Abstract
Many bluegill (Lepomis macrochirus) populations are characterized by a stunted adult size structure and consist primarily of individuals that mature at a relatively young age and small size. Little is known about maternal effects in bluegill populations and whether they may explain the occurrence of stunted growth. We spawned bluegill from both stunted and non-stunted populations in experimental tanks and collected eggs and larvae from each clutch. We determined whether differences in egg and oil globule diameter and larval growth existed between eggs collected from females from each population. Egg and oil globule diameter were not related to female length and did not differ between stunted and non-stunted populations. Larvae length after 21 d was positively related to egg diameter but was not related to either female length or oil globule diameter. Consequently, egg diameter had a greater influence on larval growth than female source population or size. No differences were observed between source populations indicating that some aspects of early life history are more heavily influenced by egg properties than adult life history differences between parental source populations.
Introduction
Phenotypic variation in life-history traits can be influenced by a variety of factors. Long-term selective pressures of an environment can result in differences in genotype among populations (Naevdal Citation1983; Rijnsdorp Citation1993) or, alternatively, phenotypic differences can be plastic and respond to immediate changes in environmental conditions (Kaplan & Cooper Citation1984). Maternal effects can also explain differences in life-history traits among populations (Heath & Blouw Citation1998) and occur when female body size, age, or condition influences offspring fitness (Heath & Blouw Citation1998). Nutrients or toxins assimilated in the environment can be transferred to offspring during development (Rossiter Citation1996) and some consider the transfer of cytoplasmic genes a maternal effect (Whitehouse Citation1973; Wolf & Wade Citation2009). Maternal effects are prevalent among many species of fish with the most commonly reported being a positive correlation between egg diameter and female length (e.g., rainbow trout Oncorhynchus mykiss, Springate & Bromage Citation1985; Arctic char Salvelinus alpinus, Wallace & Aasjord Citation1984; channel catfish Ictalurus punctatus, Reagan & Conley Citation1977). Another common maternal effect is a positive relationship between egg size and larval growth (Marteinsdottir and Steinarsson Citation1998; Venturelli et al. Citation2010). The majority of maternal effect studies in fish have focused on species that readily reproduce in the laboratory, species of commercial interest, or commonly cultured species with few studies conducted on other fishes.
Bluegill sunfish Lepomis macrochirus are a popular sport fish (Drake et al. Citation1997). The egg diameter in bluegill is 1.2–1.4 mm and are similar in diameter to other species in the genus Lepomis (Meyer Citation1970). Larval bluegill growth rates vary with latitude and range from approximately 55 mm during the first year of life in Canada (Cargnelli & Gross Citation1996) to approximately 80 mm in the southern United States. Relationships between egg size and larval growth appear not to have been described for any Lepomis species. Also, over the past 50 years, there has been a gradual decline in the size of bluegill harvested by anglers (Coble Citation1988; Drake et al. Citation1997). Managers categorize many bluegill populations as stunted (Drake et al. Citation1997), where individuals have a maximum size that is much smaller than conspecifics in other populations (Aday & Graeb Citation2012). In bluegill, a significant proportion of fish in these stunted populations also matures at an earlier age than is typical in non-stunted populations (Coble Citation1988; Drake et al. Citation1997). Traditionally, stunting in bluegill has been attributed to excessive angler harvest (Goedde and Coble Citation1981; Coble Citation1988), low predator densities (Swingle Citation1950; Guy & Willis Citation1990), or inadequate food supplies (Gerking Citation1962, Jennings et al. Citation1997). It has also been demonstrated that non-stunted populations tend to occur in populations where male bluegill delay maturation (Aday, Wahl, et al. Citation2003). Multiple studies have demonstrated that plasticity in size and age of maturation are more influenced by environmental differences among populations than genetic differences (Belk Citation1995; Aday, Wahl, et al. Citation2003; Oplinger et al. Citation2011). No studies have evaluated whether differences in early life history exist between populations with stunted and non-stunted size structures. In addition, maternal influences on life history are not known.
Herein, we assess factors that influence bluegill egg properties (egg and oil globule diameter) and larval growth. We compared these properties between females from both stunted and non-stunted populations to determine whether life-history traits (e.g., size and age of maturation) influence these relationships. In addition, we determined whether female size has an influence on early bluegill life history. We hypothesized that egg properties are larger and larval growth is faster among individuals produced by females from the non-stunted population. We also hypothesize that larger diameter eggs with bigger oil globules tend to produce faster growing larvae.
Methods
Spawning was monitored from May 15–August 30 using twice-daily egg inspection in each of three years (2005–2007). Each year, bluegill were collected in late April using an AC boat electrofishing unit (240 V, 6–10 A) from one stunted (Pana Lake, IL) and one non-stunted (Forbes Lake, IL) population. The classification of each population as stunted or non-stunted is based on average fish length, timing of sexual maturation, and fisheries manager experience (Diana et al. Citation2007). Bluegill in the non-stunted population were on average greater in length and matured later than those in the stunted population. Average fish lengths and timing of sexual maturation in Pana Lake and Forbes Lake are similar to those of other stunted and non-stunted populations in Illinois (; Diana et al. Citation2007). Beyond these differences in bluegill size and age of maturation, both lakes were similar in terms of size, depth, fish species composition, and invertebrate prey community composition (), and are representative of other stunted and non-stunted populations in the region (Diana et al. Citation2007). Routine bluegill samples from 1996 to 2006 showed that over that time period, there has been little change in the size and the age of maturation of bluegill in these populations (Diana et al. Citation2007).
Table 1. Total length (based on creel surveys), proportion of sexually mature males longer than 170 mm total length (PQM), and age of maturation of male and female bluegill in Forbes Lake (non-stunted), Pana Lake (stunted), and compared to other stunted and non-stunted populations in Illinois (n = 16 of each population; Diana et al. Citation2007). Values represent means with standard errors in parentheses.
Table 2. Reservoir morphometry, primary production, prey community composition, fish community composition, and watershed data from Forbes and Pana Lakes, IL, USA. Values are averages from biweekly samples collected between 1996 and 2006. Further description of the methods used to collect these data can be found in Diana et al. (Citation2007).
Upon collection, fish were transported to the Kaskaskia Biological Station (Illinois Natural History Survey) and placed in 1300-L, circular (1.8 m diameter × 0.7 m deep) plastic tanks. In the first year, 24 tanks (12 per female source population) were established, and 36 tanks (18 per female source population) were established in the two subsequent years with newly collected fish. Three females (all from the same source population) were placed into each tank in late April (prior to the start of the spawning season). Two males, both from the same population, were also placed into each tank. A low percentage of fish spawned (see Results) and several fish were placed in each tank to increase the odds of collecting eggs. One circular, plastic nesting tray (25 cm diameter × 8 cm deep) filled with pea gravel was also placed into each tank (Mischke & Morris Citation1997). Four white, ceramic tiles (5 × 5 cm) were placed into each nesting tray to aid in egg observation and collection. Bluegill spawned in these nesting trays were never observed to nest elsewhere in the tanks. The three females in each tank were similar in size with the difference in length between the smallest and largest females never exceeding 4 mm. The females were selected to represent the entire size range of mature females present in each population (110–180 mm total length, TL). To prevent inherent differences in size structure between the two populations from biasing our results, this size range was divided into 10 mm increments (110–119, 120–129, 130–139 mm, etc.), and an equal number of tanks from each population were created from each increment. Since paternal size effects are seldom reported in the literature, no attempt was made to group males on the basis of size; however, all of the males were between 150 and 170 mm TL.
Adult bluegill were fed ad libitum with commercial fish pellets (fed all, they could consume in 3 min, fed five times/d). At the beginning of the experiment each year, a 0.5 m diameter, 64-μm mesh net was used to collect zooplankton from several area lakes to inoculate the tanks and establish a zooplankton population to provide prey for larval bluegill. Prior to inoculation, the plankton sample was passed through a 500-μm sieve to remove any inadvertently collected fish. Zooplankton samples were collected from each tank daily by passing 1.0 L of water from each tank through a 63-μm sieve. The collected sample was re-suspended in 10 mL of water. Three, 1-mL aliquots were then enumerated under a microscope. Additional zooplankton were added to the tanks when densities declined below 500 zooplankton/L to ensure ad libitum feeding (Hoxmeier et al. Citation2004).
Upon spawning, a sample of 50–100 eggs was removed from the ceramic tiles and preserved in 5% unbuffered formalin (Markle Citation1984). The remaining eggs were left in the tank and were allowed to hatch. The parental male was left in the tank to care for his eggs and larvae (all other adults removed) but was removed once the larvae became free swimming. Because fish were collected prior to the start of the spawning season, it was assumed that the adults were participating in their first spawn of the season. No adults were allowed to participate in multiple spawning bouts. Twenty-one days after the eggs were deposited (the earliest age larvae could be handled without damage), a sample of 15 larvae was removed from each tank and preserved in 95% ethanol.
Using the preserved egg and larvae samples, egg diameter, oil globule diameter, and larval length were measured using a calibrated digitizing tablet running SigmaScan software (Jandel Scientific Software) attached to a dissecting microscope. All egg measurements were based on eggs collected 2–5 h after deposition and the embryo was yet visible within the eggs. All egg and larval metrics were averaged to produce a mean value for each tank. In addition, because we did not know which female deposited eggs in a tank, the lengths of all of the females were averaged. Because the difference in length between the largest and smallest females in a tank never exceeded 4 mm, this average total length is close to the length of the female that released the eggs. The effect of female age was not considered because stunting has been associated with differences in female age of maturation among populations (Aday, Wahl, et al. Citation2003). In addition, saggital otolith samples revealed that even though each tank contained similar-sized fish, there was variation in female age (otoliths read whole view at 25x magnification by two experienced readers). Paired t-tests comparing average age of females used in experiments (broken into 10 mm increments from 110 to 180 mm TL) revealed no differences in age-at-length between populations (T1,7 = 0.72, p = 0.49).
Two sets of correlations were performed using these data. First, we determined how egg and oil globule diameter were related to female total length. Second, we determined how female length and the egg properties were related to larvae size. Regressions first were performed separately for each maternal source population and then the data from both sources were combined to determine whether more general patterns occurred. ANCOVA was used to determine differences in slopes of regression lines and intercepts between maternal source populations. All analyses were performed using the MIXED procedure in SAS (Citation2003) and were considered statistically significant at p ≤ 0.05. There were no differences in egg size or oil globule diameters among years (ANOVA, all p > 0.20). Therefore, the data from all years were combined for analysis.
Results
We successfully collected eggs from 35 of the 96 tanks that were established in the experiment (Year 1: 13 of 24 tanks, Year 2: 18 of 36 tanks, Year 3: 4 of 36 tanks). Twenty-two of these egg clutches were spawned from females from the stunted population and the remaining 13 were from the non-stunted population. The eggs from all 35 tanks hatched; unfortunately, larval numbers and survival were low in some tanks (<30 fry surviving after 21 d). Thus, we collected 21-d larval samples from 16 tanks (12 stunted and 4 non-stunted). We monitored the tanks for spawning activity between 15 May and 30 August each year; however, no spawning activity was observed after 30 June of each year and 30 of the 35 clutches of eggs collected were produced prior to 15 June.
Overall, the mean diameter of the collected eggs was 1.31 ± 0.02 mm (mean ± 1 SE, n = 33 clutches) and did not differ between source populations (t1,31 = 1.14, p = 0.26; stunted: 1.30 ± 0.02 mm; non-stunted: 1.34 ± 0.03 mm). There was more variation in egg diameter between clutches (coefficient of variation, CV = 0.09) than within individual clutches (CV = 0.05). There was no significant relationship between female length and egg diameter for either source population (linear regression; both p ≥ 0.19; ). Slopes and intercepts did not differ between source populations (slope: ANCOVA, F1,31 = 1.36, p = 0.25; intercept: ANOVA, F1,32 = 1.15, p = 0.29). In addition, when the data for both source populations were combined, there was no relationship between egg diameter and female length (linear regression: n = 35, r2 < 0.01, p = 0.97).
Figure 1. Relationship between egg diameter (mm, panel A), oil globule diameter (mm, panel B), and female total length (mm) of bluegill eggs from both non-stunted (closed circles) and stunted populations (open circles). Points denote mean values for each tank. Trend lines (solid for non-stunted, dashed for stunted) show the best-fit least-squares linear regression line for each source population.
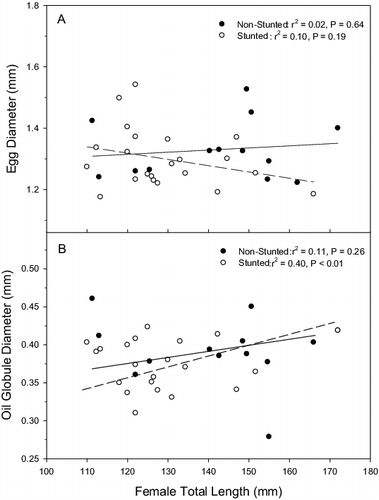
The mean oil globule diameter of the eggs was 0.38 ± 0.01 mm with more variation within (CV = 0.13) than between clutches (CV = 0.09). The relationship between female length and oil globule diameter was significant for only the stunted population (linear regression; ; stunted: oil diameter = 0.19 + (0.0014 * female TL), p < 0.01; non-stunted: oil diameter = 0.28 + (0.0008 * female TL), p = 0.26). The slopes and intercepts also did not differ between source populations (slope: F1,31 = 0.95, p = 0.34; intercept: F1,32 = 2.08, p = 0.10). When the data for both source populations were combined, there was also no relationship between female length and oil globule diameter (n = 35, r2 < 0.01, p = 0.77).
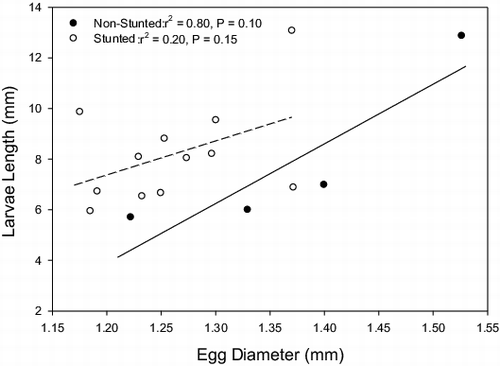
At 21 d of age, larval total length averaged 7.97 ± 0.51 mm and did not differ between maternal source populations (t-test: t1,12 = 1.15, p = 0.27; stunted: 8.3 ± 0.66 mm, non-stunted: 7.1 ± 0.43 mm). For both source populations, there was no relationship between female length and larval length (both p ≥ 0.22). The slopes and intercepts of the relationship between larval size and female size did not differ between source populations (slope: F1,15 = 0.53, p = 0.48; intercept: F1,15 = 0.02, p = 0.90). In addition, when both source populations were combined, there was no significant relationship between female length and larval size (n = 16, r2 = 0.19, p = 0.09, p = 0.76). Similarly, the larval size at 21 d was not related to either egg or oil globule diameter and did not vary between source populations (p ≥ 0.10 in all cases). For both source populations combined, there was a significant, positive relationship between egg diameter and larval length (, n = 16, r2 = 0.28, p = 0.03); however, the relationship between oil globule diameter and larval total length was not significant (n = 16, r2 = −0.06, p = 0.38).
Figure 2. Relationship between egg diameter and the total length of 3-week-old bluegill larvae that were spawned in experimental tanks. Points denote mean values for each tank. Trend lines (solid for non-stunted, dashed for stunted) show the best-fit least-squares linear regression line for each source population.
Discussion
Our findings indicate that larval properties in bluegill are not caused by genetic differences in females between stunted and non-stunted populations. These results are consistent with other studies that have shown that other life-history traits (the age and size of maturation) are more heavily influenced by factors such as population, social structure, or predation pressure rather than genetic differences among populations (Belk Citation1995; Aday, Wahl, et al. Citation2003; Oplinger et al. Citation2011). We expected that small environmental differences between stunted and non-stunted populations could have influenced the egg properties. Environmental factors such as nutritional status (Izquierdo et al. Citation2001) and stress (Schreck et al. Citation2001) can cause plasticity in egg properties. In the wild, the larval bluegill growth has been shown to vary with zooplankton community composition (Wittenrich et al. Citation2009), and generally increases with declining adult density (Rettig & Mittelbach Citation2002), increasing zooplankton abundance (Welker et al. Citation1994), decreasing gizzard shad (Dorosoma cepedianum) density (Oplinger et al. Citation2013), and increasing temperature (Belk and Hales Citation1993). Such environmental effects may be important in determining the growth of larval bluegill in the wild but were likely obscured by the controlled conditions (i.e., similar temperature, food resources, and predation pressure) in our experimental mesocosms. The females spawned within 4–6 weeks after collection from the wild in our experiment. Even though the females from each population were housed under identical conditions, the potential exists for domestication prior to spawning. However, given the short time period until spawning, it is likely that the environmental differences between the stunted and non-stunted populations would have had some carry-over effect and would thus have had an influence on the egg properties if they existed.
Even though genetic and environmental differences between source populations had little effect on early bluegill life history, maternal effects did influence the growth of larval bluegill. We found a positive relationship between egg diameter and larval growth. Similar to our findings, the majority of previous studies with fish that have assessed the relationship between egg diameter and larval growth have shown the two to be positively correlated (e.g., Bagenal Citation1969; Elliott Citation1984; Springate & Bromage Citation1985; Marsh Citation1986). Bluegill larvae that hatched from larger eggs were longer at hatch than those produced by smaller eggs, translating into larger, better developed larvae. Egg effects may have a significant influence on the early life history of bluegill since individuals that hatch from larger eggs will outgrow the gape of predators at a younger age and are less prone to starvation (Marsh Citation1986; Jonas & Wahl Citation1998). In addition, individuals that hatch from larger eggs may begin to forage on prey items earlier and optimize growth at a younger age (Mayer & Wahl Citation1997; Claramunt & Wahl Citation2000) allowing for greater larval size.
In addition to maternal influences, other factors including diet, stress, and female endocrine status can influence the size of eggs that are produced (Brooks et al. Citation1997). Genetic influences on egg size are poorly understood (Brooks et al. Citation1997), although for walleye, environmental factors influence egg size more than genetic factors (Johnston and Leggett Citation2002). We found that the egg size had a significant influence on larval growth of bluegill. No differences in egg properties between bluegill populations indicate that neither genetic nor environmental effects have a significant influence on egg size. In addition, egg size was not related to female length. Consequently, it appears that egg diameter varies by individual. We could not assess the effect of female condition on the egg properties because with multiple females in each tank, we were not certain of the specific female that spawned. Regardless, it is unlikely that there were large differences in condition between fish from both source populations because at the beginning of the experiment average relative weights (Wr) of the three females added to each tank were similar between the stunted (96.2 ± 10.4) and non-stunted (95.3 ± 8.3) populations.
The diameters of the eggs that we collected were similar to other Lepomis spp. (1.0–1.6 mm). We found that female length had little influence on egg and oil globule diameter and that the relationship did not differ between stunted and non-stunted populations. Although numerous studies have shown a positive relationship between egg size and female length in fishes (e.g., Wilson & Millemann Citation1969; Beacham & Murray Citation1985), several other studies have shown this relationship to be either negative (e.g., Iguchi & Yamaguchi Citation1994) or non-significant (e.g. Marsh Citation1984; Mire & Millett Citation1994). Most studies that have found a positive correlation between egg size and female size were conducted on larger fish species that have a wide range of adult lengths. Positive correlations are observed less frequently among smaller species such as bluegill. In our study, the lack of a difference in egg diameter between source populations could possibly be attributed to a small overall difference in length (<30 mm) among females from each source population. Some of the variation in egg properties could be attributed to differences in female age, but we were not able to simultaneously control for the effects of both female size and age, and further research on maternal age effects is warranted.
Little is known about the egg properties in fractional spawning fish species with multiple spawning bouts such as bluegill. The egg diameters observed in our study (1.31 ± 0.02 mm) fall within the range that has been previously reported for bluegill (1.2–1.4 mm, Morgan Citation1951; Meyer Citation1970). Similarly, the mean oil globule diameter we observed was identical to other published findings on bluegill (Childers Citation1967). However, we recovered three clutches of eggs that had a mean diameter of 1.50–1.55 mm, which are larger than that has been previously reported. Only a portion of the eggs are released from the ovaries during each bout and females may regulate the amount of energy invested into each clutch based on the perceived potential for survival and growth of each clutch (Cargnelli & Neff Citation2006). Thus, it is possible that egg and oil globule size varies among clutches. Egg diameter has been shown to decline through successive spawns in Atlantic cod (Gadus morhua) (Chambers & Waiwood Citation1996). It is not known if egg size decreases across successive bouts in bluegill, which would indicate less energy investment into reproduction later in the spawning season in anticipation of poor survival among later spawned eggs. All of our samples were collected during the first spawning bout of the season as the fish were collected from the lakes prior to the spawning season. As a result, we controlled for spawning bout and the effect that it may have on egg diameter.
Other studies have tried to determine why differences in size and age of maturation occur among bluegill populations. These other studies have attributed these differences to predator density (Oplinger et al. Citation2011), competitor density (Aday, Hoxmeier, et al. Citation2003), gizzard shad (Oplinger et al. Citation2013), and angling pressure (Coble Citation1988). In addition, much recent research has emphasized the role that population social structure has on determining adult size structure (Oplinger et al. Citation2013), and juvenile male bluegill tend to delay maturation and mature at a larger size in populations that have a high density of larger males (Aday, Wahl, et al. Citation2003). Population-specific influences that female bluegills have on growth rates were previously reported and we observed no differences in egg properties or larval growth between the stunted and non-stunted maternal source populations. Differences in other maternal characteristics between populations may have a stronger influence on these egg and larval properties. Although bluegill egg size was not related to female length, it is possible that the egg size is related to other phenotypic factors we did not assess such as female age, weight, or body condition. Larval growth was most heavily influenced by egg and oil globule size; however, it is not known what, if any, maternal characteristics determine these egg properties. It is also not clear if maternal egg properties have an effect on ultimate size and age of maturation or if these effects could have a stronger influence on adult life history of progeny than those found in other studies. The rearing of bluegill in experimental mesocosms potentially obscured the effect that environment may have on early bluegill life history. Studies comparing the growth of larval bluegill among lakes with stunted and non-stunted bluegill populations would help elucidate the effect that rearing environment may have on early development.
Acknowledgements
We thank L. Einfalt, C. DeBoom, and the staff of the Kaskaskia and Sam Parr Biological Stations (Illinois Natural History Survey) for their assistance in the laboratory and field. The manuscript was improved with comments by reviewers and the Aquatic Ecology Discussion Group of the Kaskaskia Biological Station. We thank S. Pallo, L. Dunham, M. Conlin, and S. Stuewe for coordinating activities with the IDNR.
Additional information
Funding
References
- Aday DD, Graeb BDS. 2012. Stunted fish in small impoundments: an overview and management perspective. In: Neal JW, Willis DW, editors. Small impoundment management in North America. Bethesda (MD): American Fisheries Society; p. 215–232.
- Aday DD, Hoxmeier RJH, Wahl DH. 2003. Direct and indirect effects of gizzard shad on bluegill growth and population size structure. Trans Am Fish Soc. 132:47–56.
- Aday DD, Wahl DH, Philipp DP. 2003. Assessing population-specific and environmental influences on bluegill life histories: a common garden approach. Ecology. 84:3370–3375.
- Bagenal TB. 1969. Relationship between egg size and larval survival in brown trout Salmo trutta L. J Fish Biol. 1:349–353.
- Beacham TD, Murray CB. 1985. Effect of female size, egg size, and water temperature on developmental biology of chum salmon (Oncorhynchus keta) from the Nitinat River, British Columbia. Can J Fish Aquat Sci. 42:1755–1765.
- Belk MC. 1995. Variation in growth and age at maturity in bluegill sunfish: genetic or environmental effects? J Fish Biol. 47:237–247.
- Belk MC, Hales LS. 1993. Predation-induced differences in growth and reproduction of bluegills (Lepomis macrochirus). Copeia. 1993:1034–1044.
- Brooks S, Taylor CR, Sumpter JP. 1997. Egg quality in fish: what makes a good egg? Rev Fish Biol Fish. 7:387–416.
- Cargnelli LM, Gross MR. 1996. The temporal dimension in fish recruitment: birth date, body size, and size-dependent survival in a sunfish (bluegill: Lepomis macrochirus). Can J Fish Aquat Sci. 53:360–367.
- Cargnelli LM, Neff BD. 2006. Condition-dependant nesting in bluegill sunfish Lepomis macrochirus. J Anim Ecol. 75:627–633.
- Chambers RC, Waiwood KG. 1996. Maternal and seasonal differences in egg sizes and spawning characteristics of captive Atlantic cod, Gadus morhua. Can J Fish Aquat Sci. 53:1986–2003.
- Childers WF. 1967. Hybridization of four species of sunfishes (Centrarchidae). Ill Nat Hist Surv Bull. 29:159–214.
- Claramunt RM, Wahl DH. 2000. The effects of abiotic and biotic factors in determining larval fish growth rates: a comparison across species and reservoirs. Trans Am Fish Soc. 129:835–851.
- Coble DW. 1988. Effects of angling on bluegill populations: management implications. North Am J Fish Manag. 8:277–283.
- Diana MJ, Stein J, Oplinger RW, Aday DD, Hoxmeier JW, Claussen JE, Philipp DP, Wahl DH. 2007. Quality management of bluegill: factors affecting population size structure. Champaign (IL): Division of Fisheries, Illinois Department of Natural Resources, Federal Aid in Fish Restoration. ( F-128-R, Aquatic Ecology Final Technical Report 2007(17)).
- Drake MT, Claussen JE, Philipp DP, Pereira DL. 1997. A comparison of bluegill reproductive strategies and growth among lakes with different fishing intensities. North Am J Fish Manag. 17:496–507.
- Elliott JM. 1984. Growth, size, biomass and production of young migratory trout Salmo trutta in a lake district stream, 1966–83. J Anim Ecol. 53:979–994.
- Gerking SD. 1962. Production and food utilization in a population of bluegill sunfish. Ecol Monogr. 32:31–78.
- Goedde LE, Coble DW. 1981. Effects of angling on a previously fished and unfished warmwater community in two Wisconsin lakes. Trans Am Fish Soc. 110:594–605.
- Guy CS, Willis DW. 1990. Structural relationships of largemouth bass and bluegill populations in South Dakota ponds. North Am J Fish Manag. 10:338–343.
- Heath DD, Blouw DM. 1998. Are maternal effects in fish adaptive or merely physiological side effects? In: Mousseau TA, Fox CW, editors. Maternal effects as adaptations. New York (NY): Oxford University Press; p. 178–201.
- Hoxmeier RJH, Wahl DH, Hooe ML, Pierce CL. 2004. Growth and survival of larval walleyes in response to prey availability. Trans Am Fish Soc. 133:45–54.
- Iguchi KI, Yamaguchi M. 1994. Adaptive significance of inter- and intrapopulational egg size variation in ayu Plecoglossus altivelis (Osmeridae). Copeia. 1994:184–190.
- Izquierdo MS, Fernandez-Palacios H, Tacon AGJ. 2001. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture. 197:24–42.
- Jennings MJ, Claussen JE, Philipp DP. 1997. Effect of population size structure on reproductive investment of male bluegill. N Am J Fish Manag. 17:516–524.
- Johnston TA, Leggett WC. 2002. Maternal and environmental gradients in the egg size of an iteroparous fish. Ecology. 83:1777–1791.
- Jonas JL, Wahl DH. 1998. Relative importance of direct and indirect effects of starvation for young walleyes. Trans Am Fish Soc. 127:192–205.
- Kaplan RH, Cooper WS. 1984. The evolution of developmental plasticity in reproductive characteristics: an application of the “adaptive coin-flipping” principle. Am Nat. 123:393–410.
- Markle DF. 1984. Phosphate buffered formalin for long term preservation for formalin fixed ichthyoplankton. Copeia. 1984:525–528.
- Marsh E. 1984. Egg size variation in central Texas populations of Etheostoma spectabile (Pisces: Percidae). Copeia. 1984:291–301.
- Marsh E. 1986. Effects of egg size on offspring fitness and maternal fecundity in the orangethroat darter, Etheostoma spectabile (Pisces: Percidae). Copeia. 1986:18–30.
- Marteinsdottir G, Steinarsson A. 1998. Maternal influence on the size and viability of Iceland cod Gadus morhua eggs and larvae. J Fish Biol. 52:1241–1258.
- Mayer CM, Wahl DH. 1997. The relationship between prey selectivity and growth and survival in a larval fish. Can J Fish Aquat Sci. 54:1504–1512.
- Meyer FA. 1970. Development of some larval centrarchids. Prog Fish Cult. 32:130–136.
- Mire JB, Millett L. 1994. Size of mother does not determine size of eggs or larval in the Owens pupfish, Cyprinodon radiosus. Copeia. 1994:100–107.
- Mischke CC, Morris JE. 1997. Out-of-season spawning of sunfish Lepomis spp. in the laboratory. Prog Fish Cult. 59:297–302.
- Morgan GD. 1951. The life history of the bluegill sunfish, Lepomis macrochirus, of Buckeye Lake (Ohio). Ohio J Sci. 42:21–59.
- Naevdal G. 1983. Genetic factors in connection with age at maturation. Aquaculture. 33:97–106.
- Oplinger RW, Diana MJ, Wahl DH. 2013. Population social structure and gizzard shad density influence the size-specific growth of bluegill. Can J Fish Aquat Sci. 70:1278–1288.
- Oplinger RW, Wahl DH, Nannini MA. 2011. Comparison of the effect of predators on the life history of bluegill sunfish from populations with a differing size and age of maturation. Trans Am Fish Soc. 140:1532–1539.
- Reagan RE, Conley CM. 1977. Effect of egg diameter on growth of channel catfish. Prog Fish Cult. 41:202–204.
- Rettig JE, Mittelbach GG. 2002. Interactions between adult and larval bluegill sunfish: positive and negative effects. Oecologia. 130:222–230.
- Rijnsdorp AD. 1993. Fisheries as a large-scale experiment on life-history evolution: disentangling phenotypic and genetic effects in changes in maturation and reproduction of North Sea plaice, Pleuronectes platessa L. Oecologia. 96:391–401.
- Rossiter M. 1996. Incidence and consequences of inherited environmental effects. Annu Rev Ecol Syst. 27:451–476.
- SAS Institute. 2003. SAS/STAT user's guide, version 9.1. Vol. 1. Cary (NC): SAS Institute.
- Schreck CB, Contreras-Sanchez W, Fitzpatrick MS. 2001. Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture. 197:3–24.
- Springate JRC, Bromage NR. 1985. Effects of egg size on early growth and survival in rainbow trout (Salmo gairdneri Richardson). Aquaculture. 47:163–172.
- Swingle HS. 1950. Relationships and dynamics of balanced and unbalanced fish populations. Auburn (AL): Alabama Agricultural Experiment Station, Auburn University. ( Bulletin 274).
- Venturelli PA, Murphy CA, Shuter BJ, Johnston TA, van Coeverden de Groot PJ, Boag PT, Casselman JM, Montgomerie R, Wiegand MD, Leggett WC. 2010. Maternal influences on population dynamics: evidence from an exploited freshwater fish. Ecology. 91:2003–2012.
- Wallace JC, Aasjord D. 1984. An investigation of the consequences of egg size for the culture of Arctic char (Salvelinus alpinus). J Fish Biol. 24:427–435.
- Welker MT, Pierce CL, Wahl DH. 1994. Growth and survival of larval fishes: roles of competition and zooplankton abundance. Trans Am Fish Soc. 123:703–717.
- Whitehouse HLK. 1973. Towards an understanding of the mechanisms of inheritance. 3rd ed. New York (NY): St. Martins.
- Wilson DC, Millemann RE. 1969. Relationships of female age and size to embryo number and size in the shiner perch, Cymatogaster aggregata. J Fish Res Board Can. 26:2339–2344.
- Wittenrich ML, Rhody NR, Turingan RG, Main KL. 2009. Coupling osteological development of the feeding apparatus with feeding performance in common snook, Centropomus undecimalis, larvae: identifying morphological constraints to feeding. Aquaculture. 294:221–227.
- Wolf JB, Wade MJ. 2009. What are maternal effects (and what are they not)? Philos Trans R Soc B. 364:1107–1115.
