Abstract
The study of the diet of tadpoles can provide important information about the maintenance and dynamics of populations and communities of tropical amphibians. Tadpoles are in general herbivores and consume mostly algae. Despite this, little is known about how specific guilds use resources that are available in the same space and at the same time. It is thought that species of tadpoles segregate based on food use, especially in lotic environments where food resources may be a limiting factor. Conversely, it is believed that the use of food resources is similar among tadpoles with the same ecomorphotype. This study examined the diet of Aplastodiscus cavicola, Aplastodiscus sibilatus and Bokermannohyla capra syntopic tadpoles, as similarities may exist between these benthic species, all of which are adapted to the flow of water. This study was undertaken in the Michelin Ecological Reserve, municipality of Igrapiúna, state of Bahia, Brazil. To analyze the diet of the tadpoles, nonmetric multidimensional scaling (NMDS) was performed to evaluate the similarity between the diets of the species. Analysis of similarity (ANOSIM) was carried out to detect whether diet differed among tadpole species. The diet of these tadpoles consists mainly of algae, especially diatoms, plants, protozoa, fungi and microscopic animals. The NMDS identified three clusters representing the three species studied. ANOSIM detected significant differences among the diets of the species, which indicates partition of food resources.
Introduction
The coevolution of sympatric species and the adaptation of these species to the environment can reduce overlap in the use of available resources, thereby determining species abundance and diversity in a community. Resource allocation, therefore, is important for the coexistence of two or more species and spatial and temporal dimensions, and food availability, are considered the most important factors for specific niche delineation (Pianka Citation1974; Schoener Citation1974). For tropical frogs, spatial and temporal dimensions have traditionally been considered the most important factors, particularly the choice of breeding sites (Crump Citation1971; Toft Citation1985), although some studies have identified food resource partition among adults of sympatric and phylogenetically closely related species (e.g., Menin et al. Citation2005).
The temporal dimension can be decisive, especially for tadpoles, since bodies of water are often subject to periodic droughts. Additionally, competition for food can be fierce when many species use the same body of water, leading to resource allocation. Tadpoles are considered specialized filter feeder herbivores (Duellman & Trueb Citation1994), as they consume a large variety and quantity of algae, especially in the early stages of their development (Wickramasinghe et al. Citation2007). However, tadpoles can also consume protozoa, fungi and microscopic animals available in their habitat, which characterizes them additionally as omnivores (Altig & Johnston Citation1989).
This scenario suggests that in a given community of tadpoles, food resource allocation may be detected indirectly through the different ecomorphotypes (sensu Altig & Johnston Citation1989) of these organisms. Each ecomorphotype is associated with a specific microhabitat and utilizes different food resources, which are also associated, therefore, with the respective microhabitat (Rossa-Feres et al. Citation2004).
The benthic tadpole is one of the most representative ecomorphotypes of tropical environments and is found in both lentic and lotic ecosystems (McDiarmid & Altig Citation1999). However, lotic environments can undergo more drastic natural perturbations than lentic environments (e.g., flood pulses, Resh et al. Citation1988), resulting in lower algae diversity and primary production (Rodrigues & Bicudo Citation2001; Cardinale et al. Citation2005) and consequently affecting food availability for tadpoles. Therefore, at least two challenges are faced by tadpoles in lotic environments: low availability of food resources and water flow.
With some exceptions (e.g., Kupferberg Citation1997; Dutra & Callisto Citation2005; Sousa Filho et al. Citation2007), there are relatively few studies of the diet of anuran larvae in lotic environments. Studies of the composition of the diet of tadpoles are especially important in the tropics, where despite taxonomic diversity being severely threatened by factors that can cause irreparable declines; little is known of the trophic importance of tadpoles in many aquatic environments (Altig et al. Citation2007). This study investigated the allocation of food resources among benthic tadpoles of three hylid species (Aplastodiscus cavicola, Aplastodiscus sibilatus and Bokermannohyla capra) from lotic communities based on the hypotheses that similar ecomorphotypes use similar food resources and that species that coexist in the same micro-habitat must share food sources.
Methods
Tadpole collection
Tadpoles were collected in December 2008, February, April, July and September 2009, and January 2010 from the Michelin Ecological Reserve (MER) (13°50′ S, 39° 10′ W), located in the municipality of Igrapiúna, in south-eastern Bahia, Brazil. The air temperature in the region varies between 21°C and 30°C and annual rainfall is approximately 2000 mm (Camurugi et al. Citation2010).
The MER includes two fragments of Atlantic rainforest and a variety of natural ecosystems. Both fragments are surrounded by agroforestry systems, including areas of rubber silviculture governed by two different understory management systems: overall management, in which the understory is totally removed, and partial management, in which only the understory of the main access route to the rubber trails is removed. The understory of the stream margins has been maintained. Several springs, rivers and streams run through the reserve.
Tadpoles of 12 anuran species (Allobates olfersioides, A. cavicola, Aplastodiscus ibirapitanga, A. sibilatus, B. capra, Dendropsopus haddadi, Phasmahyla timbo, Proceratophrys renalis, Proceratophrys schirchi, Rhinella hoogmoedi, Scinax strigilatus and Vitreorana spp.) were found in the streams. While some of these species are benthic, only A. cavicola, A. sibilatus and B. capra are of similar size and possess similar morphological characteristics, some of which are closely related to swimming in flowing water (visible lateral line, narrow fins, long tail and developed caudal musculature) (Mercês & Juncá Citation2010; Mercês et al. Citation2011). These three species are widely distributed among the streams of MER and present for large parts of the year. The analyzed tadpoles were taken from four streams of one of the Atlantic rainforest fragments [forest stream, FS: FS1 (13° 50′ 29.8″ S 39° 15′ 2.2″ W), FS2 (13° 50′ 24.8″ S 39° 15′ 0.8″ W), FS3 (13° 50′ 28″ S 39° 14′ 28.3″ W) and FS4 (13° 51′ 3.2″ S 39° 13′ 47.4″ W)] and three streams located in an area of partially managed rubber silviculture adjacent to the forest fragment [rubber plantation stream, RPS: RPS1 (13° 52′ 12.5″ S 39° 14′ 3.2″ W), RPS2 (13° 52′ 36.4″ S 39° 14′ 10.8″ W) and RPS3 (13° 53′ 0.3″ S 39° 14′ 31.9″ W)], where the understory had been partially removed. Various types of nets with different mesh sizes and thicknesses (1, 2 and 3 mm2) were used to capture the individuals. After collection, the tadpoles were anesthetized, immediately killed in 10% alcohol, and preserved in 6% formaldehyde in order to conserve food items.
A. cavicola and B. capra tadpoles were found in both FS and RPS streams, while A. sibilatus tadpoles were found only in FS streams. The diets of 20 tadpoles of A. cavicola (FS = 9, RPS = 11), 10 tadpoles of A. sibilatus (FS) and 17 tadpoles of B. capra (FS = 11, RPS = 6) were analyzed. All tadpoles were from developmental stages 26 to 28 (Gosner Citation1960). The occurrence of tadpoles from two or three of the species studied was recorded for all streams sampled. However, tadpoles of more than one species from development stages 26 to 28 occurred in only four streams: tadpoles of three species were found in FS2, B. capra and A. sibilatus tadpoles were found in FS1 and FS4, and B. capra and A. cavicola tadpoles were found in FS3 and RPS1. In addition to these streams, tadpoles of A. cavicola in development stages of 26–28 were found in RP2 and RP3.
Diet analysis
The intestine of each tadpole was removed through an incision from the cloaca to the oral disk. Each intestine was then opened in a Petri plate and its contents were removed by longitudinal cuts. After the intestinal contents were removed, 1.5 ml of 4% formaldehyde was added and the mixture was placed into a microtubule. Approximately 0.05 ml of this solution was analyzed on 75 × 24 mm glass slides and 50 × 20 mm coverslips under a light microscope (Leica DMLS2) at 10× magnification and an Optovar 2×. When necessary, 40× lens magnification was used. The food items were identified to the lowest possible taxonomic level and quantified. The identification of the items was based on examination of specialized bibliographies. The items ingested by tadpoles were quantified based on numerical frequency (%NF) and frequency of occurrence (%FO). The importance index was obtained by calculating the %NF plus the %FO divided by two, in accordance with Colli et al. (Citation2003). The collected specimens were deposited in the Museu de Zoologia da Universidade Estadual de Feira de Santana.
The nonmetric multidimensional scaling method (NMDS) was performed to evaluate the similarity between the diets of tadpoles of the three species under analysis, using a matrix of relative abundance of the food items taken from each individual (relative abundance of each matrix cell = number of items of food category y consumed by individual a divided by total number of items consumed by individual a). The Sorensen index was used as a distance measure and two dimensions were selected. In accordance with Xavier and Napoli (Citation2011), it was performed with 50 starting configurations, the instability value of 0.0005 for the stability criterion, 100 iterations to evaluate the stability of the solution and 500 as the maximum number of iterations. The Monte Carlo test was used to evaluate whether NMDS extracted a stronger axis than expected by chance. Analysis of similarity (ANOSIM) was then performed in order to detect whether the diet of tadpoles differed among species, using 10,000 permutations and the Bray-Curtis similarity index. The significance level was 0.05, adjusted according to the Bonferroni method (Tabachnick & Fidell Citation2007). NMDS and ANOSIM were performed using the PCord 4.1 (McCune & Mefford, Citation1999) and Past 2.17C (Hammer et al. Citation2001) softwares, respectively.
Results
The diet of the three species was primarily based on algae, protozoa and plant fragments. A total of 898 sample items of the diet of A. sibilatus tadpoles were collected, distributed in 19 food categories. Samples of the diet of A. cavicola tadpoles totaled 1958 items distributed into 20 food categories, while samples of the diet of B. capra tadpoles totaled 2815 items distributed among 24 categories (). There were 16 food categories that occurred in the diet of the three species. Based on the importance index scores of over 50, Bacillariophyceae, Trinema and Spermatophyta fragments were important food items for the three species. Other food categories were important for tadpoles of only two species, although consumed by tadpoles of the three species. Oscillatoria was important for the tadpoles of two species of Aplastodiscus. Plancktonlyngbya and Arcela were important categories for tadpoles of A. sibilatus, although in tadpoles of A. cavicola, they scored 47.8 and 46.6, respectively. Nebela was important for the diet of A. cavicola tadpoles and fungi were important for the diets of A. sibilatus and B. capra.
Table 1. Items found in the diet of Aplastodiscus cavicola, Aplastodiscus sibilatus and Bokermannohyla capra tadpoles collected from Atlantic Forest streams of the MER, and rubber silviculture streams. %NF – numerical frequency, %FO – frequency of occurrence, I – importance index, UND – undetermined.
Based on the tadpole diets, the NMDS arranged the sample into three clusters representing the three studied species (). However, there was an overlap between the two clusters that represented the diets of the two Aplastodiscus species. The NMDS stress was 26.0 and the Monte Carlo test indicated that the NMDS result was stronger than expected by chance (p = 0.002).
Figure 1. Clusters formed from NMDS analysis of the diet of tadpoles studied in the MER. Triangle – Bokermannohyla capra, circle – Aplastodiscus sibilatus, square – Aplastodiscus cavicola.
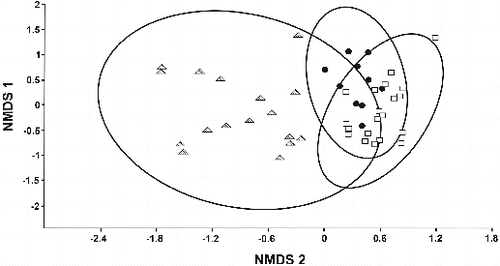
ANOSIM confirmed that there were significant differences among the diets of the three species of tadpoles (r = 0.536, p < 0.0001).
Discussion
Food items found in the diet of tadpoles of A. cavicola, A. sibilatus and B. capra were similar to those of other tadpoles, with their diets consisting primarily of algae, plants, protozoa (e.g., Inger Citation1986; Sekar Citation1992), fungi and microscopic animals (e.g. Rossa-Feres et al. Citation2004; Sousa Filho et al. Citation2007).
A high diversity and abundance of algae is usual in tadpoles of different anuran species (Dutra & Callisto Citation2005; Sousa Filho et al. Citation2007). However, the ingestion of a large amount of algae by anuran larvae will not necessarily result in nutritional gain (Pryor Citation2003; Akers et al. Citation2008). In contrast, at least one experimental study has found that tadpoles that ingested algae associated with diatoms showed increased growth and rapid development (Kupferberg et al. Citation1994). Diatoms are one of the items of major importance in the tadpole diet of the three species in this study, and in the diet of all benthic tadpoles (e.g., Rossa-Feres et al. Citation2004).
While protozoa diversity was relevant in the diet of the three species of tadpoles in this study (nine genera of protozoa were found), some of which had a high importance index (e.g., Trinema); in general, the importance of these organisms was low compared with other taxa (e.g., Bacillariophyceae, Fungi). Studies that address the importance of protozoa in the diet of tadpoles are rare (e.g., Nathan & James Citation1972) and few taxa of the group have been documented in the diet of amphibian tadpoles (Arias et al. Citation2002; Rossa-Feres et al. Citation2004).
Fungi were an important item in the diet of B. capra and A. sibilatus tadpoles. The presence of fungi in the diet of tadpoles is also rarely reported in literature even though it is an important component in oligotrophic habitats (Altig et al. Citation2007). Studies that address the importance of fungi in the diet of tadpoles were not found, however, Rossa-Feres et al. (Citation2004) identified mycophagous eating habits in Leptodactylus fuscus.
Food items such as rotifers, crustaceans and mites found in the diet of tadpoles in this study have been reported for other species (e.g., Candioti Citation2005; Dutra & Callisto Citation2005; Heinen & Abdella Citation2005; Pfennig et al. Citation2006; Sousa Filho et al. Citation2007; Wickramasinghe et al. Citation2007), and were usually associated with the diet of tadpoles in the later stages of development, unlike the three species of this study, which were in the early stages of development. The high nutritional value of invertebrates in terms of protein and energy is worthy of note (Bowen et al. Citation1995).
A. sibilatus, A. cavicola (Mercês & Juncá Citation2010) and B. capra (Mercês et al. Citation2011) can be considered benthic tadpoles (Altig & Johnston Citation1989), a guild characterized by Wells (Citation2007) as having general eating habits. The results of this study confirmed similarity in eating habits but also illustrated differences according to NMDS analysis, which could be the result of resource allocation. This study confirmed the hypothesis that there was allocation of available food sources by these species, which coexist in the same micro-habitat. One must consider, however, that differences in food supply may emerge. Besides, in some streams, tadpoles from developmental stages 26 to 28 (sensu Gosner Citation1960) from only one species were found. Nevertheless, considering the occurrence in streams of tadpoles in stages 26–28, there was more overlap between B. capra and A. sibilatus (two streams) and B. capra and A. cavicola (two streams), than A. sibilatus and A. cavicola (one stream). Despite this, there was considerable similarity between the diets of the two Aplastodicus species, while the greatest difference in diets was between these species and B. capra tadpoles, indicating the possible importance of the phylogenetic evolution of food habits.
Acknowledgements
The authors would like to thank the Michelin Center for Biodiversity and the UEFS Post-graduate Zoology Program for logistical support, Ivania Batista for help in identifying food items, Ednei de Almeida for help in the field and laboratory work, Arielson Protázio for his review of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Michelin Center for Biodiversity; the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship awarded to ASP e FJMS; the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the scholarship awarded to FAJ [305457/2009-8]; Fundação de Amparo a Pesquisa do Estado de São Paulo [2010/52321-7]; Conselho Nacional de Desenvolvimento Científico e Tecnológico [563075/2010-4].
References
- Akers EC, Taylos CM, Altig R. 2008. Effects of clay-associated organic material on the growth of Hyla chrysoscelis tadpoles. J Herpetology. 42:408–410.
- Altig R, Johnston GF. 1989. Guilds of anuran larvae: relationships among developmental modes, morphologies, and habitats. Herpetology Monogr. 3:81–109.
- Altig R, Whiles MR, Taylor CL. 2007. What do tadpoles really eat? Assessing the trophic status of an understudied and imperiled group of consumers in freshwater habitats. Freshwater Biol. 52:386–395.
- Arias MM, Peltzer PM, Lajmanovich RC. 2002. Diet of the giant tadpoles Pseudis paradoxa platensis (Anura, Pseudidae) from Argentina. Phyllomedusa. 1:97–100.
- Bowen SH, Lutz EV, Ahlgren MO. 1995. Protein and energy as determinants of quality: trophic strategies compared. Ecology. 76:899–907.
- Camurugi F, Lima TM, Mercês EA, Juncá FA. 2010. Anurans of the Reserva Ecológica da Michelin, municipality of Igrapiúna, state of Bahia, Brazil. Biota Neotropica. [Internet]. [cited 2014 Mar 29];29. Available from: http://www.biotaneotropica.org.br/v10n2/pt/fullpaper?bn02810022010+en
- Candioti MFV. 2005. Morphology and feeding in tadpoles of Ceratophrys cranwelli (Anura: Leptodactylidae). Acta Zoologica. 86:1–11.
- Cardinale BJ, Palmer MA, Ives AR, Brooks SS. 2005. Diversity-productivity relationships in streams vary as a function of the natural disturbance regime. Ecology. 86:716–726.
- Colli GR, Mesquita DO, Rodrigues PVV, Kitayama K. 2003. Ecology of the Gecko Gymnodactylus geckoides amarali in a neotropical savanna. J Herpetology. 37:694–706.
- Crump M. 1971. Quantitative analysis of the ecological distribution of a tropical herpetofauna. Occas Pap Museum Nat Hist. 3:1–62.
- Duellman WE, Trueb L. 1994. Biology of amphibians. Baltimore: The Johns Hopkins University Press.
- Dutra SL, Callisto M. 2005. Macroinvertebrates as tadpoles food: importance and body size relationships. Revista Brasileira Zoologia. 22:923–927.
- Gosner KL. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 16:183–190.
- Hammer Ø, Harper DAT, Ryan PD. 2001. Past: paleontological statistics software package for education and data analysis. Version 2.12. Available from: http://folk.uio.no/ohammer/past/
- Heinen JT, Abdella JA. 2005. On the advantages of putative cannibalism in American toad tadpoles (Bufo a. americanus): is it active or passive and why?. Am Midland Naturalist. 153:338–347.
- Inger RF. 1986. Diets of tadpoles living in a Bornean rain forest. Alytes. 5:153–164.
- Kupferberg SJ, Marks JC, Power ME. 1994. Effects of variation in natural algal and detrital diets on larval anuran (Hyla regilla) life-history traits. Copeia. 1994:446–457.
- Kupferberg SJ. 1997. The role of larval diet in anuran metamorphosis. Am Zoologist. 37:146–159.
- McCune B, Mefford, MJ. 1999. Multivariate analysis of ecological data. Version 4.10. Available from: http://home.centurytel.net/∼mjm/
- McDiarmid R, Altig R. 1999. Tadpoles: the biology of anuran larvae. Chicago, IL: University of Chicago Press.
- Menin M, Rossa-Feres DC, Giareta AA. 2005. Resource use and coexistence of two syntopic hylid frogs (Anura, Hylidae). Revista Brasileira Zoologia. 22(1):61–72.
- Mercês EA, Juncá FA. 2010. Girinos de três espécies de Aplastodiscus Lutz, 1950 (Anura - Hylidae) ocorrentes no Estado da Bahia, Brasil. Biota Neotropica. [Internet]. [cited 2012 Mar 29];29. Available from: http://www.biotaneotropica.org.br/v10n4/pt/fullpaper?bn03410042010+pt
- Mercês EA, Protázio AS, Juncá FA. 2011. The tadpole of Bokermannohyla capra Napoli and Pimenta, 2009 (Anura, Hylidae). Zootaxa. 3167:66–68.
- Nathan JM, James VG. 1972. The role of protozoa in the nutrition of tadpoles. Copeia. 1972:669–679.
- Pfennig DW, Rice AM, Martin RA. 2006. Ecological opportunity and phenotypic plasticity to promote character displacement and species coexistence. Ecology. 87:769–779.
- Pianka ER. 1974. Niche overlap and diffuse competition. Proc Natl Acad Sci USA. 71:2141–2145.
- Pryor GS. 2003. Growth rates and digestive abilities of bullfrog tadpoles (Rana catesbiana) fed algal diets. J Herpetology. 37:560–566.
- Resh VH, Brown AV, Covich AP, Gurtz ME, Li HW, Minshall GW, Reice SR, Sheldon AL, Wallace JB, Wissmar RC. 1988. The role of disturbance in stream ecology. J North Am Benthological Soc. 7:433–455.
- Rodrigues L, Bicudo DC. 2001. Similarity among periphyton algal communities in a lentic-lotic gradient of the upper Paraná river floodplain, Brazil. Rev Braz Bot. 24:235–248.
- Rossa-Feres DC, Jim J, Fonseca MG. 2004. Diets of tadpoles from a temporary pond in southeastern Bazil (Amphibia, Anura). Revista Brasileira Zoologia. 21:745–754.
- Schoener TW. 1974. Resource partition in ecological communities. Science. 18:27–39.
- Sekar AG. 1992. A study of the food habits six anuran tadpoles. J Bombay Nat Hist Soc. 89:210–214.
- Sousa Filho IF, Branco CC, Carvalho-E-Silva AMPT, Silva GR, Sabagh LT. 2007. The diet of Scinax angrensis (Lutz) tadpoles in an area of the Atlantic Forest (Mangaratiba, Rio de Janeiro) (Amphibia, Anura, Hylidae). Revista Brasileira Zoologia. 24:965–970.
- Tabachnick BG, Fidell LS. 2007. Using multivariate statistics. Boston, MA: Pearson Education.
- Toft CA. 1985. Resource partitioning in amphibians and reptiles. Copeia. 1985:1–21.
- Wells KD. 2007. The ecology and behavior of amphibians. Chicago, IL: University of Chicago Press.
- Wickramasinghe DD, Oseen KL, Wassersug RJ. 2007. Ontogenetic changes in diet and intestinal morphology in semi-terrestrial tadpoles of Nannophrys ceylonensis (Dicroglossidae). Copeia. 4:1012–1028.
- Xavier AL, Napoli MF. 2011. Contribution of environmental variables to anuran community structure in the Caatinga Domain of Brazil. Phyllomedusa. 10:45–64.
