Abstract
While taxonomic metrics are common indicators for assessing impact of acid mine drainage (AMD) on macroinvertebrates, functional diversity metrics are scarcely used. We tested the hypothesis that functional diversity metrics could be used as indicators for assessing impact of AMD on macroinvertebrates. Differences in both taxonomic metrics and functional diversity metrics were examined among sites with varying levels of AMD impact. AMD led to loss of sensitive functional groups and resulted in low functional diversity in the impacted sites. Functional richness, functional Shannon's index and functional Brillouin's index were significantly correlated with all key AMD parameters. Compared to taxonomic metrics, functional diversity metrics had higher correlations with most key AMD parameters. Functional diversity metrics were more informative than taxonomic metrics for assessing impact of AMD on macroinvertebrates since they were more effective at indicating mild or moderate AMD pollution. Functional Shannon's index and functional Brillouin's index were the most precise metrics.
Introduction
As one of the most widely demonstrated consequences of mining activities, acid mine drainage (AMD) has become a worldwide environmental issue because of its impacts on both surface and ground waters (Hogsden and Harding Citation2012a). AMD is formed when sulfide-bearing rocks are exposed to air and water during mining and processing of metal ores and coals (Kim & Chon Citation2001). Characterized by low pH and high concentration of metals, AMD imposes complex effects (e.g., acidity, metal toxicity, metal precipitation, and salinization) on streams (Gray Citation1997; DeNicola & Stapleton Citation2002; Akcil & Koldas Citation2006) and results in species loss and structural changes to freshwater organisms (Niyogi et al. Citation2002; Bray et al. Citation2009; DeNicola & Stapleton Citation2014). AMD pollution can be a long-term environmental problem as abandoned mines may continue to generate highly acidic and metal-rich leachate for many years after they are closed (Johnson & Hallberg Citation2005).
Benthic macroinvertebrates are sensitive to a broad range of environmental stressors and they have been widely used as indicators in stream assessments because of their diversity and life-history characteristics (Miserendino et al. Citation2011; Li et al. Citation2010, Citation2012; Stranko et al. Citation2012; Helson & Williams Citation2013). Many studies have documented the negative effects of AMD on species richness and abundance of macroinvertebrates (Battaglia et al. Citation2005; MacCausland & McTammany Citation2007; Gray & Delaney Citation2008). Macroinvertebrate communities are devastated in severely impacted streams; either only a few individuals remain or macroinvertebrates are completely absent (Winterbourn Citation1998; Soucek et al. Citation2003). AMD exerts complex and species-specific effects on macroinvertebrates (Courtney & Clements, Citation2002). In AMD-affected streams, macroinvertebrate communities often are dominated by tolerant species (e.g., chironomids, beetles), while sensitive species (e.g., mayflies, caddisflies) are excluded due to low pH and elevated metal concentrations which exceed the organisms' physiological limits (Winterbourn Citation1998; Hogsden & Harding Citation2012a). The metal hydroxides have a smothering effect on macroinvertebrates by clogging their gill surfaces (Soucek et al. Citation2000). Furthermore, the substrata covered by metal hydroxides may lead to loss of habitat and result in low richness and abundance of macroinvertebrates (McKnight & Feder Citation1984; Gray Citation1998; DeNicola & Stapleton Citation2002).
Functional diversity is based on the value and range of biological traits that influence their performance and thus functioning (Villéger et al. Citation2008). Functional diversity indicates not only number and dominance of species but also their functional roles in the ecosystem (Gallardo et al. Citation2011). There is an increasing amount of literature using the functional approach that involves primary production (Niyogi et al. Citation2002; Rowe et al. Citation2007) and decomposition (Schlief & Mutz Citation2005; Simmons et al. Citation2005) to assess AMD impact on stream health. Many studies have also demonstrated the impact of AMD on taxonomic diversity of macroinvertebrates (Courtney & Clements Citation2002; Van Damme et al. Citation2008; Gray & Delaney Citation2010; Gray & Harding Citation2012). However, studies focusing on the impact of AMD on functional diversity are lacking.
The functional trait approach has a long history in ecological research since traits are very important to ecosystem functioning in streams (Statzner et al. Citation2001; Heino Citation2008). Functional feeding groups are defined by the feeding habits of macroinvertebrates while habit traits include information on where and how the foods are obtained (Allan & Castillo Citation2007; Heino Citation2008). These two traits are perhaps most directly related to stream ecosystem functioning and have been widely used as parts of the functional composition of macroinvertebrate communities (Bady et al. Citation2005; Heino Citation2005, Citation2008; Martínez et al. Citation2013). Studies focusing on responses of functional diversity to environmental stressors can improve our understanding of the relationship between stream biodiversity and ecosystem functioning (Heino Citation2005; Péru & Dolédec Citation2010). Our study addresses impact of AMD on functional diversity of macroinvertebrates. Our main hypotheses are as follows: (1) AMD has a negative impact on functional diversity of macroinvertebrates; (2) functional diversity metrics can be used as indicators to monitor impact of AMD on macroinvertebrates; (3) functional diversity metrics are more informative than taxonomic metrics for assessing impact of AMD on macroinvertebrates.
Methods
Gaolan River is located in the upper reaches of the Three Gorge Reservoir and drains one of the most famous mining regions in China. The major source of water flowing into the river is rainfall. AMD caused by mining activities in the watershed results in serious pollution of the river before it finally flows into the Xiangxi Bay of the Three Gorges Reservoir. In order to protect the water quality of Three Gorges Reservoir, the pyrite mines were closed in 2004. However, the AMD contamination remained active after the mines were shut down (Jiang et al. Citation2008).
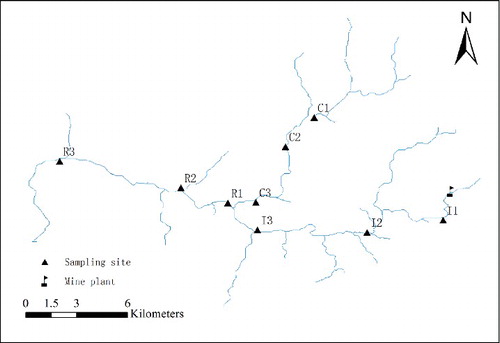
Water samples and macroinvertebrates were collected in October 2006 and January 2007, during the periods of lowest discharge. The control sites (C1–C3) were located in the upper reaches of Xiayang River which is a tributary of Gaolan River (). The impacted sites (I1–I3) were located in the upper reaches of the Gaolan River and all of them showed visual signs of AMD impact. The recovery sites (R1–R3) were located immediately after the mixing of the Xiayang and Gaolan rivers. Details of each site were shown in and they were also reported by Jiang et al. Citation(2008) and Jia et al. Citation(2009).
Stream water pH was measured with a multi-parameter meter (W-23, Horiba, http://www.horiba.com) in the field. Three water samples were collected with polyethylene bottles at each site and transported back to the laboratory in an icebox. Water samples were filtered through a MilliporeTM cellulose nitrate membrane (0.45 μm). The samples were refrigerated (4 °C) in the dark for later analysis. Al, Cu, Fe, Mn, and Zn were analyzed by an inductively coupled plasma-atomic emission spectrometer.
Benthic algae biomass was estimated from chlorophyll a (Chl a) of algal biomass on the stones. Nine to fifteen stones were picked randomly in the selected reaches by walking along the shoreline and transect. A 27-mm-diameter corer was placed on the upper surface of the stone. The area around the corer was brushed thoroughly with a nylon brush and washed with stream water. The algae beneath the corer was brushed and washed into a 355-mL sample container with filtered stream water. A 80–100 mL aliquot of the pooled sample was filtered through a Whatman™ glass fiber filter (GF/F). The filters were stored in the dark at −20 °C until analyzed for chlorophyll a. Absorbance was measured after extraction with 10 mL of 90% buffered acetone for 24 hours at 750, 665, 645 and 630 nm with a spectrophotometer (UV-1601, Shimadzu Corp., http://www.shimadzu.com).
At each site, three benthic macroinvertebrate samples were collected from riffles using a Surber sampler (30 cm × 30 cm, 0.43 mm mesh size) within a 100-m reach. The samples were pooled and preserved in 10% formalin (Cai Citation2007). In the laboratory, macroinvertebrates were fully sorted and identified to the lowest feasible taxonomic level following Kawai Citation(1985) and Morse et al. Citation(1994), and the numbers of individuals were counted.
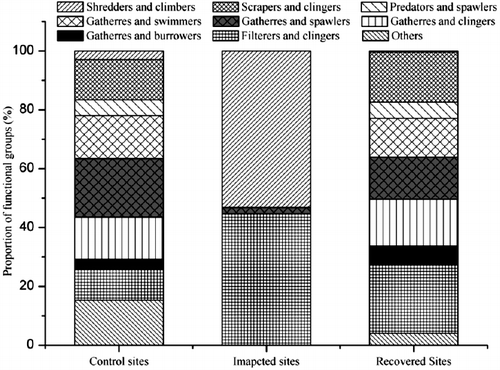
Macroinvertebrates were categorized into different functional groups according to a combination of functional feeding groups and habit trait groups (Merritt et al. Citation2008). Nineteen functional groups were observed in sampling sites (). Functional feeding groups included gatherers, filterers, scrapers, shredders, and predators. Habit trait groups included burrowers, climbers, clingers, sprawlers, and swimmers. Functional feeding groups are related to morphological and behavioral adaptations of feeding whereas habit trait groups contain information on mobility and where food is obtained (Allan & Castillo Citation2007; Heino Citation2008). Both of these two characteristics are directly related to stream ecosystem functioning (Heino Citation2005).
Table 2. Observed functional groups at sampling sites.
Four taxonomic metrics were selected based on their previous use for AMD assessment. The taxonomic metrics selected were richness, Shannon's index, Brillouin's index, and Biological Monitoring Working Party (BMWP) score (Hawkes Citation1998). Three functional diversity metrics were calculated. Functional richness was the number of functional groups. Functional Shannon's index and functional Brillouin's index were based on Shannon's index and Brillouin's index. The diversity metrics were calculated as
(Pielou Citation1966)
(Brillouin Citation1962)
(Grey & Delaney Citation2010)
Not all the biological and abiotic variables were normally distributed; thus Mann–Whitney U test was used to examine the differences of biological and abiotic variables among three groups. Six chemical parameters (i.e., pH, Al, Cu, Fe, Mn, and Zn) were selected as key AMD parameters based on the previous study of Jiang et al. Citation(2008). Relationships between biological metrics and key AMD parameters were determined using Pearson correlation. Average r values for each metric using individual values for key AMD parameters, allowed the performance of the metrics to be compared (Gray & Delaney Citation2008). Paired-samples t test was also used to examine the differences in correlation between biological metrics and key AMD parameters. All statistical tests were done using SPSS 16.0.
Results
Impacted sites were severely affected by AMD compared to control sites and recovered sites (). pH in impacted sites was significantly lower than control sites and recovered sites (p < 0.05), while concentrations of Al, Fe, and Zn in impacted sites was significantly higher than control sites and recovered sites (p < 0.05). Concentration of Mn showed significant differences among control sites (p < 0.05), impacted sites and recovered sites, whereas concentration of Cu was not significantly different among those three groups (p > 0.05).
Table 3. Mean and standard deviation (SD) of pH and metal concentrations at sampling sites.
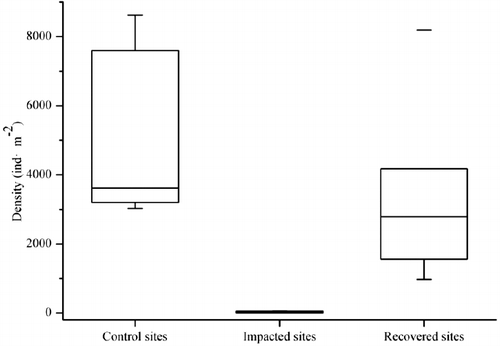
AMD had a significantly negative effect on macroinvertebrates. Densities of macroinvertebrates in impacted sites were significantly lower than control sites and recovered sites (p < 0.05; ). The impact of AMD as measured by the functional diversity metrics is summarized in . Functional richness, functional Shannon's index, and functional Brillouin's index of impacted sites were significantly lower than both control sites and recovery sites (p < 0.01). Functional Shannon's index and functional Brillouin's index of recovery sites were significantly lower than control sites (p < 0.05), while functional richness showed no significant differences between control sites and recovery sites (p > 0.05).
Table 4. Mean and standard deviation (SD) of macroinvertebrate metrics at sampling sites.
In general, functional diversity metrics showed a high degree of correlation with the AMD parameters (). All of them were strongly correlated with pH (p < 0.001), Mn (p < 0.001), Al (p < 0.01), and Zn (p < 0.01), while they showed weaker correlations with Fe (p < 0.05). The correlation of Cu with functional Shannon's index (p < 0.001) was higher than both functional richness (p < 0.01) and functional Brillouin's index (p < 0.01).
Table 5. Pearson correlations of functional diversity metrics against AMD parameters.
Functional Shannon's index and functional Brillouin's index showed significant differences between control sites and recovery sites. However, no taxonomic metrics showed significant differences between control sites and recovery sites. All functional diversity metrics were significantly correlated with every AMD parameter (p < 0.05), while richness, Brillouin's index, AMD' index, and BMWP score showed no significant correlation with Fe. Functional richness had higher correlation with every AMD parameter than richness. Functional Shannon's index had weaker correlation with pH than Shannon's index, but it had stronger correlations with Al, Cu, Fe, Mn, and Zn, as in the same case of functional Brillouin's index and Brillouin's index. In addition, the average r value of functional diversity metrics against key AMD parameters was higher than their taxonomic counterpart. The pair-samples t test also showed that corrections of functional diversity metrics against key AMD parameters were higher than their taxonomic counterpart, AMD' index and BMWP score (p < 0.05).
Discussion
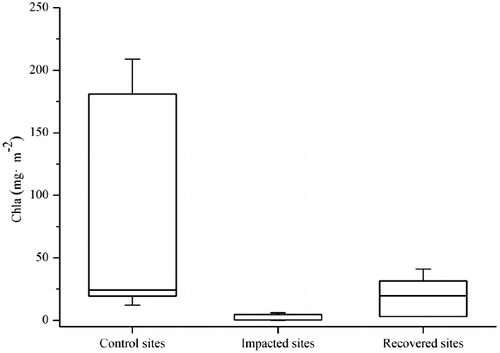
Compared to control sites, the functional diversity of macroinvertebrates was lower at impacted sites, and the functional structure was much simpler. Gatherers were the dominant group at both control sites and recovery sites ( 3). However, they were only collected at the I2 site with extremely low abundance. This was similar to the findings of Schultheis et al. Citation(1997) who demonstrated that gatherers were most responsive to the contamination of a pyrite mine. Jiang et al. Citation(2008) reported that the gatherers at control sites and recovery sites mainly consisted of mayflies and caddisflies. Both of them were very sensitive to AMD and vulnerable in severely affected streams (Winterbourn Citation1998). Scrapers were absent at impacted sites partly because of their low tolerance to contamination (Barbour et al. Citation1996). Besides, benthic algae was an important food resource for scrapers, while its biomass was very low at impacted sites due to AMD impact (), thus limiting the growth of scrapers (Niyogi et al. Citation2002; Jia et al. Citation2009). At impacted sites, the proportion of filterer-clingers was high, which might be resulted from reduced competition and adequate foods such as small particles retained in nets coated by metal hydroxides (Hünken & Mutz Citation2007). Shredder-climbers were the dominant group at impacted sites. They could be more adaptive in AMD affected streams than other functional groups since leaves coated with Fe hydroxide and Fe-loving bacteria might be a suitable food resource for them (Schlief & Mutz Citation2006). Predators were absent at impacted sites. The predators growth might be limited by reduced basal resources (e.g., periphyton) and low numbers of invertebrate prey (Hogsden & Harding Citation2014). The food web was expected to be much simpler and shorter in AMD impacted streams because of loss of species and declines of biomass. The declines of both consumers (e.g., macroinvertebrate) and primary producer (e.g., benthic algae) limited the number of trophic links, thus weakening the complexity of both structure and functioning of stream ecosystem (Schmid-Araya et al. Citation2002; Hogsden & Harding Citation2012b).
Table 1. Elevation, mean of channel width and water depth at sampling sites.
Functional Shannon's index and functional Brillouin's index incorporated both functional richness and abundance. At impacted sites, loss of functional richness and abundance caused by AMD impact resulted in low functional diversity measured by functional Shannon's index and functional Brillouin's index. In AMD affected streams, macroinvertebrate communities gradually recover when the contamination of AMD is sufficiently diluted (Battaglia et al. Citation2005). Compared to impacted sites, the functional diversity of recovery sites was much higher. Besides, the functional structure of recovery sites was more similar to control sites than impacted sites. Clean water from the Xiayang River diluted acid and metal concentrations in Gaolan River, thereby weakening the impact of AMD on macroinvertebrates at recovery sites. This suggested that clean water might be the key to recovery of AMD affected streams.
Both functional diversity metrics and taxonomic metrics were capable of indicating the effect of AMD on macroinvertebrates at impacted sites. Functional Shannon's index and functional Brillouin's index indicated that AMD also had negative impacts on macroinvertebrates at recovery sites. Taxonomic metrics were not as sensitive as functional diversity metrics; none of them showed significant differences between control sites and recovery sites. When the regional species pool contained acid/metal tolerant species, the taxonomic diversity in AMD affected streams could remain high due to replacement of acid/metal sensitive taxa by acid/metal tolerant species (Gerhardt et al. Citation2004; Hogsden & Harding Citation2012a). Furthermore, the BMWP score was also strongly related to species richness (Gray and Delaney Citation2008). The additional tolerant species could cover up the disappearance of sensitive species. Consequently, the taxonomic metrics were inadequate to indicate mild or even moderate pollution of AMD. However, the reduced functional richness could not be covered up by the additional tolerant species. Functional diversity metrics revealed not only abundance and dominance of species but also each species' functional role in the community (Gallardo et al. Citation2011). Therefore, functional diversity metrics were more informative than taxonomic metrics for assessing impact of AMD on macroinvertebrates. Nevertheless, using a combination of taxonomic and functional metrics will help to gain a more thorough understanding of structure and function of stream ecosystem and their responses to anthropogenic disturbances.
Acknowledgements
We thank Xinghuan Jia, Fengqing Li, Naicheng Wu, and Shugui Duan for their assistance with laboratory analysis and field sampling. The constructive comments of two anonymous reviewers greatly improved our manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Akcil A, Koldas S. 2006. Acid Mine Drainage (AMD): causes, treatment and case studies. J Clean Prod. 14:1139–1145.
- Allan JD, Castillo MM. 2007. Stream ecology: structure and function of running waters. 2nd ed. Dordrecht: Springer.
- Bady P, Dolédec S, Fesl C, Gayraud S, Bacchi M, Schöll F. 2005. Use of invertebrate traits for the biomonitoring of European large rivers: the effects of sampling effort on genus richness and functional diversity. Freshwater Biol. 50(1):159–173.
- Barbour M, Gerritsen J, Griffith G, Frydenborg R, McCarron E, White J, Bastian M. 1996. A framework for biological criteria for Florida streams using benthic macroinvertebrates. J North Am Benthol Soc. 15(2):185–211.
- Battaglia M, Hose G, Turak E, Warden B. 2005. Depauperate macroinvertebrates in a mine affected stream: clean water may be the key to recovery. Environ Pollut. 138:132–141.
- Bray JP, Broady PA, Niyogi DK, Harding JS. 2009. Periphyton communities in New Zealand streams impacted by acid mine drainage. Marine Freshwater Res. 59:1084–1091.
- Brillouin L. 1962. Science and information theory. New York (NY): Academic Press.
- Cai QH. 2007. Protocols for standard observation and measurement in aquatic ecosystems. Beijing: China Environmental Science Press.
- Courtney LA, Clements WH. 2002. Assessing the influence of water and substratum quality on benthic macroinvertebrate communities in a metal-polluted stream: an experimental approach. Freshwater Biol. 47:1766–1778.
- DeNicola DM, Stapleton MG. 2002. Impact of acid mine drainage on benthic communities in streams: the relative roles of substratum vs. aqueous effects. Environ Pollut. 119:303–315.
- DeNicola DM, Stapleton MG. 2014. Benthic diatoms as indicators of long-term changes in a watershed receiving passive treatment for acid mine drainage. Hydrobiologia 732(1):29–48.
- Gallardo B, Gascón S, Quintana X, Comín FA. 2011. How to choose a biodiversity indicator–Redundancy and complementarity of biodiversity metrics in a freshwater ecosystem. Ecological Indicators 11:1177–1184.
- Gerhardt A, Janssens de Bisthoven L, Soares A. 2004. Macroinvertebrate response to acid mine drainage: community metrics and on-line behavioural toxicity bioassay. Environ Pollut. 130:263–274.
- Gray DP, Harding JS. 2012. Acid Mine Drainage Index (AMDI): a benthic invertebrate biotic index for assessing coal mining impacts in New Zealand streams. N Z J Marine Freshwater Res. 46(3):335–352.
- Gray NF. 1997. Environmental impact and remediation of acid mine drainage: a management problem. Environ Geol. 30:62–71.
- Gray NF. 1998. Acid mine drainage composition and the implications for its impact on lotic systems. Water Res. 32:2122–2134.
- Gray NF, Delaney E. 2008. Comparison of benthic macroinvertebrate indices for the assessment of the impact of acid mine drainage on an Irish river below an abandoned Cu–S mine. Environ Pollut. 155:31–40.
- Gray NF, Delaney E. 2010. Measuring community response of bentic macroinvertebrates in an erosional river impacted by acid mine drainage by use of a simple model. Ecological Indicators 10:668–675.
- Hawkes HA. 1998. Origin and development of the biological monitoring working party score system. Water Res. 32(3): 964–968.
- Hünken A, Mutz M. 2007. On the ecology of the filter-feeding Neureclipsis bimaculata (Trichoptera, Polycentropodidae) in an acid and iron rich post-mining stream. Hydrobiologia 592(1):135–150.
- Heino J. 2005. Functional biodiversity of macroinvertebrate assemblages along major ecological gradients of boreal headwater streams. Freshwater Biol. 50:1578–1587.
- Heino J. 2008. Patterns of functional biodiversity and function-environment relationships in lake littoral macroinvertebrates. Limnol Oceanography 53:1446.
- Helson JE, Williams DD. 2013. Development of a macroinvertebrate multimetric index for the assessment of low-land streams in the neotropics. Ecological Indicators 29:167–178.
- Hogsden KL, Harding JS. 2012a. Consequences of acid mine drainage for the structure and function of benthic stream communities: a review. Freshwater Sci. 31:108–120.
- Hogsden KL, Harding JS. 2012b. Anthropogenic and natural sources of acidity and metals and their influence on the structure of stream food webs. Environ pollut. 162:466–474.
- Hogsden KL, Harding JS. 2014. Isotopic metrics as a tool for assessing the effects of mine pollution on stream food webs. Ecological Indicators 36:339–347.
- Jia XH, Jiang WX, Li FQ, Tang T, Duan SG, Cai QH. 2009. The response of benthic algae to the impact of acid mine drainage. Acta Ecol Sin. 29(9):4620–4629.
- Jiang WX, Tang T, Jia XH, Wu NC, Duan SG, Li DF, Cai QH. 2008. Impacts of acid pyrite drainage on the macroinvertebrate community in Gaolan River. Acta Ecol Sin. 28(10):4805–4814.
- Johnson DB, Hallberg KB. 2005. Acid mine drainage remediation options: a review. Sci Total Environ. 338:3–14.
- Kawai T. 1985. An illustrated book of aquatic insects of Japan. Tokyo: Tokai University Press.
- Kim JY, Chon HT. 2001. Pollution of a water course impacted by acid mine drainage in the Imgok creek of the Gangreung coal field, Korea. Appl Geochemistry 16:1387–1396.
- Li FQ, Cai QH, Ye L. 2010. Developing a benthic index of biological integrity and some relationships to environmental factors in the subtropical Xiangxi River, China. Int Rev Hydrobiologia 95:171–189.
- Li FQ, Cai QH, Qu XD, Tang T, Wu NC, Fu XC, Duan SG, Jahnig SC. 2012. Characterizing macroinvertebrate communities across China: Large-scale implementation of a self-organizing map. Ecological Indicators 23:394–401.
- MacCausland A, McTammany M. 2007. The impact of episodic coal mine drainage pollution on benthic macroinvertebrates in streams in the Anthracite region of Pennsylvania. Environ Pollut. 149:216–226.
- Martínez A, Larrañaga A, Basaguren A, Pérez J, Mendoza-Lera C, Pozo J. 2013. Stream regulation by small dams affects benthic macroinvertebrate communities: from structural changes to functional implications. Hydrobiologia 711(1):31–42.
- McKnight DM, Feder GL. 1984. The ecological effect of acid conditions and precipitation of hydrous metal oxides in a Rocky Mountain stream. Hydrobiologia 119(2):129–138.
- Merritt RW, Cummins KW, Berg MB. 2008. An introduction to the aquatic insects of North America, 4th ed. Dubuque: Kendall/Hunt Publishing Company.
- Miserendino, ML, Casaux R, Archangelsky M, Di Prinzio CY, Brand C, Kutschker AM. 2011. Assessing land-use effects on water quality, in-stream habitat, riparian ecosystems and biodiversity in Patagonian northwest streams. Sci Total Environ. 409:612–624.
- Morse JC, Yang L, Tian L. 1994. Aquatic insects of China useful for monitoring water quality. Nanjing: Hohai University Press.
- Niyogi DK, Lewis Jr WM, McKnight DM. 2002. Effects of stress from mine drainage on diversity, biomass, and function of primary producers in mountain streams. Ecosystems 5:554–567.
- Pielou EC. 1966. Shannon's formulae as a measure of specific diversity: its use and misuse. Am Nat. 100:463–465.
- Péru N, Dolédec S. 2010. From compositional to functional biodiversity metrics in bioassessment: a case study using stream macroinvertebrate communities. Ecological Indicators 10:1025–1036.
- Rowe OF, Sánchez‐España J, Hallberg KB, Johnson DB. 2007. Microbial communities and geochemical dynamics in an extremely acidic, metal-rich stream at an abandoned sulfide mine (Huelva, Spain) underpinned by two functional primary production systems. Environ Microbiol. 9(7):1761–1771.
- Schlief J, Mutz M. 2005. Long-term leaf litter decomposition and associated microbial processes in extremely acidic (pH< 3) mining waters. Arch Hydrobiol. 164(1):53–68.
- Schlief J, Mutz M. 2006. Palatability of leaves conditioned in streams affected by mine drainage: a feeding experiment with Gammarus pulex (L.). Hydrobiologia 563(1):445–452.
- Schmid-Araya JM, Schmid PE, Robertson A, Winterbottom J, Gjerløv C, Hildrew AG. 2002. Connectance in stream food webs. J Anim Ecol. 71(6):1056–1062.
- Schultheis AS, Sanchez M, Hendricks AC. 1997. Structural and functional responses of stream insects to copper pollution. Hydrobiologia 346(1-3):85–93.
- Simmons JA, Lawrence ER, Jones TG. 2005. Treated and untreated acid mine drainage effects on stream periphyton biomass, leaf decomposition, and macroinvertebrate diversity. J Freshwater Ecol. 20(3):413–424.
- Soucek DJ, Cherry DS, Trent G. 2000. Relative acute toxicity of acid mine drainage water column and sediments to Daphnia magna in the Puckett's Creek watershed, Virginia, USA. Arch Environ Con Tox. 38:305–310.
- Soucek DJ, Cherry DS, Zipper, CE. 2003. Impacts of mine drainage and other nonpoint source pollutants on aquatic biota in the upper Powell River system, Virginia. Hum Ecol Risk Assess. 9(4):1059–1073.
- Statzner B, Hildrew AG, Resh VH. 2001. Species traits and environmental constraints: entomological research and the history of ecological theory. Annu Rev Entomol. 46(1):291–316.
- Stranko SA, Hilderbrand RH, Palmer MA. 2012. Comparing the fish and benthic macroinvertebrate diversity of restored urban streams to reference streams. Restoration Ecol. 20:747–755.
- Van Damme PA, Hamel C, Ayala A, Bervoets L. 2008. Macroinvertebrate community response to acid mine drainage in rivers of the High Andes (Bolivia). Environ Pollut. 156:1061–1068.
- Villéger S, Mason NWH, Mouillot D. 2008. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89(8):2290–2301.
- Winterbourn MJ. 1998. Insect faunas of acidic coal mine drainages in Westland, New Zealand. N Z Entomologist 21:65–72.