Abstract
The aim of this study was to assess the impacts of different land use practices on physicochemical characteristics and macroinvertebrate functional feeding groups (FFGs) in the Dongjiang River basin, southeast China and also to evaluate if macroinvertebrate FFGs match the river continuum concept (RCC) predictions. For this aim, a total of 30 sampling sites were selected that comprised three different land use types (10 forest, 10 agricultural, and 10 urban sites) and sampled during the dry season in January 2013. Analysis of variance results showed evident differences in the environmental factors among the three land use types, particularly between the forest and urban sites. The forest sites had significantly lower water temperature, lower stream order, higher pH, higher dissolved oxygen, higher elevation, and coarser substrates than the other land use sites. Conversely, the urban sites showed significantly higher mean values for electrical conductivity, nitrogen and phosphorus compounds. Significant differences in the shredder and predator richness and density were observed among land uses with more shredders and predators found in forest sites. Redundancy analysis showed a clear separation of forest sites from riparian modified areas (agriculture and urban use sites). Our results were broadly in accordance with the central RCC theme. However, the longitudinal distribution of predators and collectors did not completely match the prediction of the RCC. These results confirm that macroinvertebrate FFG structure has been altered by agricultural and urbanization practices in the Dongjiang River basin. Moreover, shredders and predators could be potential candidates for monitoring and assessing land use impacts on water quality in this basin to improve future watershed management.
Introduction
The ecological consequences of land use change for the health of rivers and streams is a prominent environmental issue worldwide (Moore & Palmer Citation2005; Bu et al. Citation2014). Different land use practices in river basins, such as urbanization and agricultural activities, affect the hydrological characteristics, substratum availability, and water quality and alter the complex biotic and abiotic processes governing the functioning and attributes of macroinvertebrate communities (Hepp & Santos Citation2009). In turn, the ecological responses of macroinvertebrate communities, in terms of taxon richness and abundance, in streams and rivers reflect the land use and physico-chemical conditions at each study site (Allan Citation2004).
Macroinvertebrates are key components of aquatic food webs that link organic matter and nutrient resources (e.g., leaf litter, algae, and detritus) with higher trophic levels (Yoshimura et al. Citation2006). Cummins (Citation1974) categorized five functional feeding groups (FFGs) according to the different food sources utilized by macroinvertebrates: collector-gatherers (GC), collector-filterers (FC), shredders (SH), scrapers (SC), and predators (PR). In recent years, compared with the taxonomical approach, the functional approach was reported to be more appropriate and rapid for characterizing ecosystem conditions (Cummins et al. Citation2005; Menezes et al. Citation2010), detecting land use change (Nautiyal & Mishra Citation2013), and assessing river health (Yoshimura et al. Citation2006). Anthropogenic alteration of riparian zones and land use change are likely to override geomorphological controls on the distribution of macroinvertebrate FFGs (Maridet et al. Citation1998). Compin & Céréghino (Citation2007) detected significant responses of FFGs to landscape alterations and found that collector-gatherers exhibited higher percentages in urban landscapes whereas scrapers and predators prevailed in agricultural landscapes. Miserendino and Masi (Citation2010) found that among guild structural measures, those indicators based on FFG attributes, like shredder richness and collector density, were the best candidates for assessing land use impacts in Patagonian streams.
The river continuum concept (RCC) is a framework describing the gradually changing gradient of physical characteristics and the resulting FFG responses from headwaters to river mouth (Vannote et al. Citation1980). The RCC considers a river system in its entirety; it considers the system not only as a geographical continuum but also as a continuum linking function and ecological process. The RCC, which breaks the classic idea of a river system, quickly became a popular means for community studies in river ecosystems (Jiang et al. Citation2011). However, because physical factors (e.g., elevation and stream order) may vary differently in the longitudinal axes of rivers across the world and enormous environmental factors could influence FFG composition, the global value of the RCC is still uncertain, particularly for biomes outside of temperate North America (Tomanova et al. Citation2007). In recent years, some tests for the RCC in subtropical Asian river systems have been conducted. Jiang et al. (Citation2011) found that the RCC generally applies to running waters in the Chishui River, southwestern China. Nautiyal and Mishra (Citation2013) found that human modified riparian land use can disrupt the river continuum by examining the macroinvertebrates in the Ken River, central India. There is, however, a need for more Chinese or subtropical Asian river systems to be examined to test whether the RCC is an appropriate concept for understanding longitudinal biotic patterns.
The Dongjiang River is a major tributary of the Pearl River (Zhu Jiang, the second largest river in China by discharge). It lies predominantly within the east-central part of Guangdong province of southeast China (Zhang et al. Citation2008; ). The lower Dongjiang valley belongs to the Pearl River Delta region, which has been undergoing rapid economic development since the 1980s. Over the last few decades, the region has been transformed from predominantly fishing villages into urban centers and its land use has changed dramatically due to a variety of human activities including agriculture, logging, sand dredging, industrial, and domestic sewage discharges, and flow regulation. Zhang et al. (Citation2010) reported that the land use disturbance and water quality degradation could significantly reduce the benthic biodiversity in the Dongjiang River by investigating macroinvertebrate community composition. However, the impacts of different land uses on macroinvertebrate FFGs are not explicitly known in the Dongjiang River basin. Moreover, the relationship among land uses, environmental factors, and macroinvertebrate FFGs has not been explicitly discussed.
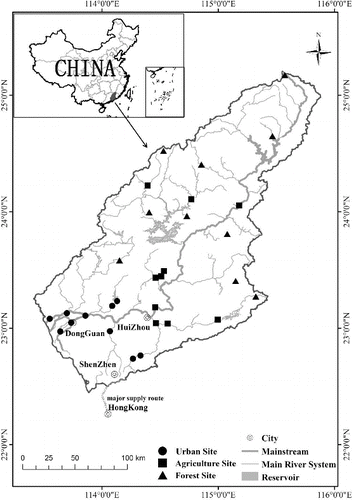
Figure 1. Map of the Dongjiang River basin showing the location of the study area and sampling sites of three land use sites.
The aim of this study was to assess the impacts of different land use practices and environmental characteristics on macroinvertebrate FFGs and evaluate if macroinvertebrate FFGs match the RCC predictions in the Dongjiang River basin.
Methods
Study area
The Dongjiang River basin (22° 21′ N to 25° 12′ N, 113° 04′ E to 115° 50′ E) encompasses a watershed area of 35,340 km2. It is the primary source of drinking water for Hong Kong and other regions of the Pearl River Delta in China (Ho et al. Citation2003). The main river channel is 562 km long, originating in the northeast in Jiangxi province and extending southwest in Guangdong province with a decline in altitude, eventually discharging into the Pearl River estuary. The Dongjiang River basin has a subtropical climate with a mean annual temperature of approximately 21 °C and a mean annual precipitation of approximately 1900 mm (Liu et al. Citation2010). Forests cover the headwater mountain areas and intensive cultivation dominates the hills and plains (Jiang et al. Citation2012). Large cities (e.g., Shenzhen, Dongguan, and Huizhou) with rapid development and growing economies are primarily located in the southern downstream area of the basin ().
Macroinvertebrate procedures
A total of 74 sites were sampled during the dry season in January 2013 under stable flow conditions. We first examined their surrounding land use cover by geographic information system (GIS) technology and then selected the most representative sites for each land use type (forest, agriculture, and urban). We also considered the site's surrounding environment by field survey records and watershed scale comprehensively. Finally, three land use types were selected: (1) forest (FOR), n = 10, located on the mountains with predominant forest cover, sparse human intervention and largely undisturbed conditions,% forest cover = 85%–98%; (2) agriculture (AGR), n = 10, predominantly crops and orchards, % agriculture cover = 49%–78%; and (3) urban (URB), n = 10, highly disturbed sites that exhibited obvious degradation signs, frequent human activities, % urban cover, 43%–74%.
Quantitative macroinvertebrate samples were taken using multi-habitat sampling procedures (Lenat Citation1988). At each sampling site (50–100 m reach, near the bank), a single sample of macroinvertebrates was taken from one or more microhabitats: silt/sand, gravel/cobble, boulder, macroalgae/macrophyte, and marginal plant. The number of microhabitats sampled varied according to the number present at each site. Silt/sand, macroalgae/macrophyte, and marginal plant microhabitats were sampled using D-frame nets (0.3 m, mesh size 500 μm) approximately 3–5-m long whereas gravel/cobble and boulder microhabitats were sampled using a Surber net sampler (30 cm × 30 cm, mesh size: 500 μm). The total composite sample was taken from an area of approximately 3 m2. The collecting gear was washed thoroughly between collections. The sampled materials were sieved through a 500-mm mesh sieve in the field for approximately 30–60 min. The specimens were manually separated from the sediment on a porcelain plate and preserved in 100-mL plastic bottles containing 10% formaldehyde solution.
In the laboratory, macroinvertebrates were keyed to genus or family and counted using identification keys (Morse et al. Citation1994; Thorp & Covich Citation2009). They were then partitioned into FFGs based on the invertebrate morphological and behavioral adaptations to acquire their food resources (Merritt & Cummins Citation1996). Identifications at the genus or family levels allow realistic functional descriptions of lotic macroinvertebrate communities (Dolédec et al. Citation2000; Gayraud et al. Citation2003).
Environmental factors
At each sampling site, water samples were collected prior to macroinvertebrate sampling to measure the physicochemical factors of the site. Water samples were collected below the water surface. For nutrient analyses water samples were kept at 4 °C and transported to the laboratory. The water temperature (WT, °C), electrical conductivity (EC, μs/cm), dissolved oxygen (DO, mg/L), and pH were measured using a portable YSI probe (YSI 6600). The water velocity (VEL, m/s) was measured by a hydrometric propeller placed near the riverbed for 60 seconds. The chemical parameters, total nitrogen (TN, mg/L), ammoniacal nitrogen (NH3-N, mg/L), nitrate-nitrogen (NO3-N, mg/L), and total phosphorus (TP, mg/L) were analyzed using a UV spectrophotometer immediately upon the arrival of the samples at the laboratory.
The substrate type and percentage cover of particle classes were also measured based on the improved Wentworth scale. These included sand/silt (diameter 0.1–2 mm), gravel (2–16 mm), pebble (16–64 mm), cobble (64–256 mm), and boulder ( > 256 mm). Using the substrate data, the average substrate score (MSUBST), a quantitative indicator of substrate constitution, was calculated (WFD-UKTAG Citation2008) as follows:(1)
(2) where BOLDCOBB, PEBBGRAV, SAND, and SILTCLAY indicate the percentage cover of bolder/cobble, pebble/gravel, sand, and silt/clay, respectively. A higher score indicates a high proportion of sand and silt; conversely, a lower score indicates a high proportion of large rocks and cobble.
The elevation (ELE, m) of the sampling site was recorded with GPS. Landsat 5 Thematic Mapper (TM)images taken in December 2009 at a spatial resolution of 30 m were used for land use pattern characterization. Land use was analyzed in ArcGIS 10 using spatial visualization and basic statistics and a characteristic value for each factor was derived for each sampling point by averaging the values of the factor over a 1-km radius of the survey point (Kong et al. Citation2013). The stream order (ORD) was coded by the Strahler stream order system (Strahler Citation1957) with a river system map layer at a scale of 1:250,000 in ArcGIS 10. All of the measured environmental factors are shown in .
Table 1. Summary of the environmental factors investigated in the present study. The data are presented as the mean ± SE for three land use categories. Minimum and maximum values are shown in brackets.
Data analysis
One-way analysis of variance (ANOVA) and non-parametric, rank-based, one-way ANOVA (Kruskal–Wallis) tests were used to test the significance of environmental factors and FFG differences among the three land use sites. The mean values of normally-distributed datasets were analyzed by one-way ANOVA. In those cases in which homogeneity of variance (Levene's tests) was not achieved, the data were log10 (x + 1) transformed. A non-parametric Kruskal–Wallis test was applied if normality was not achieved after transformation (Zar Citation1984). If significant differences were observed by ANOVA, post hoc pair-wise comparisons were performed using Tukey's test. The associations between the significantly different FFGs and environmental factors were tested using Spearman's rank correlations. All analyses were conducted using statistical product and service solutions (SPSS) 19.0.
To assess the relationships between environmental factors and FFG structure, constrained ordination was used. Detrended correspondence analysis (DCA) was used to determine the appropriate type of model for direct gradient analysis (ter Braak & Verdonschot Citation1995). DCA indicated that a linear model (gradient lengths of < 2 standard units) would best fit the data, and hence redundancy analysis (RDA) was used. The forward selection procedure was performed in RDA to filter key environmental factors (p < 0.05, Monte Carlo randomization test with 999 permutations). All analyses were conducted with log10(x+1)-transformed abundance data using CANOCO version 5 (ter Braak & Šmilauer Citation2012).
Results
Environmental features
A degradation gradient was observed among the study sites in the forest-agriculture-urban direction (). Significant differences in environmental factors were observed among the three land use types by site, with the exception of velocity (). This result may be because our sampling was carried out in the dry season during stable flow conditions. Post hoc comparisons revealed that the significant difference of environmental factors was between the forest sites and urban sites (Tukey test, p < 0.05). The forest sites had significantly lower water temperature (F(2,27) = 10.2, p < 0.01), lower stream order (F(2,27) = 16.74, p < 0.01), higher pH (F(2,27) = 5.639, p < 0.01), higher dissolved oxygen (F(2,27) = 7.71, p < 0.01), higher elevation (Kruskal–Wallis = 21.51, p < 0.001), and coarser substrates (F(2,27) = 6.91, p < 0.01) than the other types of sites. Conversely, the urban sites showed significantly higher mean values for the chemical pollutant factors than the forest and agriculture sites, including, electrical conductivity (Kruskal–Wallis, H = 18.43, p < 0.001), TP (Kruskal–Wallis, H = 11.799, p < 0.01), TN (Kruskal–Wallis, H = 7.256, p < 0.05), NH3-N (Kruskal–Wallis, H = 11.11, p < 0.01), and NO3-N (Kruskal–Wallis, H = 10.07, p < 0.01; ).
Table 2. Results of ANOVAs of environmental factors of different land use sites. Asterisks indicate P < 0.05. The results of post hoc tests among the three land use sites are indicated by superscripts, and significant differences (p < 0.05) are indicated by different letters.
FFG distribution and differences among land uses
A total of 63 taxa were identified in the survey including 5 shredders, 16 collector-gatherers, 5 collector-filterers, 12 scrapers, and 25 predators (Appendix ). Shredders and predators were mainly composed of insects, and scrapers mainly composed of gastropods. The taxa richness of each site ranged between 1 and 19 taxa and the average taxa richness values were 10 for forest sites, 6 for agriculture sites and 3 for urban sites, as expected.
The percentage contributions of different macroinvertebrate FFGs (relative abundance data) for the three land use types by site are shown in . Collector-filterers (27.2%), predators (28.8%), and scrapers (28.2%) had the highest percentage contributions to the FFG composition in forest sites, and collector-gatherers had similarly large contributions to the FFG composition in the agriculture (80.9%) and urban (79.7%) sites. Overall, the percentage contributions of these 4 FFG compositions in the agriculture and urban sites were similar.
Figure 2. Stacked bar plots showing the percentage contributions to the macroinvertebrate FFGs (relative abundance) of different land use sites. SH = shredders, FC = collector-filterers, GC = collector-gatherers, SC = scrapers, PR = predators.
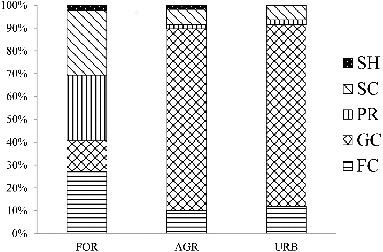
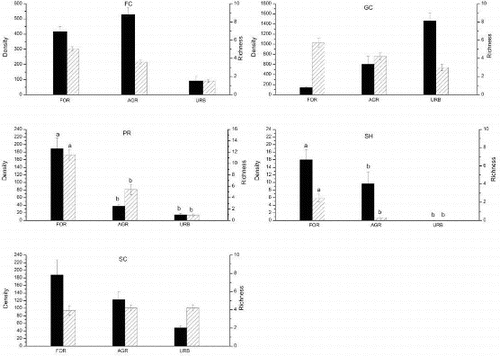
The shredders showed rather low contributions (<3%) in the three land use types. In particular, there were no shredders present in the surveyed urban sites. Significant differences in the shredders and predators (richness and density) were observed among land uses (Kruskal--Wallis test, p < 0.05), with more shredder and predator species found in the forest sites (). The post hoc comparisons revealed that the difference between the forest sites and the other human-disturbed sites (agriculture and urban sites) of shredders (H = 6.544, p < 0.05) and predators (H = 9.51, p < 0.01) was remarkably significant ().
Figure 3. Density (mean ± SE, individual m−2, left bar) and richness (mean ± SE, right bar) for FFGs of different land uses. Data with no superscript letters show there is no significant differences among sites (p < 0.05). SH = shredders, FC = collector-filterers, GC = collector-gatherers, SC = scrapers, PR = predators.
Redundancy analysis
Four important environmental factors (TP, elevation, stream order, and substrates) were retained in the RDA model by the forward selection procedure ( and ). All four axes explained 40% of the total variation in the FFG structure. The first axis explained 21.5% (eigenvalue = 0.215) of the total variation of functional structure and TP (r = 0.66) was the most important factor from the first axis, which highlighted a water quality impairment gradient. The second axis accounted for 14.8% of the variation (eigenvalue = 0.148) and was mainly determined by elevation (ELE, r = 0.60), reflecting the presence of a gradient from narrow headwater streams with coarse substrates to high stream order with fine substrates ().
Figure 4. Redundancy analysis (RDA) tri-plot relating macroinvertebrate FFG communities to environmental factors of 30 sites. Significant environmental factors (p < 0.05) were TP, ELE, ORD and SUB. ELE = elevation; ORD = stream order; SUB = substrates. Different symbol represent different land use sites (forest: △; agriculture:□; urban:○). SH = shredders, FC = collector-filterers, GC = collector-gatherers, SC = scrapers, PR = predators.
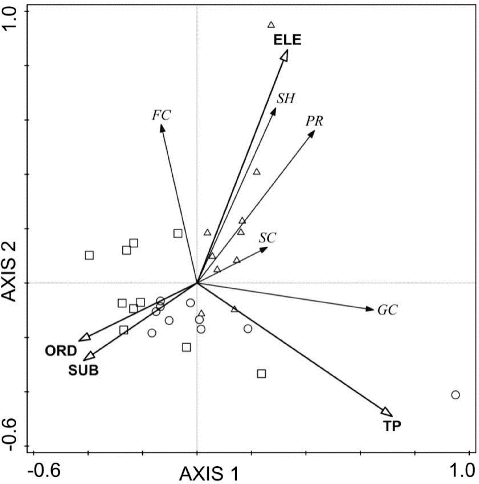
Table 3. Summary statistics for the redundancy analysis (RDA) relating macroinvertebrate FFG communities to environmental factors. The important factors in axis 1 and axis 2 are shown in bold. ELE = elevation; ORD = stream order; SUB = substrates.
Table A1. Identified macroinvertebrate taxa with respect to their corresponding FFGs.
In the RDA tri-plot (), the forest sites could be clearly distinguished from the agriculture sites and urban sites. The shredders, predators, and scrapers characterized headwater assemblages with coarse substrate at high altitude forest sites. Many collector-gatherers were present in urban sites with high organic pollution levels, which were associated with large rivers where substrates were mainly composed of silt and clay.
Discussion
Differences in FFGs among land uses
Our results suggest that the distribution of shredders and predators were significantly different among land uses and that these two FFGs were significantly more abundant in forest headwater streams than in human disturbed agriculture and urban sites ().
Shredders are an important ecological guild in headwater streams, playing a vital role in the process of leaf litter decomposition (Jonsson et al. Citation2001). They facilitate the release of nutrients into stream ecosystems and provide other invertebrates with food sources in the form of coarse particulate organic matter (CPOM) and fine particulate organic matter (FPOM) (Li & Dudgeon Citation2009; Yule & Gomez Citation2009). Jung et al. (Citation2008) found that shredders were relatively more abundant (approximately 14% of total macroinvertebrates) within the headwater reach in a subtropical mountain stream in northern Vietnam. Che Salmah (Citation2013) documented a higher proportion of shredders (18.22% of total macroinvertebrates) in Malaysian forest headwater streams. Shade conditions and substrate are important factors for shredder habitat. Li and Dudgeon (Citation2009) found that shading did not affect the densities or relative abundances of FFGs other than shredders. Couceiro et al. (Citation2011) found that anthropogenic sediment inputs to streams particularly affected shredders due to the burial of their primary substrate (CPOM). Our results were in accordance with the general conclusion that shredders are abundant in forest headwater streams and rapidly decrease under land use practices.
The density and richness of shredders in our study were much lower compared with other FFGs (). Some studies have shown the occurrence of shredders in tropical streams but in low densities (Gonçalves et al. Citation2006; Li & Dudgeon Citation2009). Moreover, the scarcity of shredders (a total of 5 taxa) in our study area seems to be reasonable when compared to other tropical areas: Hong Kong, 10 taxa (Li & Dudgeon Citation2009); New Guinea, 7 taxa (Yule Citation1996); and Kenya, 5 taxa (Dobson et al. Citation2002). Despite low density and richness of shredders in the Dongjiang River basin, they are sensitive to land use change and human disturbance.
The conclusions about predator distribution in different land uses were inconsistent. Miserendino and Pizzolon (Citation2003) found that predators had a high density in forest areas, which is similar to our results. However, Miserendino and Masi (Citation2010) found that predators occurred more often in urban sites, whereas Hepp and Santos (Citation2009) found that predators showed similar distributions with different land uses.
Our results showed that the FFG communities in forest sites were significantly different from the agriculture and urban sites ( and ). The agriculture and urban land use practices reduce water quality due to inputs of fine sediments, nutrients, and pesticides. Alterations to the physical properties of the river channel cause a loss in riparian vegetation, which would be expected to produce a significant effect on FFG structure (Paul & Meyer Citation2001; Genito et al. Citation2002; Allan Citation2004). The agriculture and urban sites are mainly located in the southern region of the Dongjiang River basin (), where urbanization and high population density led the FFG composition structure to be controlled and impacted by multiple land use practices with complicated interactions. A similar conclusion was found by Miserendino and Masi (Citation2010), who reported that macroinvertebrate FFG assemblages showed a clear separation between forested and riparian-modified areas (pasture, urban and harvest sites) in Patagonian streams.
FFGs and environmental relationships
A degradation gradient of environmental factors was observed among the study sites in the forest-agriculture-urban direction (). Our results agreed with those of Zhang et al. (Citation2010) and Zhou et al. (Citation2012), who reported the impact of different land uses on water quality in the Dongjiang River basin.
Our results showed that TP, elevation, stream order, and substrates were important factors in deciding the FFG community distribution ( and ). TP was the most important factor from the first axis and is associated with water quality. Collector-gatherers were most abundant with the increasing TP gradient in downstream urban sites where strong human activities led to high sewage and fertilizer loading inputs to rivers. Many collector-gatherers are tolerant to disturbance and organic pollution, such as Tubifex spp., Limnodrilus spp., Branchiura sp., and Chironomidae and abundant in polluted rivers. Mccormick et al. (Citation2004) found that TP is an important factor controlling the macroinvertebrate FFG assemblages.
In addition, elevation and stream order are widely known for their strong relationship to FFG variability. Tomanova et al. (Citation2007) suggested that altitude combined with position along the longitudinal gradient is an important factor controlling the FFG assemblages of stream invertebrates in neotropical streams. Jung et al. (Citation2008) also found that the composition of aquatic insects and their functional groups are related to variability in elevation and stream order.
The RDA showed that the abundance of shredders, predators and collector-filterers increased in sites with increasing elevation and coarse substrate in the forest sites (). This indicated that these FFGs were better suited to inhabit riparian vegetation and better water quality habitats, which are sensitive to disturbance and organic pollution. The abundance of scrapers was negative with high substrate scores in the RDA plot because scrapers graze or scrape materials, such as periphyton and epilithic algae, on the stream cobble substrates, depending upon the availability of the substrate (Heino Citation2000).
The RCC test in the Dongjiang River basin
The RCC predicts that gradually changing physical components from headwaters to downstream habitats of rivers, mainly related to elevation and stream order, affect the type and availability of food resources, which in turn drives the shift in the macroinvertebrate FFGs (Vannote et al. Citation1980). Our results were broadly accordant with the expectations of the RCC. Elevation and stream order were selected as important factors controlling the FFG assemblages in the RDA ordination ( and ).
The RCC also predicts that shredders are dominant in low-order forested streams and that the predator component changes little in relative dominance with stream order (Vannote et al. Citation1980). Our results further showed that both the shredders and predators were dominant in low-order forest streams but not exactly as predicted by the RCC ( and ).
The original RCC (Vannote et al. Citation1980) united collector-gatherer and collector-filterers into a single collector category and did not particularly predict the longitudinal pattern for collector-gatherers. Our results showed that the percent of collector-gatherers increased with longitudinal gradient and also confirmed the increase in collector-gatherers with a forest-agriculture-urban direction due to the relative abundant food resources. This is in agreement with Compin and Céréghino (Citation2007) who reported collector-gatherers showing higher percentages in urban landscapes in southwestern France streams. The results above were also congruent with some studies in China that reported the increase in collector-gatherers from upstream to downstream (Hu et al. Citation2005; Qu et al. Citation2007; Jiang et al. Citation2011). The conclusion that collector-gatherers are more abundant in middle and lower stream reaches is characteristic of temperate and subtropical rivers in East Asia (Jung et al. Citation2008). Nautiyal and Mishra (Citation2013) concluded that changes in FFG composition show disruption of the river continuum due to anthropogenic stress and agricultural practices in tropical Asia.
Conclusions
The impacts of land use and environmental factors on macroinvertebrate FFGs were studied in the Dongjiang River Basin, southeast China. In general, the FFG composition showed evident differences between the forest sites and human-disturbed sites (agriculture and urban sites). Significant differences in the shredders and predators were observed among land uses, with more shredders and predators found in the forest sites. The TP, elevation, stream order, and substrate type were significant drivers of the FFG community variance. Moreover, our results were broadly accordant with the RCC prediction. In conclusion, agricultural and urbanization practices had an impact on the FFG composition and water quality in the Dongjiang River basin. Shredders and predators may serve as potential candidates to monitor and assess land use change on water ecosystem health in this basin to improve future watershed management.
Acknowledgements
The authors thank Professor Dayong Wu for his help with the specimen identifications. We are grateful to Bo Wang, Chenglong Tang, Kai Wu, and Tinghai Li for their help with the fieldwork.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Allan JD. 2004. Landscapes and riverscapes: the influence of land use on stream ecosystems. Annu Rev Ecol Syst. 35:257–284.
- Bu H-M, Meng W, Zhang Y, Wan J. 2014. Relationships between land use patterns and water quality in the Taizi River basin, China. Ecol Indic. 41:187–197.
- Che Salmah MR, Al-Shami SA, Abu HA, Madrus MR, Nurul HA. 2013. Distribution of detritivores in tropical forest streams of peninsular Malaysia: role of temperature, canopy cover and altitude variability. Int J Biometeorol. 58:679–690.
- Compin A, Céréghino R. 2007. Spatial patterns of macroinvertebrate functional feeding groups in streams in relation to physical variables and land-cover in Southwestern France. Landsc Ecol. 22:1215–1225.
- Couceiro SR, Hamada N, Forsberg BR, Padovesi FC. 2011. Trophic structure of macroinvertebrates in Amazonian streams impacted by anthropogenic siltation. Aust Ecol. 36:628–637.
- Cummins KW. 1974. Structure and function of stream ecosystems. Bioscience. 24:631–641.
- Cummins KW, Merritt RW, Andrade PC. 2005. The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil. Stud Neotrop. Fauna and Environ. 40:69–89.
- Dobson M, Magana A, Mathooko JM, Ndegwa, FK. 2002. Detritivores in Kenyan highland streams: more evidence for the paucity of shredders in the tropics? Freshwater Biol. 47:909–919
- Dolédec S, Olivier J, Statzner B. 2000. Accurate description of the abundance of taxa and their biological traits in stream invertebrate communities: effects of taxonomic and spatial resolution. Archiv für Hydrobiologie. 148:25–43.
- Gayraud S, Statzner B, Bady P, Haybachp A, Schöll F, Usseglio‐Polatera P, Bacchi M. 2003. Invertebrate traits for the biomonitoring of large European rivers: an initial assessment of alternative metrics. Freshw Biol. 48:2045–2064.
- Genito D, Gburek WJ, Sharpley AN. 2002. Response of stream macroinvertebrates to agricultural land cover in a small watershed. J Freshw Ecol. 17:109–119.
- Gonçalves JR, França JS, Medeiros AO, Rosa CA, Callisto M. 2006. Leaf breakdown in a tropical stream. Int Rev Hydrobiol. 91:164–177.
- Heino J. 2000. Lentic macroinvertebrate assemblage structure along gradients in spatial heterogeneity, habitat size and water chemistry. Hydrobiologia. 418:229–242.
- Hepp LU, Santos S. 2009. Benthic communities of streams related to different land uses in a hydrographic basin in southern Brazil. Environ Monit Assess. 157:305–318.
- Ho K, Chow Y, Yau J. 2003. Chemical and microbiological qualities of The East River (Dongjiang) water, with particular reference to drinking water supply in Hong Kong. Chemosphere. 52:1441–1450.
- Hu B-J, Yang L-F, Wang B-X, Shan L-N. 2005. Functional feeding groups of macro invertebrates in 1-6 order tributaries of the Changjiang River. Chin J Appl Environ Biol. 11:463–466. (in Chinese with English abstract).
- Jiang Y, Ding Z-Y, Peng Q-Z, Liao J-Y, Lv L-L. 2012. Spatial distribution and corresponding factors of heavy metals concentrations in the Dongjiang River basin, southeast China. Res J Environ Earth Sci. 4:448–459.
- Jiang X-M, Xiong J, Xie Z-C, Chen Y-F. 2011. Longitudinal patterns of macroinvertebrate functional feeding groups in a Chinese river system: a test for river continuum concept (RCC). Quat Int. 244:289–295.
- Jonsson M, Malmqvist B, Hoffsten PO. 2001. Leaf litter breakdown rates in boreal streams: does shredder species richness matter? Freshw Biol. 46:161–171.
- Jung SW, Nguyen QH, Bae YJ. 2008. Aquatic insect faunas and communities of a mountain stream in Sapa Highland, northern Vietnam. Limnology. 9:219–229.
- Kong W-J, Meng W, Zhang Y, Gippel C, Qu X-D. 2013. A freshwater ecoregion delineation approach based on freshwater macroinvertebrate community features and spatial environmental data in Taizi River Basin, northeastern China. Ecol Res. 28:581–592.
- Lenat DR. 1988. Water quality assessment of streams using a qualitative collection method for benthic macroinvertebrates. J N Am Benthol Soc. 7:222–233.
- Li AO, Dudgeon D. 2009. Shredders: species richness, abundance, and role in litter breakdown in tropical Hong Kong streams. J N Am Benthol Soc. 28:167–180.
- Liu D-D, Chen X-H, Lian Y-Q, Lou Z-H. 2010. Impacts of climate change and human activities on surface runoff in the Dongjiang River basin of China. Hydrol Process. 24:1487–1495.
- Maridet L, Wasson J G, Philippe M, Amoros C, Naiman RJ. 1998. Trophic structure of three streams with contrasting riparian vegetation and geomorphology. Archiv Fur Hydrobiologie. 144:61–85.
- Mccormick PV, Shuford III RB, Rawlik PS. 2004. Changes in macroinvertebrate community structure and function along a phosphorus gradient in the Florida Everglades. Hydrobiologia. 529:113–132.
- Menezes S, Baird DJ, Soares AM. 2010. Beyond taxonomy: a review of macro invertebrate trait‐based community descriptors as tools for freshwater biomonitoring. J Appl Ecol. 47:711–719.
- Merritt RW, Cummins KW. 1996. An introduction to the aquatic insects of North America. 3rd ed. Dubuque, IA, : Kendall Hunt Publishing Company.
- Miserendino ML, Masi CI. 2010. The effects of land use on environmental features and functional organization of macroinvertebrate communities in Patagonian low order streams. Ecol Indic. 10:311–319.
- Miserendino ML, Pizzolon LA. 2003. Distribution of macroinvertebrate assemblages in the Azul‐Quemquemtreu river basin, Patagonia, Argentina. NZ J Mar Freshwater Res. 37:525–539.
- Moore AA, Palmer MA. 2005. Invertebrate biodiversity in agricultural and urban headwater streams: implications for conservation and management. Ecol Appl. 15:1169–1177.
- Morse JC, Yang L-F, Tian L-X.1994. Aquatic insects of China useful for monitoring water quality. Nanjing, : Hohai University Press.
- Nautiyal P, Mishra A. 2013. Variations in benthic macroinvertebratefauna as indicator of land use in the Ken River, central India. J Threat Taxa 5:4096–4105.
- Paul MJ, Meyer JL. 2001. Streams in the urban landscape. Annu Rev Ecol Syst. 32:333–365.
- Qu X-D, Cai Q- H, Xie Z-C, Tang T, Cao M, Li D-F. 2007. Research of functional of feeding groups of macro invertebrate in the stony habitat of the Xiangxi River system. Resour Environ Yangtze Basin. 16:738–743. (in Chinese with English abstract)
- Strahler AN. 1957. Quantitative analysis of watershed geomorphology. Transactions Am Geophysical Union. 38:913–920.
- ter Braak CJ, Šmilauer P. 2012. Canoco Reference Manual and User's Guide: Software for Ordination (version 5.0). Ithaca, NY: Microcomputer Power.
- ter Braak CJ, Verdonschot P F. 1995. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat Sci. 57:255–289.
- Thorp JH, Covich AP. 2009. Ecology and classification of North American freshwater invertebrates. New York, NY: Academic Press.
- Tomanova S, Tedesco PA, Campero M, Van Damme PA, Moya N, Oberdorff T. 2007. Longitudinal and altitudinal changes of macroinvertebrate functional feeding groups in neotropical streams: a test of the River Continuum Concept. Fundam Appl Limnol. 170:233–241.
- Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE. 1980. The river continuum concept. Can J Fish AquatSci, 37:130–137.
- Water Framework Directive-United Kingdom Advisory Group (WFD-UKTAG). 2008. River assessment methods benthic invertebrate fauna, river invertebrate classification tool (RICT). Doi: 978-1-906934-07-1.
- Yoshimura C, Tockner K, Omura T, Moog O. 2006. Species diversity and functional assessment of macroinvertebrate communities in Austrian rivers. Limnology. 7:63–74.
- Yule CM. 1996. Trophic relationships and food webs of the benthic-invertebrate fauna of two aseasonal tropical streams on Bougainville Island, Papua New Guinea. J Trop Ecol. 12:517–534.
- Yule CM, Gomez LN. 2009. Leaf litter decomposition in a tropical peat swamp forest in Peninsular Malaysia. Wetlands Ecol Manage. 17:231–241.
- Zar JH. 1984. Biostatistical analysis. 2nd ed. Englewood Cliffs, NJ: Prentice Hall.
- Zhang Y, Dudgeon D, Cheng D, Thoe W, Fok L, Wang Z, Lee JHW. 2010. Impacts of land use and water quality on macroinvertebrate communities in the Pearl River drainage basin, China. Hydrobiologia. 652:71–88.
- Zhang S, Lu X-X, Higgitt DL, Chen C-T, Han J, Sun H. 2008. Recent changes of water discharge and sediment load in the Zhujiang (Pearl River) Basin, China. Global Planet Change. 60:365–380.
- Zhou T, Wu J-G, Peng S-L. 2012. Assessing the effects of landscape pattern on river water quality at multiple scales: A case study of the Dongjiang River watershed, China. Ecol Indic. 23:166–175.
