ABSTRACT
Stable isotope analysis (SIA) is a powerful tool for assessing food webs and diet. However, the underlying assumptions that stable isotope ratios in an organism's tissues reflect that of its present diet may be complicated by isotopic turnover and retention time. Quantification of in situ isotopic turnover of study organisms and different tissues via field studies is essential to inform scientifically sound food web and diet studies utilizing SIA. Rainbow Trout white muscle and liver tissue were collected monthly from North Twin Lake, Washington during the 2013 growing season. Using ANCOVAs, we estimated stable carbon retention time of 154 (95% CI: 106–224) and 292 (95% CI: 257–763) days for Rainbow Trout liver and white muscle, respectively. For nitrogen, we estimated a retention time of 186 (95% CI: 114–329) days for both white muscle and liver. Our analyses suggest longer isotopic retention times (and therefore slower isotope turnover rates) for adult fish in a natural system relative to those commonly reported in the literature. These findings can improve interpretation of SIA in future aquatic food web and diet studies involving adult coldwater fish in freshwater systems.
Introduction
Stable isotope ratios of carbon (C) and nitrogen (N) have been used extensively to quantify diet by tracing pathways of organic matter among consumers (Schwarcz & Schoeninger Citation1991; McCutchan et al. Citation2003) and assessing trophic level within a given food web (Macko et al. Citation1999). As a result of biosynthesis and the incorporation of diet-derived N and C into consumer tissues, there is a strong parallel between isotopic composition of an organism and that of its diet (Macko et al. Citation1999). Specifically, the stable-C isotope ratio of tissue (13C/12C) reflects sources of organic C available to the consumer over time with little tissue fractionation (DeNiro & Epstein Citation1978), while stable-N isotope ratios (15N/14N) become enriched with successive trophic levels, allowing estimates of consumer trophic position (Post Citation2002).
Although the statement ‘you are what you eat’ generally describes patterns of C and N isotope ratios between predator and prey (DeNiro & Epstein Citation1976), there is a time-lag before dietary changes are reflected in tissue (Hobson & Clark Citation1993; Maruyama et al. Citation2001). Isotopic turnover is the time required for stable isotope ratios associated with past diet to be diluted or replaced with ratios from more recent diets. Specifically, isotopic turnover is defined as the isotopic change due to growth and metabolic tissue replacement associated with a change in diet (MacAvoy et al. Citation2001). Studies of juvenile fish have attributed over 90% of observed isotopic turnover in muscle tissue to growth, while metabolic replacement was the dominant process governing isotopic turnover in older fish with slower growth rates (Hesslein et al. Citation1993; Maruyama et al. Citation2001; Sakano et al. Citation2005). Organisms with high growth rates tend to have high isotope turnover rates and short isotopic retention times (Fry & Arnold Citation1982). Conversely, adult fish have relatively slow growth rates compared to other organisms, contributing to slow isotopic turnover and longer isotopic retention times in muscle tissue (Hesslein et al. Citation1993; MacAvoy et al. Citation2001).
Relative to muscle tissue, isotopic turnover occurs quickly in liver tissue (Hobson & Clark Citation1992; Philips & Eldridge Citation2006; Buchheister & Latour Citation2010) and is associated with metabolism rather than growth (Hobson & Clark Citation1992; MacNeil et al. Citation2006). In fish species with slow growth rates, the use of liver in SIA is becoming increasing popular to make frequent diet assessments over relatively short intervals (Philips & Eldridge Citation2006; Buchheister & Latour Citation2010). However, quantification of liver and muscle isotopic turnover rates and retention times, particularly those reflective of the age and environmental conditions pertinent to study organisms, is essential to inform sound food web and diet studies. Relatively few studies have compared muscle and liver turnover in coldwater fish and even fewer have examined in situ multiple-tissue turnover in adult Rainbow Trout (Oncorhynchus mykiss; RBT). The purpose of this study was to (Equation1(1) ) estimate isotopic turnover in RBT white muscle and liver tissue and (2) compare retention times between the two tissue types.
Methods
Study site
North Twin Lake is a mesotrophic lake located about 8.5 miles west of Inchelium, Washington on the Reservation of the Colville Confederated Tribes (CCT). The lake is dimictic with strong summer thermal stratification and winter ice cover. The CCT typically stocks North Twin Lake annually with marked triploid RBT (Cross et al. Citation2015). Very few stocked fish survive the winter and the 2013 year class had a unique mark (adipose and pectoral fin clips) relative to previous year-classes (Cross et al. Citation2015). Trout used in this study were reared on 4 mm EWOS® vita fish feed at the CCT Resident Fish Hatchery in Bridgeport, WA and released into the lake in April 2013 at an average weight of 426 g. After release, RBT typically feed on trichoptera and diptera larva, and Daphnia in the littoral zone of the lake until late June (Skinner et al. Citation2014). High epilimnetic temperatures, exceeding 21 °C, tend to confine RBT in deep hypolimnetic and pelagic waters from late June to early September (Moore et al. Citation2014). To maintain a relatively constant external temperature, RBT adjust their depth distributions throughout the sampling season (Moore et al. Citation2014). When confined to the pelagic zone of the lake, RBT feed heavily on Golden Shiner (Notemigonus crysoleucas), Daphnia and larvae of the Chironomidae and Chaoboridae families (Skinner et al. Citation2014).
Sample collection and processing
Prior to their release in late April 2013, white muscle and liver samples were collected from 10 hatchery-reared RBT. After RBT were planted in North Twin Lake, RBT from the April 2013 stocking cohort were collected monthly from May–October 2013 using gillnets (Skinner et al. Citation2014). Golden Shiner were collected with gillnets and via electro-fishing in May and August (Skinner et al. Citation2014). Tissue samples were dissected in the field, transported to the laboratory on ice, and frozen upon arrival. Additionally, we collected zooplankton and profundal invertebrate samples from two sites in each lake during monthly fish sampling events. Zooplankton were collected with vertical hauls with a 73 μm mesh net from a depth of 13 m to the surface. Profundal invertebrates were collected from sediments with a 550 cm3 surface area Eckman dredge. Bulk zooplankton and macroinvertebrate samples were kept separately in filtered lake water until sample processing. Taxa were identified to genus (for zooplankton) or family (for profundal macroinvertebrates) isolated manually under a dissecting microscope and frozen. For zooplankton and profundal macroinvertebrates, individuals collected on the same date and at the same site were pooled for analysis such that we had a composite sample for each taxon at each site during each sampling event. Frozen prey and fish liver samples were freeze-dried for 24 hours and ground by mortar and pestle in liquid N. Rainbow Trout liver samples were lipid-extracted (Skinner et al. Citation2016).
Finally, we also collected stomach samples from a subset of fish for gut content analysis (GCA) to inform SIA model inputs. Stomach sample collection and prey enumeration followed methods outlined in Skinner et al. (Citation2014). While Skinner et al. (Citation2014) assessed gut content in the same lakes in 2012, we felt it was necessary to reanalyze a subset of fish sampled in 2013 for SIA (Skinner et al. In review) to ensure proper model inputs and to reduce uncertainty related to potential inter-annual variation in fish diet.
Stable isotope analysis
Prior to SIA, 0.3–0.5 mg of sample was weighed into tin capsules. Analysis was performed at the Washington State University Stable Isotope Core Laboratory using an Elemental Combustion System 4010 elemental analyzer (Costech Analytical, Valencia, CA, USA) to convert nitrogen and carbon compounds to N2 and CO2. These gases were separated with a 3 m gas chromatography column and analyzed with a Delta Plus XP continuous flow isotope ratio mass spectrometer (Thermofinnigan, Bremen, Germany). Isotope values are calculated as
(1)
where X is the heavy isotope (13C or 15N) and R = 13C/12C or 15N/14N (Coplen Citation2011). The primary standard for carbon and nitrogen is Vienna Pee Dee belemnite limestone and N2 (air), respectively. All primary standards are assigned δ values of 0.0‰. Internal precision for the continuous flow interface coupled with the mass spectrometer, reported here as standard deviation, was 0.03‰ for 13C and 0.07‰ for 15N. In-lab standards (acetanilide, corn, and keratin) were used to develop a two-point normalization regression for the C/N analysis; the curve was then utilized to determine sample δ values.
Statistical analysis
Given that the rate of isotopic turnover in fish tissues is often growth-dependent (Hesslein et al. Citation1993; MacAvoy et al. Citation2001; Sakano et al. Citation2005), we felt it was necessary to analyze RBT growth (measured as weight; Hesslein et al. Citation1993; Bosley et al. Citation2002) through the sampling season. We used a least squares linear regression in the R statistical software package (R Core Team Citation2014) to assess trends in fish weight.
Commonly, isotopic turnover studies are laboratory-controlled due to inherent temporal variability in prey δ13C and δ15N and fish activity and metabolism in natural systems; this variability often needs to be controlled to accurately determine isotopic change from one isotopically distinct diet to another. Given the prey being consumed by RBT in North Twin Lake, ‘lake diet’ was expressed as the weighted mean δ13C and δ15N values of Daphnia, Chaoboridae, Chironimidae, and Golden Shiner with weighting based on prey item contribution to RBT diet, determined via gut content analysis (Skinner et al. In review). Before assuming the mean would accurately reflect the season-long ‘lake diet,’ prey δ value means () were assessed using two two-way ANOVAs (one for each element), with species and month as factors (R Core Team Citation2014). All data met assumptions of normal error distribution and equal variance using Shapiro tests and residual plots generated in R.
Table 1. Summary (+/− 1 SD) statistics for prey, RBT, and hatchery feed δ13C and δ15N values by sampling date.
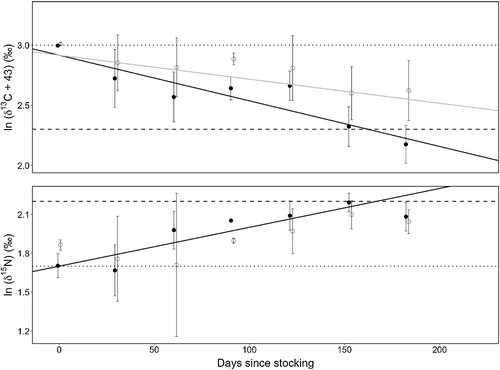
Corrected Akaike information criterion was employed to determine if linear or nonlinear models would assess turnover trends most accurately. Models with the lowest corrected Akaike information criterion value indicate the best model (Burnham & Anderson Citation2002). If an exponential model better explained the turnover trends, δ13C and δ15N would be ln-transformed with 43 added to all δ13C values (to account for negative numbers) to linearize the relationship (see for all non-transformed RBT δ values by tissue type and sampling date). To analyze retention time of 13C and 15N in RBT white muscle and liver tissue, one-way ANCOVAs were utilized with C or N isotope δ value as the dependent variable, tissue type as the independent variable, and days since stocking as the covariate (R Core Team Citation2014). Linear models generated via ANCOVAs were then used to determine how many days after release RBT tissues were fully reflective of ‘lake diet’ δ13C and δ15N (i.e. when the regression lines cross the ln-transformed weighted mean δ value of ‘lake diet’). All data met assumptions of normal error distribution and equal variance using Shapiro tests and residual plots generated in R.
Finally, to account for metabolic fractionation of stable isotopes during prey assimilation, isotopic discrimination values of 3.3‰ and 4.6‰ for 13C and 15N, respectively, were used. Discrimination was calculated as the difference between hatchery RBT muscle tissue δ value (mean −19.4 ± 0.3 and 9.9 ± 0.6 for C and N, respectively) and hatchery feed δ value (mean −22.9 ± 0.1 and 5.3 ± 0.2 for C and N, respectively). Discrimination values were within the ranges previously reported for fish (Pinnegar and Polunin Citation1999; Vander Zanden and Rasmussen Citation2001).
Results
Trends in fish weight and prey δ values
There was no significant trend in RBT weight over the sampling period (linear regression: p = 0.689). Additionally, there was no significant interaction between prey species and month for δ13C (two-way ANOVA: F14,55 = 1.115, p = 0.366) or δ15N values (two-way ANOVA: F14,55 = 1.606, p = 0.101), so only the main effects of species and sampling month will be discussed further. Between species, there was a significant difference for both δ13C (two-way ANOVA: F3,55 = 27.754, p < 0.002) and δ15N values (two-way ANOVA: F3,55 = 44.329, p < 0.002). Finally, there was no significant difference in δ13C (two-way ANOVA: F5,55 = 0.714, p = 0.366) and δ15N values (two-way ANOVA: F5,55 = 2.001, p = 0.065) when compared between sampling months. Based on these findings, the weighted mean δ values of prey captured throughout the sampling season were used to signify ‘lake diet’ and predict turnover rate. We used weighting values of 23, 51, 13, and 13 for Chaoboridae, Daphnia, Chironomidae, and Golden Shiner, respectively (Skinner et al. In review) (see for non-transformed prey δ values by sampling date).
Tissue turnover models
Corrected Akaike information criterion indicated that exponential models better explained trends in δ13C and δ15N relative to linear or polynomial models. There is a significant effect of tissue type on δ13C after controlling for time (one-way ANCOVA: F1,90 = 8.741, p = 0.004) (). The ANCOVA model (ln (δ13C + 43) = −0.004x + 2.917 for liver and ln (δ13C + 43) = −0.002x + 2.917 for white muscle) indicates liver and muscle took approximately 154 (95% CI: 106–224) and 292 (95% CI: 257–762) days, respectively, to reflect a weighted-mean lake diet of −32.7‰ (). Conversely, tissue type did not have a significant effect on δ15N after controlling for time (one-way ANCOVA: F1,90 = 2.200, p = 0.142) (). The N ANCOVA model (ln (δ15N) = 0.003x + 1.694 for both liver and white muscle) estimated a turnover to lake diet δ15N of 9.5‰ in 186 days (95% CI: 114–329 days) ().
Figure 1. Isotopic turnover trends for δ13C and δ15N in RBT liver (black line, filled circles) and white muscle (gray line, open circles). Given that tissue type did not have a significant effect on δ15N, after controlling for time (ANCOVA: F(1,90) = 2.200, p = 0.142), only one solid black regression line is shown for N. Dotted and dashed lines represent δ values of hatchery food and ‘lake diet’, respectively. Error bars represent ± 1 SD.
Discussion
There was a clear downward trend in RBT white muscle and liver δ13C values and upward trend in δ15N values during the sampling season, indicating that fish were assimilating ‘lake diet’ after stocking in April 2013. However, C retention time was shorter in liver relative to white muscle, in agreement with previous studies (Tieszen et al. Citation1983; Hobson and Clark Citation1992). Conversely, N turnover in liver and muscle was not significantly different. Other studies have found that δ15N turnover varied significantly between tissue types (MacNeil et al. Citation2006; Buchheister and Latour Citation2010), but these studies employed enriched food material in which δ15N values of food before and after diet switch differed by as much as 200‰. Indeed, Hesslein et al. (Citation1993) found that N turnover did not vary between liver and white muscle when fish were switched between diets differing by only 2.0‰. As such, it is possible that differences in isotopic retention times between tissues are only observed when organisms switch between food sources with very different δ values; in the present study, δ15N values of hatchery feed and lake diet only differed by 4.2‰.
Our estimated RBT white muscle and liver isotopic retention times generally agree with those found in past studies using a variety of fish species (Hesslein et al. Citation1993; MacAvoy et al. Citation2001), but are much longer overall than estimates from laboratory-controlled studies of juvenile or warmwater fish for both muscle (Vander Zanden et al. Citation1998; Bosley et al. Citation2002; Hoffman et al. Citation2011) and liver tissues (Bosley et al. Citation2002; Perga & Gerdeaux Citation2005 ). Faster turnover in laboratory studies is the result of using fish (often juveniles) fed high-quality diets in thermally controlled environments that promote high growth rates (Bosley et al. Citation2002; Sakano et al. Citation2005,), which is in obvious contrast to in situ conditions experienced by adult fish. Hence, our results indicate that laboratory turnover estimates may be much higher relative to those actually occurring in situ; it may be problematic to rely on literature rates when applying SIA in natural systems. The contribution of growth and metabolism in isotopic turnover must be considered (Hoffman et al. Citation2011). Growth will likely be much slower in in situ situations relative to the laboratory. Metabolism may primarily determine turnover rate in natural systems, especially in older fish (Sakano et al. Citation2005) and this appears to be the case for RBT in North Twin Lake. North Twin RBT growth was not statistically significant over the sampling period. Indeed, any substantial growth during the sampling period would likely have led to shorter isotopic retention times in these fish.
Our study indicated that liver may be an appropriate choice for quantifying diet over the short-term (i.e. around seven months) in systems with limited fish growth. White muscle is of interest in studies seeking a time-integrated diet assessment over a year or more. Liver has constant protein turnover (McMillian & Houlihan Citation1989) and turnover continues during basal metabolism phases that do not include growth (Sakano et al. Citation2005). Indeed, rate of isotopic change is driven by growth when growth efficiencies are high and is more closely related to metabolic rate when growth efficiencies are low (Sakano et al. Citation2005; Weidel et al. Citation2011 ), which is likely the case in North Twin Lake. In studies of juvenile fish or in systems with high rates of fish growth, turnover would likely proceed much more quickly than observed in our study lake. In contrast to previous studies (Perga and Gerdeaux Citation2005; MacNeil et al. Citation2006; Buchheister & Latour Citation2010), our findings do not indicate that liver can be used to quantify diet over a period of less than six months. This may be an issue when prey δ values exhibit a high level of temporal variability over the sample period or when attempting to capture temporally fleeting changes in diet. It is also important to note that no matter the time scale being considered, SIA delivers a time-integrated δ value, meaning that isotope ratios reflect fish diet assimilated over some time period that depends on the tissue used. SIA will not provide a ‘snap-shot’ of diet similar to other methods (e.g. gut content analysis). For short-term diet assessments, SIA may not be the appropriate method.
One major consideration our study did not address was how sexual maturation and spawning may affect isotopic turnover. Juvenile and sub-adult fish are likely funneling all consumed resources to somatic growth when possible, and turnover rate in these fish does not account for the influence of gonad production (Sakano et al. Citation2005). For example, in European Whitefish (Coregonus lavaretus) muscle, energy derived from prey is not necessarily destined for somatic growth year-round (i.e. during winter, energy is used for gonad growth) so white muscle turnover rates are likely altered (Perga & Gerdeaux Citation2005). Interestingly, liver turns over continuously throughout the year and thus may not be affected by sexual maturation and spawning (Perga & Gerdeaux Citation2005). Our study does not account for growth and maintenance of gonad tissues since we used triploid RBT; researchers should consider how turnover is affected by sexual maturation and spawning before applying rates from the literature that may not account for these life history events.
One additional aspect of our study that must be discussed is the large estimated range in 95% CI estimates for turnover in white muscle C. It is entirely possible that our study period was not long enough to precisely estimate the long isotopic retention time for white muscle tissue. High angler harvest yields low annual survival rates of RBT in North Twin Lake (Cross et al. Citation2015), limiting the ability to prolong the experiment. Regardless, we believe our study indicates that SIA remains a suitable method for quantifying coldwater fish diet over long timeframes. To precisely estimate isotopic turnover in fish tissues, future work in systems with slow fish growth would ideally measure turnover for a year or more, especially when considering tissues with slow isotopic turnover such as white muscle.
In conclusion, our study indicates that liver tissue isotopes turn over more quickly relative to white muscle tissue in RBT. However, the turnover in liver took several months, making weekly or monthly SIA diet assessments problematic in systems with slow fish growth. White muscle tissue appeared sufficient for SIA studies assessing diet over a year or more, but would not accurately reflect diet over a shorter time period. Additionally, our models reflected in situ turnover that was applicable to natural systems and should assist in the planning of future RBT SIA studies.
Acknowledgments
The Colville Confederated Tribes Fish and Wildlife Department partially funded this work and provided gillnets, an electroshocking boat, and all fish white muscle and liver samples. The authors thank the Washington State University Isotope Core Laboratory for sample analysis. The authors also thank Raymond Lee for his stable isotope analysis expertise. The study also received support from the Washington State Lake Protection Association Nancy Weller Student Scholarship and the Idaho Chapter of the American Fisheries Society Graduate Student Scholarship.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors

Megan M. Skinner

Benjamin K. Cross
References
- Bosley KL, Witting DA, Chambers RC, Wainright SC. 2002. Estimating turnover rates of carbon and nitrogen in recently metamorphosed winter flounder Pseudopleuronectes americanus with stable isotopes. Mar Ecol Prog Ser. 236:233–240.
- Buchheister A, Latour RJ. 2010. Turnover and fractionation of carbon and nitrogen stable isotopes in tissues of a migratory coastal predator, summer flounder (Paralichthys dentatus). Can J Fish Aquat Sci. 67:445–461.
- Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York (NY): Spring Science and Business Media.
- Coplen TB. 2011. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun Mass Spectrom. 25:2538–2560.
- Cross BK, Phillips J, Keleher BA. 2015. Colville Tribes resident fish hatchery operation and maintenance/ monitoring and evaluation 2011-2013 annual report. Annual Report to the Bonneville Power Administration, Project 1985-038-00, Portland (OR).
- DeNiro MJ, Epstein. S 1976. You are what you eat (plus a few ‰). Geol Soc Am Abs Prog. 8:834–835.
- DeNiro MJ, Epstein S. 1978. Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta. 42:495–506.
- Fry B, Arnold C. 1982. Rapid 13C/12C turnover during growth of Brown Shrimp (Penaeus aztecus). Oecologia. 54:200–204.
- Hesslein RH, Hallard KA, Ramlal P. 1993. Replacement of sulfur, carbon, and nitrogen in tissue of growing Broad Whitefish (Coregonis nasus) in response to a change in diet traced by δ34S, δ13C, and δ15N. Can J Fish Aquat Sci. 50:2071–2076.
- Hobson KA, Clark RG. 1992. Avian diets using stable isotopes I: turnover of 13C in tissues. Condor. 94:181–188.
- Hobson KA, Clark RG. 1993. Turnover of 13C in cellular and plasma fractions of blood: implications for nondestructive sampling in avian dietary studies. Auk. 110:638–641.
- Hoffman JC, Cotter AM, Peterson GS, Corry TA, Kelly JR. 2011. Rapid stable isotope turnover of larval fish in a Lake Superior coastal wetland: implications for diet and life history studies. Aquat Ecosyst Health Manage. 14:403–413.
- MacAvoy SE, Macko SA, Garman GC. 2001. Isotopic turnover in aquatic predators: quantifying the exploitation of migratory prey. Can J Fish Aquat Sci. 58:923–932.
- Macko SA, Engel MH, Andrusevich V, Lubec G, O'Connell TC, Hedges REM. 1999. Documenting the diet in ancient human populations through stable isotope analysis of hair. Phil Trans R Soc Lond. 354:65–76.
- MacNeil MA, Drouillard KG, Fisk AT. 2006. Variable uptake and elimination of stable nitrogen isotopes between tissues in fish. Can J Fish Aquat Sci. 63:345–353.
- Maruyama A, Yamada Y, Rusuwa B, Yuma M. 2001. Change in stable nitrogen isotope ratio in the muscle tissue of a migratory goby, Rhinogobius sp., in a natural setting. Can J Fish Aquat Sci. 58:2125–2128.
- McCutchan JH, Lewis WM, Kendal C, McGrath CC. 2003. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos. 102:378–390.
- McMillian DN, Houlihan DF. 1989. Short-term responses of protein synthesis to re-feeding in Rainbow Trout. Aquaculture. 79:37–46.
- Moore BC, Cross BK, Clegg EM, Lanouette BP, Skinner MM, Preece E, Child A, Gantzer P, Shallenberger E, Christensen D, Nine B. 2014. Hypolimnetic oxygenation in Twin Lakes, WA part I: distribution and movement of trout. Lake Reservoir Manage. 30:226–239.
- Perga ME, Gerdeaux D. 2005. Are fish what they eat all year round? Oecologia. 144:598–606.
- Philips DL, Eldridge PM. 2006. Estimating the timing of diet shifts using stable isotopes. Oecologia. 147:195–203.
- Pinnegar JK, Polunin NVC. 1999. Differential fractionation of δ13C and δ15N among fish tissues: implications for the study of trophic interactions. Functional Ecol. 13:225–231.
- Post DM. 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718.
- R Core Team. 2014. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.( R Studio v 0.98.1091). Available from: http://www.R-project.org/.
- Sakano H, Fujiwara E, Nohara S, Ueda H. 2005. Estimation of nitrogen stable isotope turnover rate of Oncorhynchus nerka. Environ Biol Fish. 72:13–18.
- Schwarcz HP, Schoeninger MJ. 1991. Stable isotope analyses in human nutritional ecology. Am J Phys Anthropol. 34:283–321.
- Skinner MM, Cross BK, Moore BC. In review. Using stable isotope analysis to assess effects of hypolimnetic oxygenation on diet in a mixed cold- and warmwater fish community. Environmental Biology of Fishes.
- Skinner MM, Martin AA, Moore BC. 2016. Is lipid correction necessary in the stable isotope analysis of fish tissues? Rapid Commun Mass Spectrom. 30:881–889.
- Skinner MM, Moore BC, Swanson ME. 2014. Hypolimnetic oxygenation in Twin Lakes, WA part II: feeding ecology of a mixed cold- and warmwater fish community. Lake Reservoir Manage. 30:240–249.
- Tieszen LL, Boutton TW, Tesdahl KG, Slade NA. 1983. Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia. 57:32–37.
- Vander Zanden MJ, Hulshof M, Ridgway MS, Rasmussen JB. 1998. Application of stable isotope techniques to trophic studies of age-0 Smallmouth Bass. Trans Am Fish Soc. 127:729–739.
- Vander Zanden MJ, Rasmussen JB. 2001. Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnol Oceanogr. 46:2061–2066.
- Weidel BC, Carpenter SR, Kitchell JF, Vander Zanden MJ. 2011. Rates and components of carbon turnover in fish muscle: insights from bioenergetics models and a whole-lake 13C addition. Can J Fish Aquat Sci. 68:387–399.

