ABSTRACT
During the spring 2015 sampling efforts on the Missouri River, several adult pallid sturgeon (Scaphirhynchus albus) were captured in a visually emaciated condition. As pallid sturgeon recovery efforts continue throughout the Missouri River, this may negatively impact the local population and hamper recovery. Therefore, the objectives were to (1) assess the annual variation in pallid sturgeon condition, (2) determine how condition varies amongst gender and reproductive status and if condition affects fecundity, (3) determine the annual proportion of reproductive female pallid sturgeon, and (4) test several hypothesized factors which may contribute to variations in pallid sturgeon condition. Overall, the relative condition of pallid sturgeon has significantly declined over the past 12 years, except for the large adult-sized fish (> 840 mm fork length). Pallid sturgeon condition began to decline in 2013 then rapidly declined in 2014 and even more in 2015. The mean annual pallid sturgeon condition reached record lows in 2015. Females had a higher overall Kn compared to males and sexually reproductive fish had a significantly higher Kn for both genders. Overall, 28% of the females captured have been in reproductive condition; however, that frequency appears to be declining with condition. Also, relative condition was not correlated with the number of eggs released by reproductively ready females. Correlation between pallid sturgeon condition and hypothesized variables did not result in any statistically significant relationships that would explain the variations in pallid sturgeon condition. As pallid sturgeon recovery continues throughout the lower Missouri River, concern arises over whether or not the current habitat available and river management can support a viable pallid sturgeon population.
Introduction
Historically, the Missouri River was a large, free-flowing dynamic river system that meandered extensively back and forth across the floodplain, continuously creating new habitats (Galat & Lipkin Citation2000; Galat et al. Citation2005). In its unaltered state, the Missouri River had annual cycles of high and low discharge that resulted in constantly changing depths and velocities. These conditions provided access to a wide variety of habitats for fish and acted as spawning cues for many native species. The Missouri River is now a highly modified system managed for flood control, navigation, irrigation, hydropower, water supply, water quality, recreation, and fish and wildlife (US Army Corps of Engineers Citation2001). Modifications included construction of six mainstem dams, an extensive levee system and a highly altered navigation channel from Sioux City, Iowa (river kilometer [rkm] 1178.8) to the confluence with the Mississippi River (rkm 0.0). Overall, approximately 35% of the river has been impounded by the mainstem dams, 32% has been channelized for navigation, and 33% remains relatively unaltered but is interspersed among reservoirs where temperature, flows, and turbidity have been greatly altered (Keenlyne Citation1989; Hesse & Mestl Citation1993). The total water surface of the channelized reach has been reduced by approximately 50% (Funk & Robinson Citation1974) and annual sediment discharge has decreased by 100–150 million metric tons, more than is currently transported annually by the entire Missouri–Mississippi River systems to the Gulf of Mexico (Meade & Moody Citation2010). The mainstem dams and current water management practices have altered the natural hydrograph and sediment transport system that historically created the dynamic habitat elements for the native fauna and flora (Funk & Robinson Citation1974; Hesse et al. Citation1993; Latka et al. Citation1993; Pegg et al. Citation2003). Furthermore, these modifications have eliminated the natural flow regime and created a high-energy channel that has promoted stream bed degradation and disconnected the Missouri River from much of its floodplain (Winston & Criss Citation2003).
Flows on the lower Missouri River are controlled by Gavins Point Dam (Yankton, SD; rkm 1305.2), which is the smallest and lowest dam on the mainstem Missouri River. Gavins Point Dam's purpose is to stabilize the irregular discharge from upstream hydro-peaking and to support navigation on the lower Missouri River, resulting in artificially stable flows during the March to November navigation season that do not mimic the natural hydrograph. Before dam construction, the hydrograph had an early peak in March, followed by a second peak in late June, which inundated the low-lying floodplain and islands, then continuously declining stages for the rest of the year. The current channel configuration and water management prevents floodplain connection, except during extreme water years. This lack of connection limits food availability reduces spawning areas and limits refugia area for age-0 fishes (Junk et al. Citation1989). However, high discharges through the upper channelized reach of the Missouri River in 2010 and 2011 resulted in connection to the floodplain and increased levels of macroinvertebrate and fish production (Steffensen, Eder et al. Citation2014); therefore, increased food resources.
These aforementioned dam operations and physical modifications were identified as contributing to the population decline of the federally listed pallid sturgeon (Scaphirhynchus albus) on the Missouri River (Dryer & Sandvol Citation1993; USFWS Citation2014). These same modifications and water management practices are also implicated in population declines documented for several other native fishes in the Missouri River (Hesse et al. Citation1989, Citation1993; Steffensen, Shuman et al. Citation2014; Steffensen, Shuman & Stukel Citation2014). To supplement the diminished wild pallid sturgeon population, conserve unique genotypes, and to bolster the number of reproductive fish in the system, the Pallid Sturgeon Conservation Augmentation Program was implemented in the lower Missouri River (i.e. Gavins Point Dam [rkm 1305.2] to the confluence with the Mississippi River [rkm 0.0]). Stocking occurred three times in the 1990s and annually since 2001 with more than 163,000 pallid sturgeon stocked into the lower Missouri River (Huenemann Citation2015). The primary concern of the Pallid Sturgeon Conservation Augmentation Program was to augment the suppressed pallid sturgeon population while properly managing for genetic contributions; there was little discussion about the current carrying capacity of the system and impacts to other benthic fishes.
Adult pallid sturgeons are piscivorous and dependent on the native chubs and minnows as their primary prey. Pallid sturgeon prey selectivity is unknown as the early diet studies only reported small fishes in their stomachs (Coker Citation1930; Cross Citation1967; Carlson et al. Citation1985), not species-specific information. Recent food habit studies have been more descriptive of species consumed but were conducted in modified reaches where the fish community has been highly altered. In the upper Missouri River (i.e. Fort Peck Dam [rkm 2850.9] to the headwaters of Lake Sakakawea [rkm 2523.5]), Gerrity et al. (Citation2006) found that sturgeon chub (Macrhybopsis gelida) and sicklefin chub (M. meeki) comprised 79% of all identifiable fish recovered from juvenile pallid sturgeon stomachs. Channel catfish (Ictalurus punctatus), flathead chub (Platygobio gracilis), sand shiner (Notropis stramineus), and shorthead redhorse (Moxostoma macrolepidotum) were also consumed. In the inter-reservoir reach of the middle Missouri River bordering Nebraska and South Dakota, Wanner et al. (Citation2007) found that Johnny darter (Etheostoma nigrum), channel catfish, silver chub (M. storeriana), and emerald shiner (N. atherinoides) were the primary prey selected. In the channelized reach of the lower Missouri River, just below our study area, Winders et al. (Citation2014) found Ictaluridae and Cyprinidae were the primary prey for pallid sturgeon. Specifically, the Cyprinidae family was most represented by Macrhybopsis but the level of digestion generally prohibited further identification. Finally, Hoover et al. (Citation2007) found that pallid sturgeon in the middle Mississippi River consumed Cyprinidae, Sciaenidae, and Clupeidae.
Several of these species identified as prey for pallid sturgeon are below historic levels (Meek Citation1892; Johnson Citation1942) and continue to decline locally (Hesse Citation1994; Steffensen, Shuman & Stukel Citation2014) and basin-wide (USFWS Citation2001), especially the chub species. More specifically, the range of sicklefin chub and sturgeon chub has been reduced in the mainstem Missouri River by more than 1609 km (i.e. 1287 km of impoundment and 322 km of inter-reservoir reaches), and sturgeon chub only occur in 11 of their 30 historic tributaries (USFWS Citation2001). Flathead chub populations have declined across much of the mainstem Missouri River downstream from North Dakota to Missouri (Tibbs Citation1998).
Understanding the impacts of condition on the pallid sturgeon population may be critical for the species recovery as condition affects survival, growth rates, and reproductive potential (Pope & Kruse Citation2007). Blackwell et al. (Citation2000) suggested that fish in good condition have more energy available for gamete production thus increasing relative fecundity. The relationship between condition and fecundity for pallid sturgeon from the lower Missouri River is unknown, but fork length and weight displayed a positive correlation with the number of eggs released (Steffensen et al. Citation2013a). However, this information needs to be assessed cautiously as the sample size was very limited (n = 11).
During the spring 2015 sampling efforts along the Nebraska reach of the lower Missouri River (see Welker & Drobish Citation2012a, Citation2012b for sampling details), several adult pallid sturgeon were captured in visually poor condition. Furthermore, after all potential brood fish (i.e. those great than 800 mm FL) were assessed for reproductive readiness, the proportion of reproductive females was below the previously observed levels. The majority of adult wild and hatchery-reared pallid sturgeon in the lower Missouri River resides in the upper channelized (Sioux City, IA [rkm 1178.8] to Kansas City, MO [rkm 591.4]) reach (K. Steffensen, NGPC, unpublished data). Negative changes in condition of these fish may have important implications for pallid sturgeon reproduction and ultimately recovery of the species to a naturally, self-sustaining population. Therefore, the objectives of this study were to (1) document the temporal variation in pallid sturgeon condition against time (i.e. 2003–2015) by pallid sturgeon proportional size distribution (PSD) categories; (2) assess variation in condition amongst gender and reproductive status (i.e. reproductive or non-reproductive) then determine if reproductive female condition was related to the number of eggs produced; (3) document the percentage of reproductive female pallid sturgeon compared to no non-reproductive fish; and (4) correlate the overall annual pallid sturgeon condition against several hypothesized factors which may contribute to the decline in pallid sturgeon condition.
Methods
The study area was a 423.4 rkm reach of the lower Missouri River along Nebraska's eastern border from Lower Ponca Bend (rkm 1211.8) to the Nebraska–Kansas state line (rkm 788.4). This reach has been channelized with a series of dike structures on the inside bends and limestone rock armoring the outside bends. The Platte River (rkm 957.6) intersects this reach and seasonally impacts the hydrograph of the Missouri River.
Pallid sturgeon capture data (i.e. 2003–2015) were obtained from the Nebraska Game and Parks Commission's (NGPC) Pallid Sturgeon Population Assessment (PSPA) Program sampling efforts from the upper channelized reach (rkm 1212–789) of the Missouri River (Welker & Drobish Citation2012a, Citation2012b) and NGPC's annual intensive effort directed at capturing reproductively ready pallid sturgeon for brood stock for hatchery spawning (i.e. Conservation Augmentation Program). Pallid sturgeons were classified as wild or hatchery-reared according to the presence of a hatchery mark and/or tag or by post hoc genetic determination (Schrey & Heist Citation2007; Schrey et al. Citation2007; DeHaan et al. Citation2008). Gender and gonad maturation stage were determined using ultrasound and endoscopic techniques described in Bryan et al. (Citation2007) and Divers et al. (Citation2009) and fish categorized as reproductive (stage IV, ready to spawn this season) or non-reproductive (stage II–III; Moos Citation1978; Colombo et al. Citation2007; Tripp et al. Citation2009). The number of eggs released was estimated using volumetric estimation by counting a subset of number of eggs/mL and extrapolating to the total volume extracted. Complete or incomplete spawn was noted during a post-spawn biopsy and only females that completely spawned were included in the fecundity analysis.
Relative condition (Kn) was used to assess change in pallid sturgeon condition with the pallid sturgeon weight–length relationship calculated based on the equation provided by Shuman et al. (Citation2011),where FL and W are the observed fork length and weight for each individually captured pallid sturgeon. The Shuman et al. (Citation2011) equation is based on a regional assessment (i.e. Missouri River), not the entire species range (Murphy & Willis Citation1992); therefore, relative condition factor (Kn) was calculated as
where W is the individual's observed weight and W’ is the predicted weight from the weight–length relationship as described by the Shuman et al. (Citation2011) equation.
Seasonal (i.e. pre-spawn (March–April) and post-spawn (May–November)) and origin (wild vs. hatchery-reared) effects were tested (ANOVA [analysis of variance] with a Tukey adjustment) to determine if there were differences in Kn. If treatments were significantly different, then further assessment would be analyzed by season and origin. However, if treatments did not differ, then data from each treatment were merged into a single cohort. To test the annual trends, the annual mean Kn between years was calculated and regressed by the incremental PSD indices developed for pallid sturgeon (Shuman et al. Citation2006; Guy et al. Citation2007). Pallid sturgeon length categories were stock to quality (S-Q; 330–629 mm fork length [FL]), quality to preferred (Q-P; 630–839 mm), preferred to memorable (P-M; 840–1039 mm), and memorable to trophy (M-T; 1040–1269 mm). No trophy-sized fish (>1270 mm) were collected. Next, to determine if condition varied between gender and reproductive status, annual mean and overall mean Kn by gender and reproductive condition were compared (ANOVA with a Tukey adjustment). If annual differences were detected then a pairwise comparison (LSMEANS within PROC GLM, SAS 9.4) tested for differences amongst years. In addition, the number of eggs produced was regressed against condition to determine if relative condition affects fecundity and the percent of reproductive compared to non-reproductive females was calculated. Finally, overall pallid sturgeon condition was correlated against several hypothesized factors that may contribute to the variation in pallid sturgeon condition. The hypothesized, independent variables that were acquired and tested included (1) the mean annual discharge of the Missouri River at the Omaha (USGS 06610000) and Nebraska City gages (USGS 06807000), (2) intraspecific competition which used the Steffensen et al. (Citation2013b) population viability model to estimate the current pallid sturgeon population in the lower Missouri River, (3) interspecific competition which used the standardized, long-term electrofishing catch per unit effort (CPUE) for flathead catfish Pylodictis olivaris (Porter et al. Citation2011 and M. Pegg, University of Nebraska-Lincoln, Pers. Comm.), (4) food availability by calculating CPUE from the NGPC's PSPA Program's standardized sampling efforts for (a) annual otter trawl catch rates for sturgeon and sicklefin chubs which were described as primary prey items by Gerrity et al. (Citation2006), (b) annual otter trawl catch rates for a suite of species documented by Winders et al. (Citation2014), and (c) annual mini-fyke net catch rates for all small bodied fish. Since the majority of pallid sturgeons are collected in March and April, before the independent variables were measured, independent variables were set forward one year to adjust to the observed conditions. All statistical tests were performed using SAS 9.4 (SAS Institute, Cary, NC) and significance was determined at α = 0.05.
Results
Relative condition did not differ between pre-spawn (March–April) and post-spawn (May–November) season for any of the pallid sturgeon size categories (S-Q, t = 1.41, p = 0.159; Q-P, t = 1.79, p = 0.074; P-M, t = 1.53, p = 0.128; M-T, t = 0.85, p = 0.398). Furthermore, there was no difference in mean Kn between wild-origin fish and hatchery-reared pallid sturgeon within size categories (S-Q, t = 1.06, p = 0.290; Q-P, t = 1.89, p = 0.060; P-M, t = 1.27, p = 0.204; M-T, t = 0.27, p = 0.790); therefore, wild and hatchery-reared pallid sturgeon were grouped with no season or origin effect and summarized annually.
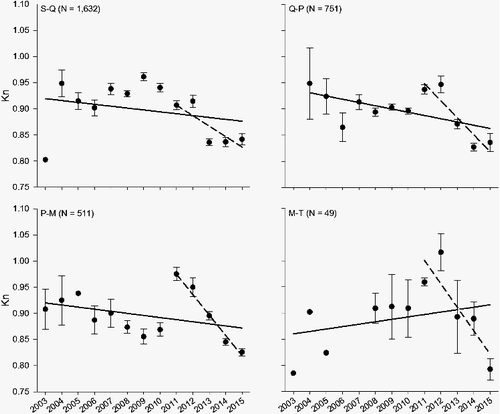
From 2003 to 2015, a significant downward trend in pallid sturgeon Kn has occurred in all size categories, except for M-T sized fish (; ) but this exception is being influenced by only three M-T-sized fish captured from 2003 to 2007. However, from 2011 to 2015, all size categories have experienced a significant decline and the annual pairwise comparison between years were significantly different for all PSD size classes, except for the M-T sized fish. Annual pairwise comparisons were significantly different for S-Q sized fish with a significant decrease in Kn from 2011 to 2012 compared 2013–2015. However, the past three years were similar. A similar trend occurred with Q-P and P-M sized fish with a significant annual decline in 2014–2015 compared to 2011–2013. Relative conditions for both size classes have reached an unprecedented low. Even though there is no long-term difference for M-T sized fish, Kn for M-T declined to an annual mean of 0.79 in 2015.
Table 1. Regression statistics (over study duration [2003–2015] and short-term [2011–2015]), ANOVA model, and annual differences in relative condition (Kn) by incremental proportional size distribution (PSD) size categories for all pallid sturgeon captured from 2003 to 2015 in the upper channelized Missouri River. Letters denote statistical comparison within each size category. PSD size categories are: S-Q = 330–629 mm fork length (FL), Q-P = 630–839 mm), P-M = 840–1039 mm, and M-T = 1040–1269 mm).
Figure 1. Annual mean (±SE) relative condition factor (Kn) by incremental proportional size distribution (PSD) size classes for all pallid sturgeon captured from 2003 to 2015 in the upper channelized Missouri River. Solid regression line indicates the overall change in Kn, while the dashed regression line displays the change over the past five years.
Pallid sturgeon appears to exhibit sexual dimorphism in Kn between males and females (). Female pallid sturgeon had a significantly higher overall Kn compared to males (t = 2.44, p = 0.015; ). Fish in reproductive condition had a significantly higher Kn compared to non-reproductive for both genders (). Furthermore, comparisons between annual mean Kn for reproductive females did not vary annually; whereas, non-reproductive females and males and reproductive males all varied annually (). Overall, Kn greatly improved in 2012 for all potential reproductive sized fish (> 800 mm) followed by a rapid decline in Kn for all fish, excluding reproductive females.
Figure 2. Annual mean (±SE) relative condition factor (Kn) by gender and reproductive assessment for adult (> 800 mm) pallid sturgeon transported to local hatcheries as potential brood fish.
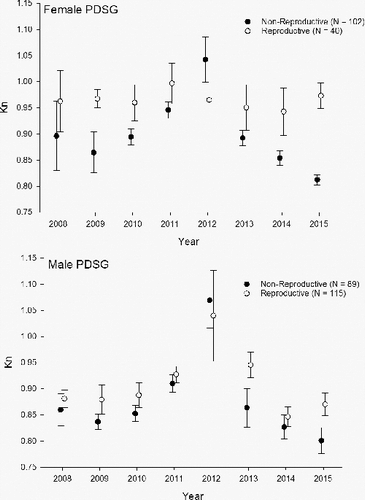
Table 2. Mean relative condition (Kn) and pairwise comparisons for pallid sturgeon by gender and reproductive assessment.
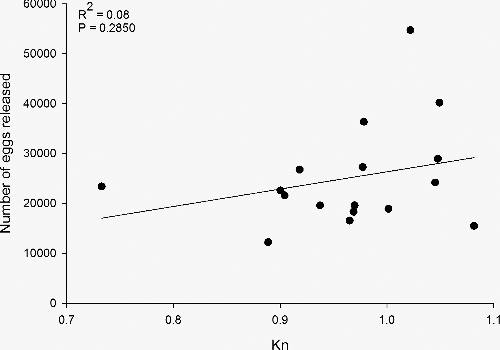
Forty reproductive female pallid sturgeons have been captured since 2008. Genetic concerns prevented 10 reproductive females from being spawned. Twenty fish successfully released eggs, but three were determined to have had an incomplete spawn. Relative condition did not differ between fish that successfully spawned (Kn = 0.97, SE = 0.02) compared to fish that did not release eggs (Kn = 0.93, SE = 0.01; t = 0.93, p = 0.360). Overall, 28% of the females captured have been in reproductive condition; however, from 2008 to 2013, 34% (n = 32) were in reproductive condition compared to only 17% (n = 8) the past two years (). For females that successfully spawned, the number of eggs was highly variable and ranged from 12,220 to 54,705 (mean = 25,087) or 4721 to 13,128 (mean = 7443) per kg of body weight. Relative condition for the reproductively ready pallid sturgeon that successfully spawned was not correlated with the number of eggs released ().
Figure 3. Percentage of reproductive females (gray bar) compared to non-reproductive females (black bar) for female pallid sturgeon collected in the upper channelized reach of the lower Missouri River.
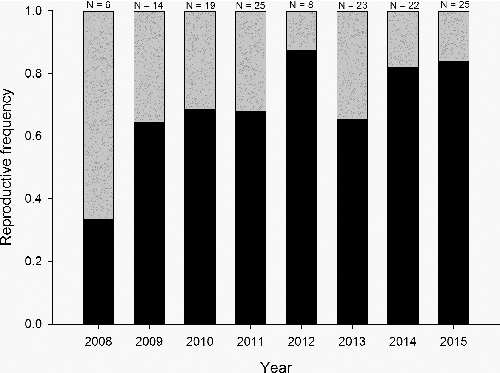
Figure 4. Number of eggs released by relative condition factor (Kn) for 17 reproductively ready female pallid sturgeon collected in the upper channelized reach of the lower Missouri River and successfully spawned at a hatchery facility.
Correlations between pallid sturgeon condition and hypothesized independent variables did not result in any significant relationships (). The annual catch rate of potential prey fish described by Winders et al. (Citation2014) with a one year offset had the strongest correlation followed by flathead catfish catch rates. Whereas, the pallid sturgeon population estimate and discharge variable showed the weakest correlation.
Table 3. Correlation statistics for independent variables hypothesized to be affecting pallid sturgeon Kn in the upper channelized reach of the Missouri River.
Discussion
The majority of the pallid sturgeon, especially the wild pallid sturgeon, resides in the upper channelized reach of the lower Missouri River. The observed decrease in condition is probably related to the increased bioenergetic demands and reduced food resources because of habitat loss caused by channelization; however, no statistical differences were determined. Karr (Citation1991) noted that higher trophic level fish (i.e. piscivorous pallid sturgeon) are more vulnerable to habitat loss and degradation, which likely affects fishes condition. This low condition has decreased maturation rates with reduced fecundity and is likely interrupting reproductive cycling. The fact that the annual condition of pallid sturgeon in Nebraska varies so much from year to year may indicate that condition may be very closely linked to the amount of prey in the system. Many native chubs appear to be as imperiled as pallid sturgeon (Hesse Citation1994; Galat et al. Citation2005; Steffensen, Shuman & Stukel Citation2014) but have not received the attention through federal listing as a threatened or endangered species. When the Conservation Augmentation Program was initiated, biologists assumed that the carrying capacity of the lower Missouri River was adequate to support the recovery goal to create a self-sustaining population (Dryer & Sandvol Citation1993; USFWS Citation2014); however, the current river's carrying capacity remains unknown. Under the current river management it is questionable if the upper reach of the lower Missouri River, in its current condition, can support a self-sustaining population of pallid sturgeon.
Keenlyne and Evenson (Citation1993) developed the original relative weight equation for pallid sturgeon and concluded that fish from the channelized Missouri River were underweight compared to fish from the upper Missouri River (i.e. Fort Peck Dam [rkm 2850.9] to the headwaters of Lake Sakakawea [rkm 2523.5]). Shuman et al. (Citation2011) detected differences in Kn by incremental PSD between the Pallid Sturgeon Recovery Priority Management Areas on the Missouri River but did not provide any temporal differences. Pallid sturgeon in the lower Missouri River (i.e. Gavins Point Dam [rkm 1305.2] to the confluence with the Mississippi River [rkm 0.0]) were always characterized as being a smaller, slender fish compared to the remnant adults in the upper Missouri River. This likely reflects the demands of living in a highly engineered channelized environment; however, pallid sturgeon relative condition in the channelized Missouri River was always assumed adequate.
Condition indices may not always provide the best assessment for the change in sturgeon condition. Beamish et al. (Citation1996) concluded that when food resources are limited, lake sturgeon (Acipenser fulvescens) increase water uptake. We acknowledge this potential bias, but Kn for pallid sturgeon along Nebraska's eastern border of the Missouri River are declining and fish are, on visual examination alone, emaciated. If pallid sturgeon can alter their body weight through water retention, then conditions in the upper channelized Missouri River may be worse than our analysis is predicting. As Kn declines, protein and lipid reserves are likely depleted and mortality may occur. Lipids tend to be the primary energy source for gonadal development (Beamish et al. Citation1996). Without sufficient lipid reserves to produce viable gametes, natural reproduction is unlikely. Until conditions can be improved, recovery of the species is unlikely and species persistence will remain dependent on hatchery supplementation. Concerns exist that the current levels of hatchery supplementation may exceed the current carrying capacity for this reach of the Missouri River but the results of this study do not support those concerns.
The mean condition factor for pallid sturgeon from the lower Missouri River along Nebraska's eastern border since 2003 was around 0.90 but highly variable. However, it is unknown what the mean condition factor should be for pallid sturgeon. Quist et al. (Citation1998) suggested relative weight target values between 80 and 90 for the sympatric shovelnose sturgeon (Scaphirhynchus platorynchus) excluding the large adults found in Montana. Although fairly similar in body shape, this recommendation may not be valid for the piscivorous pallid sturgeon, especially for the larger PSD size classes (i.e. P-M and M-T). The diets of these two species have low similarity niche (Gerrity et al. Citation2006). For the most part, only reproductively ready pallid sturgeon are achieving a Kn greater than 0.90, which is the effect of gonadal development, while the current mean population Kn is approaching the lower end of that recommendation.
Survival of hatchery-reared pallid sturgeon stocked in the lower Missouri River is similar to other sturgeon species (Steffensen et al. Citation2010). Hatchery-reared fish in the lower Missouri River were growing (Shuman et al. Citation2011), exhibiting migratory behavior and reaching sexual maturity (DeLonay et al. Citation2010; DeLonay et al. Citation2012; Steffensen et al. Citation2013a). The numbers of reproductive fish in the lower Missouri River was increasing prior to 2013; however, the proportion of reproductive fish collected annually continues to decrease. Furthermore, the number of reproductively ready female pallid sturgeon captured steadily increased from 2008 to 2013 but has decreased the past two years. A similar trend was observed with capture-recaptured, telemetry-tagged pallid sturgeon. Several females were predicted to be in reproductive condition in 2015 based on past reproductive histories but did not achieve reproductive readiness. There is also some evidence that fish may not be maturing at the same approximate size as they were prior to 2011. Prior to 2011, Steffensen et al. (Citation2013a) determined that the minimum length-of-maturity was approximately 800 mm, but over the past two years the minimum length of reproductive females sampled has been 895 mm. Several known hatchery-reared pallid sturgeon from 2001 and 2002 year classes were captured this past year and were determined to be still immature and yet to reach their first maturation cycle (J. Haas, NGPC, Pers. Comm.). Despite all of this, a few pallid sturgeon in the upper reach of the lower Missouri River are still maintaining a higher condition factor, becoming reproductively ready, and producing viable gametes.
Relative fecundity for females that reach reproductive readiness captured in the upper reach (above Kansas City, MO [rkm 591.4]) of the lower Missouri River was considerably lower than fish from the lower reach (below Kansas City, MO). George et al. (Citation2012) estimated total fecundity of two pallid sturgeon (mean weight 3.2 kg) captured in the lower Missouri River at 57,192 and 58,913 eggs or approximately 18,140 eggs per kg of body weight. The reproductive females captured during our study were slightly larger (average weight = 3.7 kg) but only produced an average of 7400 eggs per kg body weight. Additionally, Keenlyne et al. (Citation1992) reported approximately 9900 eggs per kg body weight for an old (age-41), 30.8 kg pallid sturgeon taken from North Dakota.
Even though a local difference was observed, relative fecundity for pallid sturgeon in the upper reach of the lower Missouri River was similar to other large-sized sturgeon species. Beluga sturgeon Huso huso (Linnaeus) was reported to have a relative fecundity between 2750 to 10,500 eggs per kg body weight (Raspopov Citation1987), while white sturgeon ranged from 3192 to 8582 with a mean of 5648 eggs per kg body weight (Chapman et al. Citation1996). Smaller bodied sturgeon species have been reported to produce more eggs per kg body weight with shortnose sturgeon (A. brevirostrum) having an average relative fecundity of 11,568 (Dadswell Citation1979), while shovelnose sturgeon, in the local proximity of our study, had 18,355 eggs per kg body weight in the Platte River (Hamel et al. Citation2015).
Comparatively, pallid sturgeon captured in the Platte River, a major tributary to the Missouri River, which bisects our study area, had a mean Kn ranging from 0.95 to 0.99 from 2009 to 2012 (Hamel et al. Citation2014). If our data-set is truncated to the same time period, the reported Kn values for the Platte River are still higher compared to Kn observed on the Missouri River (Kn = 0.93, SE = 0.003), but the difference is lessened. Beamesderfer (Citation1993) reported that white sturgeon (Acipenser transmontanus) relative weight (Wr) varied from 77 to 117 with the lowest Wr from areas with limited food availability and declining populations. This suggests that fish in the Platte River are likely finding additional food resources to enhance their condition as the population between the Platte and Missouri Rivers are sympatric. Furthermore, the available habitat and flow regime in the Platte River are more favorable which allow pallid sturgeon at least to maintain or improve their conditions.
We documented that pallid sturgeon in the upper channelized Missouri River can rapidly increase body weight and improve Kn when food resources become available, although not statistically correlated small bodied fishes catch rates. There was a rapid increase in body weight and Kn after the 2010 high water and 2011 major flood events when additional food and habitat resources were readily available. There was increased reproduction of many native species during the major flood in 2011 (Steffensen, Eder et al. Citation2014) and likely provided abundant food resources for pallid sturgeon. Pallid sturgeon responded and reached a mean Kn greater than 0.95 after these two years. However, the benefits of 2010 and 2011 floods were short lived and were followed by a rapid decline of pallid sturgeon condition over the past three years when food resources possibly decreased. Also during the 2011 flood, adult pallid sturgeon were utilizing the slower inundated floodplain habitats which likely reduced their biogenetics demands compared to residing in the swift currents of the main channel (Haas et al. Citation2014). Therefore, current river management needs to focus on the lower trophic levels and pallid sturgeon condition will have a positive response to increased food availability.
Acknowledgments
The authors acknowledge the Nebraska Game and Parks Commission crews from the Pallid Sturgeon Population Assessment Program, Habitat Assessment and Monitoring Program, and Pallid Sturgeon Research Program for their hard work over the past 12 years and the hundreds of volunteers who have assisted them during their annual pallid sturgeon brood stock collection efforts since 2008. Finally, the authors also thank Rick Holland and the Journal of Freshwater Ecology's reviewers for their comments on earlier drafts of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Kirk D. Steffensen
Gerald Mestl received his masters of science degree in zoology from Oklahoma State University. Gerald serves as the Missouri River Program Manager for the Fisheries Division of the Nebraska Game and Parks Commission.
Gerald E. Mestl
Kirk Steffensen gained his MS degree in fisheries science from the University of Nebraska-Lincoln. Kirk is a fisheries biologist II for the Nebraska Game and Parks Commission where he serves of the project leader of the Missouri River Pallid Sturgeon Population Assessment Project.
References
- Beamesderfer RC. 1993. A standard weight (Ws) equation for white sturgeon. Calif Fish Game. 79:63–39.
- Beamish FWH, Jebbink JA, Rossiter A, Noakes DLG. 1996. Growth strategy of juvenile lake sturgeon (Acipenser fulvescens) in a northern river. Can J Fish Aquatic Sci. 53:481–489.
- Blackwell BG, Brown ML, Willis DW. 2000. Relative weight (Wr) status and current use in fisheries assessment and management. Rev Fish Sci. 8:1–44.
- Bryan JL, Wildhaber ML, Papoulias DM, DeLonay AJ, Tillitt DE, Annis ML. 2007. Estimation of gonad volume, fecundity and reproductive state of shovelnose sturgeon using sonography and endoscopy with application to the endangered pallid sturgeon. J Appl Ichthyol. 23:411–419.
- Carlson DM, Pflieger WL, Trial L, Haverland PS. 1985. Distribution, biology and hybridization of Scaphirhynchus albus and S. platorynchus in the Missouri and Mississippi rivers. Env Bio Fish. 14:51–59.
- Chapman FA, Van Eenennaam JP, Doroshov SI. 1996. The reproductive condition of white sturgeon, Acipenser transmontanus, in San Francisco Bay, California. Fish Bull. 94:628–634.
- Coker FB. 1930. Studies of common fish of the Mississippi River at Keokuk. US Bur Fish Bull. 45:141–225.
- Colombo RE, Garvey JE, Wills PS. 2007. Gonadal development and sex-specific demographics of the shovelnose sturgeon in the middle Mississippi River. J Appl Ichthyol. 23:420–427.
- Cross FB, 1967. Handbook of fishes of Kansas. Lawrence (KS): University of Kansas Museum of Natural History (Miscellaneous Publication 45).
- Dadswell MJ. 1979. Biology and population characteristics of the shortnose sturgeon, Acipenser brevirostrum LeSueur 1818 (Osteichthyes: Acipenseridae) in the Saint John Estuary, New Brunswick, Canada. Can J Zoo. 57:2186–2210.
- DeHaan PW, Jordan GR, Arden WR. 2008. Use of genetic tags to identify captive-bred pallid sturgeon (Scaphirhynchus albus) in the wild: improving abundance estimates for an endangered species. Conserv Genet. 9:691–697.
- DeLonay AJ, Jacobson RB, Papoulias DM, Wildhaber ML, Chojnacki KA, Pherigo EK, Bergthold CL, Mestl GE. 2010. Ecological requirements for pallid sturgeon reproduction and recruitment in the lower Missouri River: annual report 2009. Columbia (MO): US Geological Survey.
- DeLonay AJ, Jacobson RB, Papoulias DM, Wildhaber ML, Chojnacki KA, Pherigo EK, Haas JD, Mestl GE. 2012. Ecological requirements for pallid sturgeon reproduction and recruitment in the lower Missouri River: annual report 2010. Columbia (MO): US Geological Survey.
- Divers SJ, Boone SS, Hoover JJ, Boysen KA, Kilgore KJ, Murphy CE, George SG, Camus AC. 2009. Field endoscopy for identifying gender, reproductive stage and gonadal anomalies in free-ranging sturgeon (Scaphirhynchus) from the lower Mississippi River. J Appl Ichthyol. 25:68–74.
- Dryer MP, Sandvol AJ. 1993. Pallid sturgeon recovery plan. Bismarck (ND): US Fish and Wildlife Service.
- Funk JL, Robinson JW. 1974. Changes in the channel of the lower Missouri River and effects on the fish and wildlife. Jefferson City (MO): Missouri Department of Conservation (Aquatic Series 11).
- Galat DL, Lipkin R. 2000. Restoring ecological integrity of great river: historical hydrographs aid in defining reference conditions for the Missouri River. Hydrobiol. 422/423:29–48.
- Galat DL, Berry CR, Gardner WM, Hendrickson JC, Mestl GE, Power GJ, Stone C, Winston MR. 2005. Spatiotemporal patterns and changes in Missouri River fishes. In: Rinne JN, Hughes RM, Calamuso B, editors. Historical changes in large river fish assemblages of the Americans. Bethesda (MD): American Fisheries Society, Symposium 45; p. 249–291.
- George SG, Slack WT, Hoover JJ. 2012. A note on the fecundity of pallid sturgeon. J Appl Ichthyol. 28:512–515.
- Gerrity PC, Guy CS, Gardner WM. 2006. Juvenile pallid sturgeon are piscivorous: a call for conserving native cyprinids. Trans Am Fish Soc. 135:604–609.
- Guy CS, Neumann RM, Wilis DW, Anderson RO. 2007. Proportional size distribution (PSD): a further refinement of population size structure index terminology. Fish. 32:348.
- Haas JD, Eder BL, Archer MW, Adams JD, Ruskamp RL. 2014. Documenting presence of pallid sturgeon on lower Missouri River floodplains. Prairie Nat. 46:97–99.
- Hamel MJ, Pegg MA, Hammen JJ, Rugg ML. 2014. Population characteristics of pallid sturgeon, Scaphirhynchus albus (Forbes and Richardson, 1905), in the lower Platte River, Nebraska. J Appl Ichthyol. 30:1362–1370.
- Hamel MJ, Rugg ML, Pegg MA, Patino R, Hammen JJ. 2015. Reproductive traits of shovelnose sturgeon Scaphirhynchus platorynchus (Rafinesque, 1820) in the lower Platte River, Nebraska. J Appl Ichthyol. 31:592–602.
- Hesse LW. 1994. The status of Nebraska fish in the Missouri River, selected chubs and minnows (Cyprinidae): sicklefin chub (Macrhybopsis meeki), sturgeon chub (M. gelida), silver chub (M. storeriana), speckled chub (M. aestivalis), flathead chub (Platygobio gracilis), plains minnow (Hybognathus placitus) and western silvery minnow (H. argyritis). Trans Neb Acad Sci. 21:99–108.
- Hesse LW, Mestl GE. 1993. An alternative hydrograph for the Missouri River based on the precontrol condition. N Am J Fish Man. 13:360–366.
- Hesse LW, Schmulback JC, Carr JM, Keenlyne KD, Unkenholz DG, Robinson JW, Mestl GE. 1989. Missouri River fishery resources in relation to past, present and future stresses. In: Dodge DP, editor. Proceedings of the International Large River Symposium; Toronto: Canadian Special Publication of Fisheries and Aquatic Sciences 106.
- Hesse LW, Mestl GE, Robinson JW. 1993. Status of selected fishes in the Missouri River in Nebraska with recommendations for their recovery. In: Heese LW, Stalnaker CB, Benson NG, Zuboy JR, editors. Restoration planning for the river of the Mississippi River ecosystem. Washington (DC): National Biological Survey, Biological Report 19; p. 327–340.
- Hoover JJ, George SG, Kilgore KJ. 2007. Diet of shovelnose sturgeon and pallid sturgeon in the free-flowing Mississippi River. J Appl Ichthyol. 23:494–499.
- Huenemann TW. 2015. Central lowlands and interior highlands pallid sturgeon spawning and stocking summary. Lincoln (NE): Nebraska Game and Parks Commission.
- Johnson RE. 1942. The distributions of Nebraska fishes [MS thesis]. Ann Arbor (MI): University of Michigan.
- Junk WJ, Bayley PB, Sparks RE. 1989. The flood pulse concept in river-floodplain systems. In: Dodge DP, editor. Proceedings of the International large River Symposium. Ottawa: Canadian Special Publications of Fisheries and Aquatic Sciences.
- Karr JR. 1991. Biological integrity: a long-neglected aspect of water resource management. Eco App. 1:66–84.
- Keenlyne KD. 1989. A report of the pallid sturgeon. Pierre (SD): US Fish and Wildlife Service.
- Keenlyne KD, Evenson PD. 1993. Standard and relative weight for the pallid sturgeon (Scaphirhynchus albus). Proc S Dak Ac Sci. 2:41–49.
- Keenlyne KD, Grossman EM, Jenkins LG. 1992. Fecundity of pallid sturgeon. Trans Am Fish Soc. 121:139–140.
- Latka DC, Nestler J, Hesse LW. 1993. Restoring physical habitat in the Missouri River: a historical perspective. In: Heese LW, Stalnaker CB, Benson NG, Zuboy JR, editors. Restoration planning for the river of the Mississippi River ecosystem. Washington (DC): National Biological Survey, Biological Report 19; p. 350–359.
- Meade RH, Moody JA. 2010. Causes for the decline of suspended-sediment discharge in the Mississippi River system, 1940–2007. Hydrol Proc. 24:35–49.
- Meek SE. 1892. Notes of the fishes of western Iowa and eastern Nebraska. Bul US Fish Comm. 10:217–248.
- Moos RE. 1978. Movement and reproduction of shovelnose sturgeon Scaphirhynchus platorynchus in the Missouri River, South Dakota [PhD thesis]. Vermillion (SD): University of South Dakota.
- Murphy BR, Willis DW. 1992. Proper distinction between relative weight and relative condition factor. N Am J Fish Man. 12:665–666.
- Pegg MA, Pierce CL, Roy A. 2003. Hydrological alteration along the Missouri River basin: a time series approach. J Aquatic Sci. 65:63–72.
- Pope KL, Kruse CG. 2007. Condition. In: Guy CS, Brown ML, editors. Analysis and interpretation of freshwater fisheries data. Bethesda (MD): American Fisheries Society, p. 423–472.
- Porter TK, Mestl GE, Porath MT. 2011. Population Characeristics of flathead catfish in channelized and unchannelized reaches of the middle river from 1997–2008. In: Michaletz PH, Travnichek VH, editors. Conservation, ecology, and management of catfish: the second international symposium. Bethesda (MD): American Fisheries Society, p. 105–118.
- Quist MC, Guy CS, Braaten PJ. 1998. Standard weight (Ws) equation and length categories for shovelnose sturgeon. N Am J Fish Man. 18:992–997.
- Raspopov VM. 1987. Fecundity of beluga sturgeon, Huso huso, of the Caspian Sea. J Appl Ichthyol. 27:140–141.
- Schrey AW., Heist EJ. 2007. Stock structure of pallid sturgeon analyzed with microsatellite loci. J Appl Ichthyol. 23:297–303.
- Schrey AW, Sloss BL, Sheehan RJ, Heidinger RC, Heist EJ. 2007. Genetic discrimination of middle Mississippi River Scaphirhynchus sturgeon into pallid, shovelnose and putative hybrids with multiple microsatellite loci. Conserv Genet. 8:683–693.
- Shuman DA, Willis DW, Krentz SC. 2006. Application of a length-categorization system for pallid sturgeon (Scaphirhynchus albus). J Freshw Eco. 21:71–76.
- Shuman DA, Klumb RA, Wilson RH, Jaeger ME, Haddix T, Gardner WM, Doyle WJ, Horner PT, Ruggles M, Steffensen KD, et al. 2011. Pallid sturgeon size structure, condition and growth in the Missouri River basin. J Appl Ichthyol. 27:269–281.
- Steffensen KD, Powell LA, Koch JD. 2010. Assessment of hatchery-reared pallid sturgeon survival in the lower Missouri River. N Am J Fish Man. 30:671–678.
- Steffensen KD, Pegg MA, Mestl GE. 2013a. Population characteristics of pallid sturgeon in the lower Missouri River. J Appl Ichthyol. 29:687–695.
- Steffensen KD, Pegg MA, Mestl GE. 2013b. Population prediction and viability model for pallid sturgeon (Scaphirhynchus albus, Forbes and Richardson, 1905) in the lower Missouri River J Appl Ichthyol. 29:984–989.
- Steffensen KD, Eder BL, Pegg MA. 2014. Fish community response to floodplain inundation in a regulated river. J Freshw Eco. 29:413–427.
- Steffensen KD, Shuman DA, Klumb RA, Stukel S. 2014. The status of fishes in the Missouri River, Nebraska: pallid sturgeon (Scaphirhynchus albus). Trans Neb Acad Sci. 34:3–15.
- Steffensen KD, Shuman DA, Stukel S. 2014. The status of fishes in the Missouri River, Nebraska: shoal chub (Macrhybopsis hyostoma), sturgeon chub (M. gelida), sicklefin chub (M. meeki), silver chub (M. storeriana), flathead chub (Platygobio gracilis), plains minnow (Hybognathus placitus), western silvery minnow (H. argyritis) and brassy minnow (H. hankinsoni). Trans Neb Acad Sci. 34:49–67.
- Tibbs J.E. 1998. Flathead chub (Platygobio gracilis) rangewide status assessment. Columbia (MO): University of Missouri.
- Tripp SJ, Phelps QE, Colombo RE, Garvey JE, Burr BM, Herzog DP, Hrabik RA. 2009. Maturation and reproduction of shovelnose sturgeon in the middle Mississippi River. N Am J Fish Man. 29:730–738.
- US Army Corps of Engineers. 2001. Missouri River mainstem reservoir system master water control manual, Missouri River Basin. Omaha (NE): US Army Corps of Engineers.
- US Fish and Wildlife Service. 2001. Updated status review of Sicklefin and sturgeon chub in the United States. Denver (CO): US Fish and Wildlife Service.
- US Fish and Wildlife Service. 2014. Revised recovery plan for the pallid sturgeon Scaphirhynchus albus. Billings (MT): US Fish and Wildlife Service.
- Wanner GA, Shuman DA, Willis DW. 2007. Food habits of juvenile pallid sturgeon and adult shovelnose sturgeon in the Missouri River downstream of Fort Randall Dam, South Dakota. J Freshw Eco. 22:81–92.
- Welker TL, Drobish MR. 2012a. Pallid sturgeon population assessment project, Volume 1.6. Yankton (SD): US Army Corps of Engineers, Omaha District.
- Welker TL, Drobish MR. 2012b. Missouri River standard operating procedures for fish sampling and data collection, Volume 1.6. Yankton (SD): US Army Corps of Engineers, Omaha District.
- Winders KR, Dattilo JE, Huffmon TR, Travnicehek VH. 2014. Season diet composition of juvenile and adult pallid sturgeon Scaphirhynchus albus (Forbes and Richardson, 1905) in the channelized lower Missouri River. J Appl Ichthyol. 30:1133–1140.
- Winston WE, Criss RE. 2003. Oxygen isotope and geochemical variation in the Missouri River. Environ Geo. 43:546–556.
