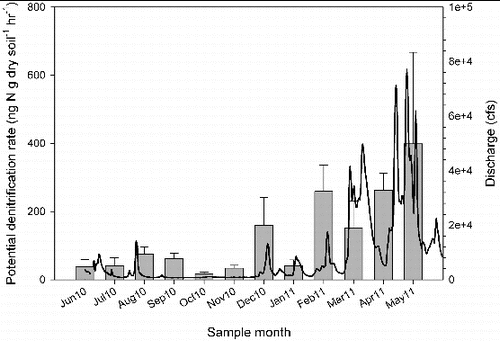
Figure 4. Average seasonal soil organic matter and standard error among study sites. Site 1 had the greatest impervious surface.
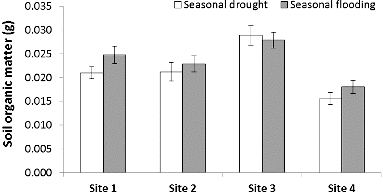
Figure 5. Average seasonal water NO3−–N concentration and standard error among study sites. Site 1 had the greatest impervious surface.
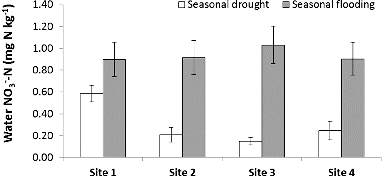
Figure 6. Average seasonal soil NH4+–N concentration and standard error among study sites. Site 1 had the greatest impervious surface.
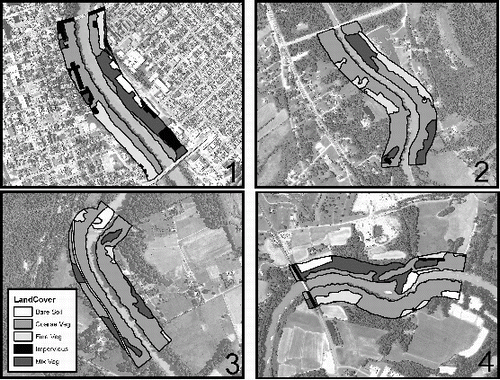
The land cover classification revealed differences in percent impervious surface, bare soil, and vegetation among the four study sites (). Study site 1 was on the banks of Newport, KY, nearly at the confluence with the Ohio River just across from downtown Cincinnati and had 24 times more impervious surface than the other study sites. Site 1 was also characterized by some bare soil and the least amount of coarse vegetation (). Site 2 had mostly vegetation with minimal impervious surface and no bare soil, while site 3 had mostly coarse vegetation with no impervious surface and some bare soil (). Site 4 had 2–10 times greater percent bare soil cover than the other sites and had some impervious surface and vegetative cover ().
Table 2. Percent cover of each cover class analyzed at each of the four study sites. Site 1 was near the confluence in Newport, KY, site 2 was 30.7 km upstream near Visalia, KY, site 3 was 39.5 km upstream near Morning View, KY, and site 4 was 59.3 km upstream in Butler, KY.
Figure 7. Aerial photographs with delineated land cover classes for study sites 1, 2, 3, and 4. Land cover classes were tabulated within a 1000 m long by 200 m wide segment upstream from each study site.
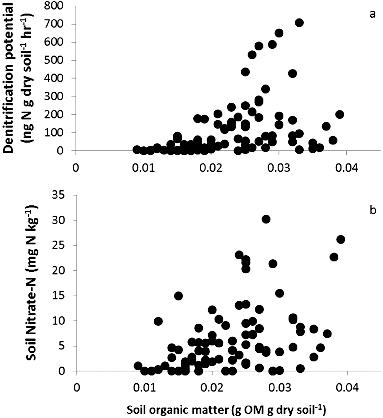
When exploring how the relationship between denitrification potential and the measured regulators varied among study sites, we found an important relationship with soil organic matter. While denitrification potential rates were greatest at site 1, the most urban site with greatest impervious surface, we found denitrification only differed among sites because it was highly related to soil organic matter (F = 26.4, p < 0.01) and slightly related to soil NO3−–N (F = 8.23, p < 0.01; ). Because site was not a factor with the relationship between denitrification potential and soil organic matter, we were able to demonstrate the increase in denitrification with soil organic matter content with all collected data (r2 = 0.33, p < 0.01; (a)). Soil NO3−–N also increased with soil organic matter content among all study sites (r2 = 0.19, p < 0.01; (b)). We did not find a relationship with denitrification potential and the regulators soil NH4+–N, soil water content, river water NO3−–N, or NH4+–N ().
Table 3. Relationship between denitrification potential rate and measured potential regulators (covariates) among study sites (analyzed using ANCOVA, n = 96, df1 = 3, df2 = 92).
Discussion
Seasonal weather events had a strong influence on denitrification potential and important regulating variables measured in our study. The record flooding in early 2011 produced our highest denitrification potential rates with the greatest rate at our urban site with the most impervious surface. In addition, flooding increased soil organic matter and water NO3−–N concentrations among our sites. High denitrification potential during flooding in urban areas is consistent with the urban watershed concept in that more nutrients are being washed into the riparian areas (Kaushal & Belt Citation2012). Kaushal et al. (Citation2011) found storm magnitude and runoff at the Baltimore Long-Term Ecological Research site to alter N contributions from various non-point sources. Urban riparian zones responding to the flooding pulse of nutrients and organic matter by increasing potential nitrogen removal rates provide an optimistic picture of the benefit of riparian zones in urban areas. Connectivity of floodplain habitats to the river can have critical impacts on N process rates in a river system (Helton et al. Citation2011) and if these riparian zones were not present, then the pulse of nutrients would simply enter the river and continue on downstream. Indeed, studies have found that urban riparian areas without a buffer zone had reduced efficiency of NO3−–N uptake (Sobota et al. Citation2012).
While some have found higher ecosystem process rates in the summer when warm temperatures stimulate microbial activity (Kadlec & Reddy Citation2001; Pina-Ochoa & Alvarez-Cobelas Citation2006), our highest rates were during the winter and spring when temperatures were cooler. Others have also found high denitrification rates in winter (Arango & Tank Citation2008; Kaushal et al. Citation2010) or no difference in rates between warm and cold seasons at all (Harrison et al. Citation2011). Denitrification rates may increase during the dormant season because the availability of NO3−–N and carbon is often higher from decomposition inputs and lack of plant assimilation (Shields et al. Citation2008). Our summer denitrification potential rates were likely reduced due to the drought but the cold season denitrification activity found during our study demonstrates the potential for year-round NO3−–N removal. Cavanaugh et al. (Citation2006) also found reduced denitrification rates due to drying of the sediments in a study in the upper Mississippi River system.
In general, the average denitrification potential rates we found in riparian areas along the Licking River were below those of other riparian areas of mid-sized rivers (). While many, including this study, have found NO3−–N to be related to denitrification (Groffman et al. Citation2002; Kaushal et al. Citation2008; Newham et al. Citation2011), perhaps the very high denitrification rates of some larger rivers, such as River Thames, are due to the high NO3−–N concentrations at these locations (). In addition, our Licking River rates were much lower than those found in urban marshes along the Mississippi and Potomac Rivers (), whereas marshes are known to have great amounts of soil organic matter compared to the clayey soils of this study. Urban riparian potential denitrification rates from this study (i.e., site 1) are lower, but within range of other urban studies ().
Table 4. Average denitrification potential (DNF) and average water nitrate concentration of soils from riparian areas and two marshes along larger rivers. Ag stands for agriculture and n.a. stands for data not available.
Table 5. Urban riparian average denitrification potential (DNF) ± standard error (SE) of soils with depth of sample used for DNF analysis. Bold indicates data from current studyand n.a. stands for data not available.
Beyond precipitation, other potential mechanisms or regulators, such as soil variables and land cover, may be contributing to the denitrification potential rates we found in our study. We found soil organic matter and soil NO3−–N to be important factors influencing denitrification potential rates. Through laboratory experiments, Newcomer et al. (Citation2012) found that when soils had plenty of available NO3−–N, organic carbon became essential in controlling rates of denitrification. Specifically, particulate organic matter comprised of grassy lawn clippings from urban areas greatly increased denitrification activity (Newcomer et al. Citation2012). Several others have also found labile organic matter inputs to partially drive denitrifier metabolism (Inwood et al. Citation2007; Arango & Tank Citation2008). Our study sites all demonstrated denitrification activity and we found soil organic matter to be the dominant driving factor of denitrification potential across sites; therefore, we suspect our sites were not NO3−–N limited.
Another potential mechanism that could explain denitrification potential patterns in our study is land cover. Study site 1, with the greatest percent impervious surface, was found to have higher denitrification potential during flooding and higher soil and water NH4+–N during drought conditions than the other study sites. These findings are consistent with the urban stream syndrome and urban watershed concepts in that greater impervious surface leads to less infiltration, greater stormwater runoff, and higher nutrient concentrations flowing through the watershed (Walsh et al. Citation2005; Kaushal & Belt Citation2012). Hoellein et al. (Citation2011) found a strong relationship between NO3−–N instantaneous yield and percent impervious surface, while others found relationships between stream water NO3−–N, a predictor of denitrification rates, and land use (Arango & Tank Citation2008). In contrast to our findings, Meyer et al. (Citation2005) found that NO3−–N decreased with increasing urbanization. They attribute this trend to low nutrient concentrations and reduced organic matter retention in highly urbanized streams; concluding that urban streams may reflect an increase of nutrient inputs, but reduction in nutrient removal (Meyer et al. Citation2005). However, we found throughout our study sites that, as soil organic matter increased, so did denitrification potential.
When comparing the process of denitrification between urban and rural areas, the literature provides mixed results for headwater streams and lacks comparative studies for larger rivers. Studies that did not find a difference in denitrification between urban and rural riparian areas attributed their findings to high variability and lack of NO3−–N at some sites (Groffman & Crawford Citation2003; Gift et al. Citation2010). While we did find variability in potential denitrification potential rates among our sites, we believe all of our study sites had enough NO3−–N and organic matter to support denitrification activity. Others found no difference in denitrification rate among land-use types but did find relationships between denitrification and carbon and nitrogen concentrations (Arango & Tank Citation2008). Similar to other studies, we also found the highest soil NH4+–N concentrations at our site with greatest percent impervious surface, perhaps demonstrating the collection of an increased nutrient load in a riparian zone along an urban river. Indeed, others have also found an increased nutrient load to stimulate denitrification activity in urban riparian zones (Groffman et al. Citation2002; Garnier et al. Citation2010; Newcomer et al. Citation2012). Newcomer et al. (Citation2012) found urbanization to influence organic carbon sources and subsequent downstream denitrification rates, while Groffman et al. (Citation2002) found hydrology and available NO3−–N to be most important.
We found seasonal precipitation affected many of our measured variables including denitrification potential, which was highest at our study site with the greatest impervious surface. However, we determined denitrification potential was mostly driven by soil organic matter content and slightly by soil NO3−–N among all study sites. These results demonstrate the importance of soil organic matter and adequate NO3−–N supply influencing denitrification potential in riparian zones along a mid-sized river; thereby, providing additional evidence of the importance of coupled carbon and nitrogen cycling enhancing NO3−–N removal along urban rivers (Sivirichi et al. Citation2011; Newcomer et al. Citation2012). In addition, we found riparian zones are not just important during the summer months, but function year-round to intercept and process excess nutrient loads.
Acknowledgements
Special thanks to Mary-Kathryn Dickerson from the Campbell and Kenton Counties Soil and Water Conservation District and Marc Hult for helping to locate our sites and providing knowledge on the Licking River. We would also like to thank the Newport Rowing Club (site 1), Mr. Meyer (site 3), and Thaxton's Canoe (site 4) for access to the river.
Additional information
Funding
References
- Alexander RB, Smith RA, Schwarz GE. 2000. Effect of stream channel size on the delivery of nitrogen to the Gulf of Mexico. Nature. 403:758–761.
- Arango CP, Tank JL. 2008. Land use influences the spatiotemporal controls on nitrification and denitrification in headwater streams. J North Am Benthol Soc. 27:90–107.
- Baker ME, Weller DE, Jordan TE. 2007. Effects of stream map resolution on measures of riparian buffer distribution and nutrient retention potential. Landscape Ecol. 22:973–992.
- Bernot MJ, Dodds WK. 2005. Nitrogen retention, removal, and saturation in lotic ecosystems. Ecosystems. 8:442–453.
- Binau S. 2011. Record breaking 2011 precipitation in the Ohio Valley – a graphical summary. National Oceanic and Atmospheric Administration (NOAA); [cited 2012 Feb 7]. Available from: http://www.erh.noaa.gov/iln/climo/summaries/wet2011/wet2011.php
- Boyer EW, Alexander RB, Parton WJ, Li C, Butterbach-Bahl K, Donner SD, Skaggs RW, Grosso SJD. 2006. Modeling denitrification in terrestrial and aquatic ecosystems at regional scales. Ecol Appl. 16:2123–2142.
- Brinson MM. 1993. A hydrogeomorphic classification for wetlands. Greenville (SC): East Carolina University.
- Burt TP, Matchett LS, Goulding KWT, Webster CP, Haycock NE. 1999. Denitrification in riparian buffer zones: the role of floodplain hydrology. Hydrol Processes. 13:1451–1463.
- Cadenasso ML, Pickett STA, Schwarz K. 2007. Spatial heterogeneity in urban ecosystems: reconceptualizing land cover and a framework for classification. Front Ecol Environ. 5:80–88.
- Campbell WH, Song P, Barbier GG. 2006. Nitrate reductase for nitrate analysis in water. Environ Chem Lett. 4:69–73.
- Cavanaugh JC, Richardson WB, Strauss EA, Bartsch LA. 2006. Nitrogen dynamics in sediment during water level manipulation on the Upper Mississippi River. River Res Appl. 22:651–666.
- Cole ML, Valiela I, Kroeger KD, Tomasky GL, Cebrian J, Wigand C, McKinney RA, Grady SP, da Silva MHC. 2004. Assessment of a delta N-15 isotopic method to indicate anthropogenic eutrophication in aquatic ecosystems. J Environ Qual. 33:124–132.
- Cooper SR, Brush GS. 1991. Long-term history of Chesapeake Bay anoxia. Science. 254:992–996.
- DeKimpe CR, Morel JL. 2000. Urban soil management: a growing concern. Soil Sci. 165:31–40.
- Elmore AJ, Kaushal SS. 2008. Disappearing headwaters: patterns of stream burial due to urbanization. Front Ecol Environ. 6:308–312.
- Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ. 2003. The nitrogen cascade. BioScience. 53:341–356.
- Garnier JA, Mounier EM, Laverman AM, Billen GF. 2010. Potential denitrification and nitrous oxide production in the sediments of the Seine River drainage network (France). J Environ Qual. 39:449–459.
- Gift DM, Groffman PM, Kaushal SS, Mayer PM. 2010. Denitrification potential, root biomass, and organic matter in degraded and restored urban riparian zones. Restoration Ecol. 18:113–120.
- Groffman PM, Bain DJ, Band LE, Belt KT, Brush GS, Grove JM, Pouyat RV, Yesilonis IC, Zipperer WC. 2003. Down by the riverside: urban riparian ecology. Front Ecol Environ. 1:315–321.
- Groffman PM, Boulware NJ, Zipperer WC, Pouyat RV, Band LE, Colosimo MF. 2002. Soil nitrogen cycle processes in urban riparian zones. Environ Sci Technol. 36:4547–4552.
- Groffman PM, Crawford MK. 2003. Denitrification potential in urban riparian zones. J Environ Qual. 32:1144–1149.
- Groffman PM, Holland EA, Myrold DD, Robertson GP, Zou X. 1999. Denitrification. In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P, editors. Standard soil methods for long-term ecological research. New York: Oxford University Press; p. 272–288.
- Groffman PM, Law NL, Belt KT, Band LE, Fisher GT. 2004. Nitrogen fluxes and retention in urban watershed ecosystems. Ecosystems. 7:393–403
- Harrison MD, Groffman PM, Mayer PM, Kaushal SS, Newcomer TA. 2011. Denitrification in alluvial wetlands in an urban landscape. J Environ Qual. 40:634–646.
- Hedin LO, Fischer JC, Ostrom NE, Kennedy BP, Brown MG, Robertson P. 1998. Thermodynamic constraints on nitrogen transformations and other biogeochemical processes at soil-stream interfaces. Ecology. 79:684–703.
- Helton AM, Poole GC, Meyer JL, Wollheim WM, Peterson BJ, Mulholland PJ, Bernhardt ES, Stanford JA, Arango C, Ashkenas LR, et al. 2011. Thinking outside the channel: modeling nitrogen cycling in networked river ecosystems. Front Ecol Environ. 9:229–238.
- Hoellein TJ, Arango CP, Zak Y. 2011. Spatial variability in nutrient concentration and biofilm nutrient limitation in an urban watershed. Biogeochemistry. 106:265–280.
- Hopfensperger KN, Kaushal SS, Findlay SEG, Cornwell JC. 2009. Influence of plant communities on denitrification in a tidal freshwater marsh on the Potomac River, U.S.A. J Environ Qual. 38:618–626.
- Howarth RW, Billen G, Swaney D, Townsend A, Jarworski N, Lajtha K, Downing JA, Elmgren R, Caraco N, Jordan T, et al. 1996. Riverine inputs of nitrogen to the North Atlantic Ocean: fluxes and human influences. Biogeochemistry. 35:75–139.
- Howarth RW, Fruci JR, Sherman D. 1991. Inputs of sediment and carbon to an estuarine ecosystem: influence of land use. Ecol Appl. 1:27–39.
- Inwood SE, Tank JL, Bernot MJ. 2007. Factors controlling sediment denitrification in midwestern streams of varying land use. Microb Ecol. 53:247–258.
- Jarrell WM, Armstrong DE, Grigal DF, Kelly EF, Monger HC, Wedin DA. 1999. Soil water and temperature status. In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P, editors. Standard soil methods for long-term ecological research. New York: Oxford University Press; p. 55–73.
- Jordan TE, Weller DE, Correll DL. 2003. Sources of nutrient inputs to the Patuxent River estuary. Estuaries. 26:226–243.
- Kadlec RH, Reddy KR. 2001. Temperature effects in treatment wetlands. Water Environ Res. 73:543–557.
- Kaushal SS, Belt KT. 2012. The urban watershed continuum: evolving spatial and temporal dimensions. Urban Ecosystems. 15:409–435.
- Kaushal SS, Groffman PM, Band LE, Elliott EM, Shields CA, Kendall C. 2011. Tracking nonpoint source nitrogen pollution in human-impacted watersheds. Environ Sci Technol. 45:8225–8232.
- Kaushal SS, Groffman PM, Band LE, Shields CA, Morgan RP, Palmer MA, Belt KT, Fisher GT, Swan CM, Findlay SEG. 2008. Interaction between urbanization and climate variability amplifies watershed nitrate export in Maryland. Environ Sci Technol. 42:5872–5878.
- Kaushal SS, Likens GE, Jaworski NA, Pace ML, Sides AM, Seekell D, Belt KT, Secor DH, Wingate RL. 2010. Rising stream and river temperatures in the United States. Front Ecol Environ. 8:461–466.
- Kemp WM, Boynton WR, Adolf JE, Boesch DF, Boicourt WC, Brush G, Cornwell JC, Fisher TR, Gilbert PM, Hagy JD, et al. 2005. Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Marine Ecolo Prog Ser. 303:1–29.
- Kohler EA, Poole VL, Reicher ZJ, Turco RF. 2004. Nutrient, metal, and pesticide removal during storm and nonstorm events by a constructed wetland on an urban golf course. Ecol Eng. 23:285–298.
- Mayer PM, Reynolds Jr. SK, McCutchen MD, Canfield TJ. 2007. Meta-analysis of nitrogen removal in riparian buffers. J Environ Qual. 36:1172–1180.
- McClain ME, Boyer EW, Dent CL, Gergel SE, Grimm NB, Groffman PM, Hart SC, Harvey JW, Johnston CA, Mayorga E, et al. 2003. Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems. 6:301–312.
- Meybeck M. 1982. Carbon, nitrogen, and phosphorus transport by world rivers. Am J Sci. 282:401–450.
- Meyer JL, Paul MJ, Taulbee WK. 2005. Stream ecosystem function in urbanizing landscapes. J North Am Benthol Soc. 24:602–612.
- Morel FMM, Hering JG. 1993. Principles and applications of aquatic chemistry. New York: John Wiley & Sons.
- Naiman RJ, Bunn SE, Nilsson C, Petts GE, Pinay G, Thompson LC. 2002. Feature legitimizing fluvial ecosystems as users of water: an overview. Environ Manag. 30:455–467.
- [NOAA] National Oceanic and Atmospheric Administration. 2010. Available from: http://www.erh.noaa.gov/iln/drought/ILNDrought.htm ( data prepared on 2010 Sep 9 by NWS Wilmington).
- Nelson DW, Sommers LE. 1996. Total carbon, organic carbon, and organic matter. In: Sparks DL, editor. Methods of soil analysis. Part 3, chemical methods. Madison (WI): Soil Science Society of America; p. 961–1010.
- Newcomer TA, Kaushal SS, Mayer PM, Shields AR, Canuel EA, Groffman PM, Gold AJ. 2012. Influence of natural and novel organic carbon sources on denitrification in forest, degraded urban, and restored streams. Ecol Monogr. 82:449–466.
- Newham MJ, Fellows CS, Sheldon F. 2011. Functions of riparian forest in urban catchments: a case study from sub-tropical Brisbane, Australia. Urban Ecosystems. 14:165–180.
- Orr CH, Stanley EH, Wilson KA, Finlay JC. 2007. Effects of restoration and reflooding on soil denitrification in a leveed Midwestern floodplain. Ecol Appl. 17:2365–2376.
- Patton CJ, Kryskall JR. 2011. U.S. Geological Survey Techniques and Methods (Book 5). Chapter B8, Colorimetric determination of nitrate plus nitrite in water by enzymatic reduction, automated discrete analyzer methods. Reston (VA): U.S. Geological Survey; p. 34.
- Paul MJ, Meyer JL. 2001. Streams in the urban landscape. Annu Rev Ecol Evol Syst. 32:333–365.
- Peterjohn WT, Correll DL. 1984. Nutrient dynamics in an agricultural watershed: observations on the role a riparian forest. Ecology. 65:1466–1475.
- Pina-Ochoa E, Alvarez-Cobelas M. 2006. Denitrification in aquatic environments: a cross-system analysis. Biogeochemistry. 81:111–130.
- Pouyat RV, Effland WR. 1998. The investigation and classification of human modified soils in the Baltimore ecosystem study. In: Kimble J, editor. Classification, correlation, and management of anthropogenic soils. Lincoln (NB): USDA Natural Resource Conservation Service; p. 141–154.
- Rhine ED, Sims GK, Mulvaney RL, Pratt EJ. 1998. Improving the Berthelot reaction for determining ammonium in soil extracts and water. Soil Sci Soc Am J. 62:473–480.
- Ringuet S, Sassano L, Johnson ZI. 2011. A suite of microplate reader-based colorimetric methods to quantify ammonium, nitrate, orthophosphate and silicate concentrations for aquatic nutrient monitoring. J Environ Monit. 13:370–376.
- Roy AH, Dybas AL, Fritz KM, Lubbers HR. 2009. Urbanization affects the extent and hydrologic permanence of headwater streams in a Midwestern US metropolitan area. J North Am Benthol Soc. 28:911–928.
- SAS Institute. 1985. SAS: statistics [CD-ROM]. Version 5.0. Cary (NC): SAS Institute, Inc.
- Shields CA, Band LE, Law N, Groffman PM, Kaushal SS, Savvas K, Fisher GT, Belt KT. 2008. Streamflow distribution of non-point source nitrogen export from urban-rural catchments in the Chesapeake Bay watershed. Water Resour Res. 44:W09416.
- Sims GK. 2006. Letter to the Editor on ‘‘Using the Berthelot Method for Nitrite and Nitrate Analysis’’. Soil Sci Soc Am J. 70:1038.
- Sims GK, Ellsworth TR, Muvaney RL. 1995. Microscale determination of inorganic nitrogen is soil and water extracts. Commun Soil Sci Plant Anal. 26:303–316.
- Sivirichi GM, Kaushal SS, Mayer PM, Welty C, Belt KT, Newcomer TA, Newcomb KD, Grese MM. 2011. Longitudinal variability in streamwater chemistry and carbon and nitrogen fluxes in restored and degraded urban stream networks. J Environ Monit. 13:288–303.
- Smith MS, Tiedje JM. 1979. Phases of denitrification following oxygen depletion in soil. Soil Biol Biochem. 11:262–267.
- Sobota DJ, Johnson SL, Gregory SV, Ashkenas LR. 2012. A stable isotope tracer study of the influences of adjacent land use and riparian condition on fates of nitrate in streams. Ecosystems. 15:1–17.
- [USGS] U.S. Geological Survey. 2011. National hydrography dataset high-resolution flowline data [ cited 2011 June 13]. Available from: http://viewer.nationalmap.gov/viewer/
- Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG. 1997. Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl. 7:737–750.
- Walsh CJ, Roy AH, Feminella JW, Cottingham PD, Groffman PM, Morgan RP. 2005. The urban stream syndrome: current knowledge and the search for a cure. J North Am Benthol Soc. 24:706–723.
- Wollheim WM, Pellerin BA, Vorosmarty CJ, Hopkinson CS. 2005. N retention in urbanizing headwater catchments. Ecosystems. 8:871–884.
- Zedler JB. 2003. Wetlands at your service: reducing impacts of agriculture at the watershed scale. Front Ecol Environ. 1:65–72.