ABSTRACT
Zooplankton communities in riverine systems are typically thought to be driven by abiotic forces. However, recent studies have shown that biological controls are capable of structuring these communities in large rivers and may become more influential as a river system becomes more lentic during low discharge. This study uses a long-term data set to examine several environmental variables as potential drivers of zooplankton community structure in a natural riverine lake. We hypothesized that water residence time would be the most important variable influencing zooplankton community structure. To test this, we used non-metric multidimensional scaling and correlation analysis to examine spatial and temporal patterns in zooplankton community structure. Analysis revealed that water residence time was the single most important environmental variable driving zooplankton abundance and community structure. The relationship between water residence time and taxa groups varied indicating that other taxa specific drivers had some influence on zooplankton community structure as well. Continued insight into the mechanisms driving zooplankton community structure will provide a basis for understanding zooplankton dynamics in large river ecosystems.
Introduction
Zooplankton dynamics in lakes are generally considered to be predictable and driven biologically (Sommer et al. Citation1986), whereas zooplankton dynamics in rivers are largely assumed to be driven by physical processes dictated by hydrological variables (Lair Citation2006). While abiotic factors have historically been considered drivers of lotic zooplankton (Hynes Citation1970), more recent research in large river systems has shown that biological controls are capable of controlling population growth and structuring crustacean zooplankton communities. These studies demonstrated that both top-down and bottom-up trophic interactions are capable of influencing zooplankton community dynamics in river systems through fish predation (Jack & Thorp Citation2002; Thorp & Casper Citation2003) and limited algal food resources (Guelda et al. Citation2005). While trophic cascades are likely to be less important in rivers than in lakes, trophic interactions are likely to fluctuate in a riverine lake, where conditions vary between lotic and lentic depending on discharge in the river it is associated with. As a system becomes more lentic the potential for increased trophic interactions and biological controls should increase. Baranyi et al. (Citation2002) found that as water residence time increased on the Danube River system, biological interactions became more important in structuring the zooplankton community rather than physical factors. Sluss and Jack (Citation2013) also suggested that zooplankton communities may have changed downriver in navigation pools on the Ohio River during lower discharge due to greater biotic interactions.
The dynamics of zooplankton community structure are likely going to be more complex in lotic systems as both physical and biological drivers may be at work. Known drivers of zooplankton dynamics analyzed in our study include water residence time (Pace et al. Citation1992; Basu & Pick Citation1996; Baranyi et al. Citation2002); water temperature (Gillooly & Dodson Citation2000); suspended material (Kirk & Gilbert Citation1990; Dejen et al. Citation2004) and phytoplankton abundance (Guelda et al. Citation2005), indicated by chlorophyll a concentrations. Other drivers capable of structuring the zooplankton community include fish predation (Jack & Thorp Citation2002) and interspecific competition (DeMott & Kerfoot Citation1982; Vanni Citation1986; Brandl Citation2005). Further complicating our understanding of zooplankton dynamics in river systems is the introduction of invasive species and their impact on trophic interactions. Since many invasive carps Hypophthalmichthys spp. are planktivorous, these fish may have immense impacts on plankton communities where they are present (Spataru & Gophen Citation1985; Xie & Yang Citation2000; Lu et al. Citation2002). In addition, climate change will undoubtedly affect discharge regimes in rivers (Miller & Russell Citation1992; Jha et al. Citation2004) altering hydrological conditions with unknown ecological consequences.
This study uses a long-term data (1995–2012) set to examine spatial and temporal dynamics of crustacean zooplankton and environmental variables that may regulate them in a natural riverine lake (Lake Pepin) on the Upper Mississippi River (UMR) (USA). A suite of simultaneously collected limnological variables and calculated water residence time were used to examine potential environmental factors driving zooplankton dynamics in Lake Pepin. We hypothesized that water residence time would be most influential among those variables considered, in controlling zooplankton communities and that taxon groups would be affected differentially by this variable. We suspected that cladocerans would be more affected by water residence time relative to the stronger swimming copepods (Richardson Citation1992). We also anticipated a community shift in zooplankton longitudinally down the lake coinciding with increasing water residence time and improved water quality (i.e. less inorganic material). The ability to assess the effect of environmental variables on the zooplankton community structure will provide a basis for understanding this important trophic level in large river ecosystems.
Methods
Study area
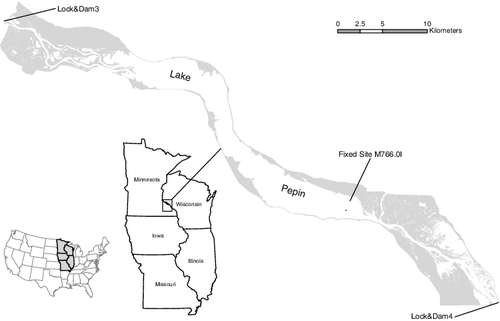
Lake Pepin is a 35 km long natural riverine lake located on the UMR that encompasses the entire floodplain (). The increase in channel depth and width in the lake creates a unique semi-lentic environment with water residence times directly related to water discharge in the river. Mean depth is 5.4 m with the downriver end of the lake deeper on average than the upper. The width of the lake ranges from approximately 1.3 to 4.0 km. The lake is located in the middle of Pool 4, one of 27 navigation pools on the UMR created by a low-head lock and dam system built for navigation. Lake Pepin was created by a tributary delta constricting the flow of the UMR and existed prior to the navigation structures. The lake is an efficient sink for sediments, retaining approximately 78% of the total suspended solids load, resulting in a twofold increase in water clarity longitudinally down the lake (Popp et al. Citation2014). Considered a eutrophic, polymictic lake, some thermal and oxygen stratification occurs during calm, lower discharge conditions.
Sample design
Zooplankton samples were collected in conjunction with routine water quality sampling in Lake Pepin by the Long Term Resource Monitoring element of the Upper Mississippi River Restoration Program. Samples were collected at sites that are selected quarterly as part of a stratified random sampling design (Soballe & Fischer Citation2004). Zooplankton is collected from only the ‘Lake’ stratum (Lake Pepin), and therefore the sampling design was treated as simple random sampling (SRS). Zooplankton included in this analysis were collected at 30 sites during summer (late-July to early-August) and fall (mid-October) sampling episodes during 1995–2012 with the exception of summer 2003 and fall 2001–2003. In addition, one fixed site in the lower part of the lake () that was sampled biweekly or monthly from May through October (1996–2012) was analyzed separately to better represent seasonal changes in the lake. This resulted in total, the analysis of over 1100 zooplankton samples spanning an 18-year period.
Environmental variables
A suite of limnological variables was collected simultaneously with all zooplankton samples (Soballe & Fischer Citation2004). Variables included in the analysis with zooplankton were water temperature, chlorophyll a (an indicator of phytoplankton abundance), total suspended solids, volatile suspended solids and percent organic matter. In addition, specific water residence times were assigned to each sample using a steady state segmented model developed with the software BATHTUB (BATHTUB, U.S. Army Corps of Engineers, Vicksburg, MS, USA). Water residence times were calculated for 3.2 km (2.0 mi) reaches of Lake Pepin using volume and discharge relationships. Discharge data from Lock and Dam 3 was obtained from the U.S. Army Corps of Engineers and converted to a 10-day mean discharge for use in the model. Twelve reaches were established and identified by the lower river mile in each reach, starting with 786 at the upper part of the lake to 764 at the lower end. Zooplankton samples were associated with the water residence time corresponding to the reach and hydraulic conditions in which they were collected.
Zooplankton sampling and enumeration
Zooplankton collections were made with an 80 μm mesh Wisconsin net, hauled vertically from 1 m above the lake bottom to the surface. The number of hauls per site was depth dependent with multiple tows collected at shallow sites and a single tow at deeper sites. Multiple shallow site hauls were composited into one sample to increase the volume of water sampled. All samples were preserved in reagent alcohol.
Sample volumes were adjusted, and a 5 ml aliquot was transferred to a counting wheel and individual zooplankters were identified, counted and measured under an Olympus SZ60 dissecting microscope at 25× magnification, with the aid of a computerized image analysis system and the ZCOUNT software program (Charpentier & Jamnick Citation1994). Biomass estimates were calculated with length/weight regression coefficients for individual taxa obtained from Culver et al. (Citation1985) and Dumont et al. (Citation1975). Adult copepods were identified to suborder and immature copepods were counted as nauplii or copepodites. A subset of 50 randomly chosen samples from the fixed site was further examined to identify adult copepods to species for a taxa list. Cladocerans were identified to either the genus or species level with the exception of Macrothricidae, which were identified to family.
Statistical analysis
Multivariate analyses were performed using various routines with PRIMER software version 6 (PRIMER-E Ltd, Plymouth, UK). Rare taxa that occurred in less than 5% of the samples were excluded from multivariate analysis, as were copepodites and nauplii. Zooplankton data were square root transformed to reduce the influence of dominant taxa and a Bray–Curtis similarity matrix was generated using biomass. Non-metric multidimensional scaling (NMDS) (Clarke Citation1993) analysis was used to examine spatial and temporal patterns in zooplankton communities, using summer and fall SRS data. Abundance was averaged over sites by year or reach for presentation in the NMDS graphs, otherwise multivariate statistical tests were performed using abundance from individual sites.
Two-way analysis of similarity (ANOSIM) was performed to determine if there were significant differences in zooplankton community structure between years and between river reaches. ANOSIM is a non-parametric permutation procedure that produces an R statistic, which is a comparative measure of the degree of separation between samples (Clarke & Warwick Citation2001). R will typically fall between 0 and 1, with values near zero indicating that assemblages are similar and support the null hypothesis of no differences between samples. R values closer to 1 indicate greater assemblage differences.
The similarity percentages routine (SIMPER) in PRIMER software was used to determine the contribution of individual species toward the separation among samples. The SIMPER routine uses average Bray–Curtis dissimilarities between all pairs of samples to produce a percent contribution from each species, which identifies the species most responsible for the dissimilarity among samples (Clarke & Warwick Citation2001).
The BIOENV procedure (Clarke & Gorley Citation2006) was used to identify which environmental variables best explained zooplankton community structure. BIOENV is a rank correlation (ρ) method between biotic and environmental (dis)similarity matrices (Clarke & Ainsworth Citation1993). Values of ρ can range from −1 to 1 with near zero values indicating the lack of correlation between the matrices. Prior to analysis, inter-correlated environmental variables were reduced to eliminate autocorrelation. Environmental data were log (x + 1) transformed when needed to approximate a normal distribution and standardized prior to generation of a Euclidean distance matrix.
Finally, Spearman's rank correlations were performed to examine the relationship of cladoceran and copepod biomass with water residence time and chlorophyll a concentrations with water residence time. Correlation analyses were performed using SAS software version 9.4 (SAS Institute Inc. Cary, NC, USA). Significance for all statistical tests was set at α = 0.05.
Results
Zooplankton composition and seasonality
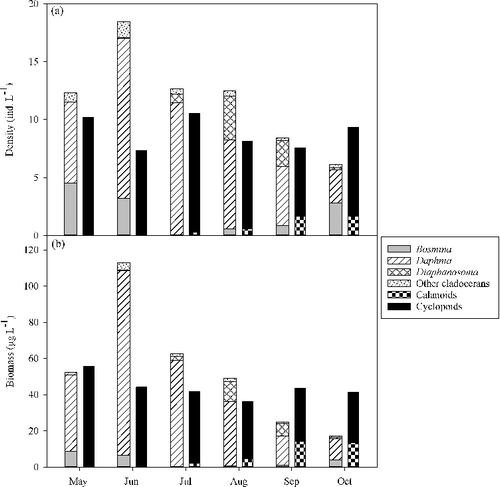
Twenty-one cladoceran taxa were identified from Lake Pepin, including six Daphnia species ( and ). Daphnia were a large component of cladoceran density and biomass during most sampling periods. Daphnia retrocurva was the most abundant caducean and was also one of the most ubiquitous cladocerans, occurring in over 95% of June through September fixed site samples, and in 86% and 78% of the summer and fall SRS episodes, respectively (). Bosmina longirostris was the second most abundant cladoceran and was most common in the spring and fall samples. Other abundant and ubiquitous cladocerans included Daphnia galeata mendotae, Diaphanosoma birgei and Chydorus sphaericus.
Table 1. Percent occurrence (percentage of samples where taxa occurred) of crustacean zooplankton collected in Lake Pepin. Fixed site data were collected at site M766.0I from 1996 through 2012. Stratified random sampling (SRS) data were collected during summer (late-July to early-August) and fall (mid-October) SRS episodes from 1995 through 2012.
Table 2. Mean density, biomass and maximum abundance of crustacean zooplankton collected in Lake Pepin. Fixed site data were collected at site M766.0I from 1996 through 2012. Stratified random sampling (SRS) data were collected during summer (late-July to early-August) and fall (mid-October) SRS episodes from 1995 through 2012. Numbers in parentheses show standard error of the mean.
Three species of calanoids, all belonging to the Diaptomidae family, were identified from the subset of samples examined for a copepod taxa list (). Skistodiaptomus oregonensis was the most common calanoid, whereas Skistodiaptomus pallidus and Leptodiaptomus siciloides were much less common. Seven species of cyclopoids were identified with Acanthocyclops vernalis and Mesocyclops edax being the most common. Diacyclops bicuspidatus thomasi was relatively uncommon and present only in samples collected during May. Other less common cyclopoids included Tropocyclops prasinus mexicanus, Macrocyclops albidus, Eucyclops prionophorus and Ectocyclops phaleratus.
Table 3. Percent occurrence (percentage of samples where taxa occurred) of copepod zooplankton collected at site M766.0I in Lake Pepin (50 randomly chosen samples were analyzed from 1996 through 2012).
Total cladoceran density and biomass at the fixed site peaked in June and declined as the season progressed (). In contrast, copepod density and biomass remained relatively consistent throughout the sampling season (). Immature copepods (copepodites and nauplii) contributed over 50% of the total copepod densities, while their relative contribution to biomass was small (). Cyclopoids, copepodites and nauplii were very common, present in over 90% of both fixed and random site samples. Calanoids were far less common, especially during early summer months (; ).
Spatial and temporal community structure
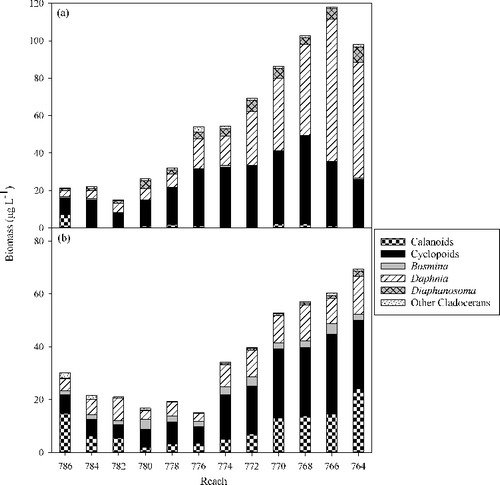
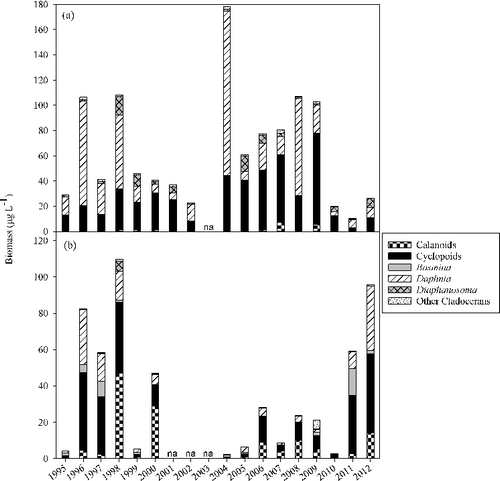
Crustacean zooplankton communities showed structural differences both annually and longitudinally through the lake ( and ). A two-way crossed ANOSIM revealed significant differences among years during summer (R = 0.56, P < 0.001) and fall (R = 0.73, P < 0.001), and also among reaches during summer (R = 0.37, P < 0.001) and fall (R = 0.31, P < 0.001). SIMPER analysis revealed that in almost all pair-wise comparisons, three of five taxa (D. retrocurva, D. galeata mendotae, cyclopoids, calanoids and D. birgei) in various combinations were the greatest contributors to dissimilarity of community structure. Given these taxa generally constitute the greatest biomass; it is understandable that they would tend to have the largest impact on community structure. There was an obvious longitudinal pattern down the lake as biomass increased during both fall and summer (). The contribution of Daphnia to the community structure increased longitudinally during summer whereas calanoids and B. longirostris made a larger contribution to community structure in fall (). Inter-annual patterns revealed that not only did community structure vary but that biomass varied substantially among years as well (). Community structure or abundance did not appear to be related between summer and fall episodes. Summer and fall zooplankton abundance were independent of each other as summer abundance had no bearing on fall abundance. Abundance patterns varied by year () where fall abundance would either be similar, lower or higher to those observed in summer.
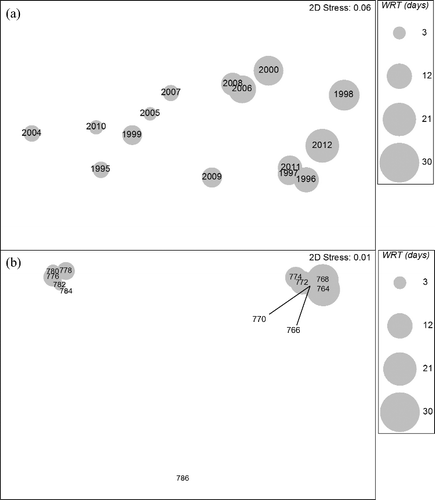
Figure 3. Non-metric multidimensional scaling (NMDS) ordination of Lake Pepin crustacean zooplankton sampled during summer stratified random sampling (SRS) episodes from 1995 through 2012. For graphical presentation, abundance was averaged over sites by (a) year and (b) 3.2 km reach (numbers are river miles) and overlaid with average water residence time.
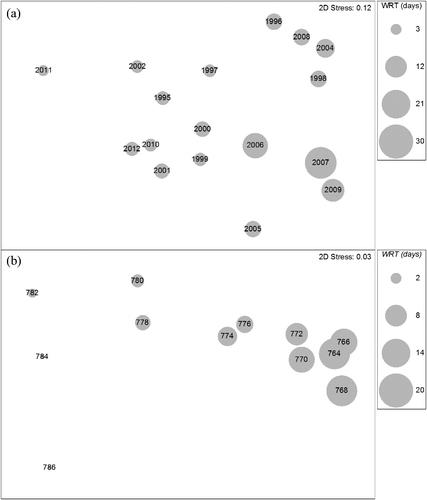
Figure 4. NMDS ordination of Lake Pepin crustacean zooplankton sampled during fall SRS episodes from 1995 through 2012. For graphical presentation, abundance was averaged over sites by (a) year and (b) reach and overlaid with average water residence time.
Environmental variables and community structure
Water residence time was the single best environmental variable that explained the patterns observed in the crustacean zooplankton community as indicated by the BIOENV procedure (). This was the case for both summer (ρ = 0.317) and fall (ρ = 0.299). No other combination of environmental variables improved the correlation between zooplankton and environmental patterns. When bubble plots of water residence time were superimposed on the NMDS graphs, easily perceived patterns emerged as similar community assemblages tended to group together based on water residence times ( and ). During this study period, mean water residence time for the entire lake ranged from 3.0 to 25.7 days during summer SRS and from 3.3 to 21.7 days during fall SRS. Maximum water residence time during the study period ranged from less than 1 day in the upper reach to over 46 days in the lower reach.
Table 4. Rank correlation coefficients (ρ) between zooplankton among-sample patterns of assemblage and associated environmental variables. Data collected during summer and fall SRS episodes (1995--2012).
The link between zooplankton abundance and water residence time was further investigated by using Spearman's rank-order correlation statistic to examine the relationship between total cladoceran and copepod biomass and water residence time. Analysis revealed significant positive correlations during both summer and fall (). Copepods had a strong correlation with residence time during both summer (r = 0.88) and fall (r = 0.87). Cladocerans also displayed significant correlations with residence time (summer r = 0.59, fall r = 0.75). Cladocerans in fact had relatively low biomass during the three summers of longest residence time ((a)) in contrast to copepods where biomass may have peaked ((c)).
Figure 7. Spearman's rank correlation between water residence time and cladocerans during (a) summer and (b) fall SRS episodes, copepods during (c) summer and (d) fall SRS episodes and chlorophyll a during (e) summer and (f) fall episodes.
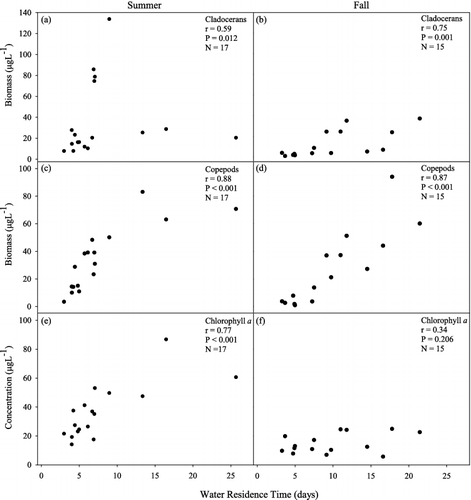
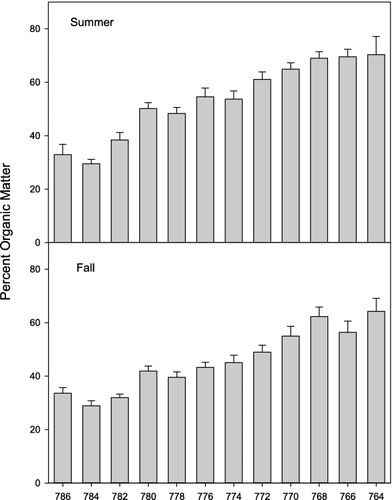
Chlorophyll a had a strong correlation with residence time during the summer episodes ((e)); however, chlorophyll a when used in NMDS analysis did not help explain patterns in zooplankton community structure in either summer or fall. Likewise, total suspended solids and percent organic matter showed distinct longitudinal patterns down the lake () due in large part to the settling of inorganic material, but also did not improve the explanation of community patterns.
Discussion
Zooplankton community patterns
Our results demonstrated that water residence time was an important environmental driver of crustacean zooplankton community structure, thus confirming our initial hypothesis. Longer water residence times were associated with a community shift toward larger-bodied crustacean zooplankton and higher overall zooplankton biomass driven primarily by Daphnia and copepods. Water residence time was not only associated with year-to-year differences but spatial differences as well. As we had hypothesized, zooplankton biomass increased from upriver to downriver in Lake Pepin and community structure shifted to one more typical of lakes. While others have shown that residence time was the primary driver controlling zooplankton biomass in river systems (Pace et al. Citation1992; Basu & Pick Citation1996; Baranyi et al. Citation2002), our results show dissimilar relationships with residence time between crustacean taxon groups. Copepods exhibited a very strong positive correlation, whereas cladocerans demonstrated a weaker correlation where biomass did not always increase with residence time. During the three summers with the longest residence times, cladoceran biomass was actually lower compared to biomass at intermediate levels of residence time. This was in contrast to copepod biomass that appeared to continue to increase with residence time. Examination of the fixed site data (sampled at two-week intervals) showed that Daphnia, the primary contributor to cladoceran biomass, was in fact abundant during these low discharge summers, but prior to the sampling in late-July, their abundance decreased. This suggests that cladocerans, particularly Daphnia, may have become driven by biological factors during late summer.
In north temperate lakes, planktonic herbivores such as Daphnia tend to be temporal, being most abundant following spring algal blooms and then decreasing over the course of the season due to food limitations and fish predation (Sommer et al. Citation1986; Vanni Citation1987). We observed similar patterns in Lake Pepin as Daphnia densities and biomass were greatest during June and decreased throughout the season. This mid-summer decline was likely the result of some combination of a decrease in quality food resources and predation by fish. Summer declines in Daphnia abundance in Lake Mendota (Luecke et al. Citation1990) and Lake Erie (Wu & Culver Citation1994) were attributed to food limitation in early summer; however, despite the return of quality food resources in late summer, Daphnia abundance remained low due to fish predation. The consumption of crustacean zooplankton by fishes in riverine systems has been well documented (Rosen & Hales Citation1981; Hoxmeier & DeVries Citation1997; Dettmers et al. Citation2001; Jack & Thorp Citation2002; Thorp & Casper Citation2003). Of the 85 species of fish collected in Pool 4 of the Mississippi River, 22 (26%) have been reported to consume zooplankton as one of their primary diet items as adults (Simon Citation1999). Van Dijk and Van Zanten (Citation1995) reported a similar food web in the Lower River Rhine where 30% of the fish community was zooplanktivorous. Additional studies in river systems have found that gizzard shad Dorosoma cepedianum (Dettmers & Stein Citation1996) and emerald shiners Notropis atherinoides (Jack & Thorp Citation2002) can reduce zooplankton abundance through predation. Gizzard shad and emerald shiners are the most abundant fishes in Lake Pepin (Meerbeek Citation2006) with larvae beginning exogenous feeding by July. Given the fish community in Lake Pepin, the potential for substantial predation on zooplankton and control of their abundance seems great and may explain in part the lower cladoceran biomass during late summer. This top-down trophic interaction may be more prevalent during periods of long water residence times.
Phytoplankton is generally considered the most important food source for herbaceous zooplankton and food availability can influence zooplankton growth (Sterner & Schulz Citation1998). Guelda et al. (Citation2005) estimated the carbon phytoplankton biomass necessary to maintain positive population growth rates of B. longirostris in a river mesocosm experiment translated to approximately 2 μg l−1 of chlorophyll a. They found that the food saturation threshold, where food resources were no longer limiting, occurred at 9 μg l−1. Similar chlorophyll a concentrations (6–15 μg l−1) required for Daphnia maximum growth were put forward by Sterner and Schulz (Citation1998). These estimates suggest that food quantity during the summer was likely not limiting population growth for cladocerans in Lake Pepin. However, fall chlorophyll a concentrations were often below these estimated thresholds required for maximum growth and therefore food resources had the potential to be a factor in zooplankton population dynamics. An abundance of phytoplankton does not guarantee saturation of quality food or increased zooplankton growth (Sterner et al. Citation1993). The nutritional content of phytoplankton has been shown to influence Daphnia growth (Sterner & Schulz Citation1998; Elser et al. Citation2001), as has phytoplankton size that can interfere and inhibit consumption (Gliwicz Citation1990). In addition, toxicity from cyanobacteria has also been reported to inhibit growth (Porter & Orcutt Citation1980). The most common copepods in Lake Pepin do not have to rely on phytoplankton as a primary food source as they can be omnivorous or carnivorous as adults. Guelda et al. (Citation2005) found that phytoplankton concentration did not correlate with cyclopoid growth and surmised that a shift to a carnivorous diet as adults may be responsible. Diet and food resources may play a role in seasonal abundance patterns within and among taxa groups in Lake Pepin. Spatially we would anticipate cladoceran growth to improve as inorganic suspended solids settle out and the percentage of organic matter increases longitudinally down the lake. Dejen et al. (Citation2004) suspected that turbidity may have played a role in the distribution of zooplankton taxa in a large tropical lake. Negative impacts of turbidity and suspended solids have been recognized on cladocerans (McCabe & O'Brien Citation1983; Kirk & Gilbert Citation1990); however, the effects on copepods are less understood. Hart (Citation1990) points out that the effects of sediment inflow on the spatial structure of zooplankton communities in reservoirs are essentially unstudied. While both cladoceran and copepod abundance increased down the lake along with decreasing turbidity and suspended solids, our analysis did not identify suspended solids as an important driver to community structure.
Another environmental variable potentially affecting community dynamics is water temperature. Gillooly and Dodson (Citation2000) suggest that the pattern of peak Daphnia abundance in water bodies is driven by water temperature. Their study looked at lakes, reservoirs and ponds across various latitudes in North America and found a strong relationship between latitude and date of maximum Daphnia abundance. Peak abundances typically occurred between 15 and 20 °C, and their reported mean temperature of 18.5 °C at maximum abundance corresponds well to Daphnia peaks observed in Lake Pepin. They also found that Daphnia abundance declined at temperatures >20 °C, a threshold that is consistently exceeded during the summer months in Lake Pepin. We suspect that water temperature does control the start of the Daphnia peak in Lake Pepin and may contribute to the late summer decline; however, other factors such as food availability, predation and water residence time likely control their ultimate abundance. Seasonal patterns were also apparent in B. longirostris with greater abundance in the spring and fall and near absence in July. This may be due to competition by the larger more efficient grazer Daphnia (Hall et al. Citation1976; Vanni Citation1986) during mid-summer. However, water temperature may also be important in controlling seasonal patterns of B. longirostris as well since they have been shown to be more successful at lower temperatures (Bhajan & Hynes Citation1972) and may explain their greater abundance in the spring and fall. The other major cladoceran contributing to biomass was D. birgei, which became most abundant in mid-summer to late summer suggesting higher optimal temperatures. Davidson et al. (Citation1998) reported D. birgei as a late summer species in the Atchafalaya River with abundance being positively related to water temperature.
Calanoid copepods also displayed a seasonal pattern where densities and biomass of adult calanoids were very low in spring and early summer, but increased in late summer and into fall. The most common calanoid species in Lake Pepin S. oregonensis was also reported to be most abundant during late summer and fall in the Great Lakes (Balcer et al. Citation1984). S. oregonensis may produce as few as one or two generations per year (Balcer et al. Citation1984; Torke Citation2001), which may in part explain the seasonal pattern observed. In addition, all of the calanoid species identified from Lake Pepin can utilize large algal cells (e.g. blue-green cyanobacteria) in their diet (Comita Citation1972; Torke Citation2001), which may give them an advantage later in the season when blue-greens are more abundant. In contrast to the calanoids, cyclopoid copepods as a group did not exhibit much temporal variation in Lake Pepin where densities and biomass remained relatively stable throughout the season. Cyclopoids also tend to have longer life cycles and fewer generations, and are less likely to attain the high rates of population growth of cladocerans, which utilize parthenogenetic reproduction and have short multivoltine life cycles (Allan Citation1976). In addition, copepods have been shown to be more able to avoid fish predation than cladocerans (Drenner & McComas Citation1980) and may not be as important in fish diets as cladocerans (Smith Citation2001). However, Jack and Thorp (Citation2002) found larval bluntnose minnows Pimephales notatus and emerald shiners readily fed on the cyclopoid Diacycops thomasi and reduced its abundance in an enclosure study on the Ohio River. While we are uncertain about which mechanisms may be contributing to seasonal patterns in the zooplankton community, it is clear that copepod abundance in Lake Pepin is more stable than that of cladocerans.
Zooplankton composition
We found a diverse crustacean zooplankton community in Lake Pepin with 31 taxa identified over the 18-year study period. Similar diversity was reported on the Missouri River from a study that spanned nearly the entire river (Dickerson et al. Citation2010). Taxa richness in Lake Pepin was approximately six times higher on average than regional lakes in Minnesota (Hirsch Citation2014). The higher diversity of zooplankton in Lake Pepin could be due to a variety of factors, including the longer sampling time frame, extensive spatial sampling coverage, including shallow sites where littoral species are more likely to be collected and the fact that Lake Pepin receives an influx of zooplankton from various upriver areas. However, much of this zooplankton diversity may simply be due to the physical complexity inherent in floodplain river systems where species diversity is expected to be high (Junk et al. Citation1989).
Six species of Daphnia were identified from Lake Pepin, one of which was the previously reported invasive D. lumholtzi (Burdis & Hirsch Citation2004). Interestingly, these six species were also the only Daphnia reported in the Missouri River (Dickerson et al. Citation2010), D. retrocurva being the most common in both rivers. Daphnia have been reported from the other large North American rivers (e.g. Illinois and Ohio) but were less abundant (Thorp et al. Citation1994; Wahl et al. Citation2008; Sluss & Jack Citation2013). The difference in Daphnia abundance among these rivers is likely due to the availability of lentic habitat. Our study was done on a large natural riverine lake in the Mississippi River; likewise, the middle section of the Missouri River includes very large impoundments that produce genera typical of lakes and lentic conditions not generally found on the Ohio and Illinois rivers. Burdis and Hoxmeier (Citation2011) investigated the zooplankton communities in the UMR above and below Lake Pepin and found the cladoceran community above the lake to be dominated by the smaller-bodied B. longirostris compared to one dominated by Daphnia below the lake. When water residence time was sufficient, the lentic habitat of Lake Pepin produced a longitudinal transition to larger-bodied crustaceans altering the zooplankton community structure through the lake. Similarly, the uppermost zones of reservoirs, where advective effects of river inflows are most prominent, are typically composed of riverine zooplankton communities, whereas communities in lacustrine zones of reservoirs resemble those of natural lakes (Hart Citation1990; Bernot et al. Citation2004).
Copepods were most abundant and the dominant taxa group in the fall. The two most common cyclopoids identified from Lake Pepin were M. edax and A. vernalis, both of which are omnivorous and oftentimes predacious, feeding on other zooplankters (Brandl & Fernando Citation1974; Kerfoot Citation1978; Williamson Citation1980). M. edax is common throughout North America and is a large copepod that will feed on rotifers, other copepods and cladocerans including Daphnia and Bosmina (Balcer et al. Citation1984; Williamson Citation1984). The most common calanoid species identified from Lake Pepin was S. oregonensis. This species is ubiquitous in the region and can utilize a wide size range of algal cells in its diet, resulting in its presence in a variety of lake types (Torke Citation2001; Van Egeren et al. Citation2011). The two less common calanoids found in Lake Pepin, S. pallidus and L. siciloides, are associated with eutrophic conditions and L. siciloides has the ability to utilize large cyanobacteria cells in its diet (Comita Citation1972). The copepod species in Lake Pepin appear to have a diverse diet that may be advantageous, particularly in the fall when phytoplankton abundance decreases.
Conclusions
Our hypothesis that water residence time would be the most influential variable structuring the zooplankton community was confirmed. Spatial and temporal patterns in the zooplankton community were best explained by water residence time while other environmental variables such as water temperature, chlorophyll a and suspended solids did not help to explain the observed patterns. Copepod abundance correlated well with residence time while cladoceran abundance did not always increase with longer water residence time suggesting that biotic controls such as fish predation or food availability may have at times influenced cladoceran dynamics. Seasonal patterns in zooplankton typical of lakes were observed in Lake Pepin; however, these patterns could be disrupted during short water residence times. As Lake Pepin became more fluvial in character, total zooplankton biomass decreased and the zooplankton community became more typical of those found in rivers where large cladocerans such as Daphnia are scarce. Biotic interactions influencing zooplankton abundance and community structure likely became more extensive during longer water residence times and appeared most conspicuous with cladocerans. Our results illustrate the diversity and complexity of zooplankton dynamics in a large riverine lake and accentuate the importance of water residence in structuring the zooplankton community. We also demonstrated that the relationship between zooplankton abundance and water residence time varied among taxa groups.
Acknowledgments
We would like to thank co-workers and the many interns, too numerous to mention, that have helped collect samples over the 18-year period. Thanks to Jim Rogala for lake morphometry data and Dave Soballe for generating a water residence time model for Lake Pepin. Thanks to Megan Moore, John Hoxmeier and Doug Dieterman for their helpful discussions regarding data analysis and interpretation. Thanks also to John Hoxmeier, Walt Popp, David Wright and two anonymous reviewers for their review of the manuscript. Funding for this project was provided by the U.S. Army Corps of Engineers’ Upper Mississippi River Restoration (UMRR) program, Long Term Resource Monitoring (LTRM) element in collaboration with the Minnesota Department of Natural Resources.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors

Robert M. Burdis
Robert M. Burdis is an Aquatic Biologist with the Minnesota Department of Natural Resources where he conducts limnological monitoring and research on the Upper Mississippi River.

Jodene K. Hirsch
Jodene K. Hirsch is a zooplankton specialist for the Minnesota Department of Natural Resources. Her work focuses on long-term zooplankton monitoring in Minnesota lakes.
References
- Allan JD. 1976. Life history patterns in zooplankton. Am Nat. 110:165–180.
- Balcer MD, Korda NL, Dodson SI. 1984. Zooplankton of the great Lakes: a guide to the identification and ecology of the common crustacean species. Madison (WI): The University of Wisconsin Press.
- Baranyi C, Hein T, Holarek C, Keckeis S, Schiemer F. 2002. Zooplankton biomass and community structure in a Danube River floodplain system: effects of hydrology. Freshwater Biol. 47:473–482.
- Basu BK, Pick FR. 1996. Factors regulating phytoplankton and zooplankton biomass in temperate rivers. Limnol Oceanogr. 41:1572–1577.
- Bernot RJ, Dodds WK, Quist MC, Guy CS. 2004. Spatial and temporal variability of zooplankton in a great plains reservoir. Hydrobiologia. 525:101–112.
- Bhajan WR, Hynes HBN. 1972. Experimental study on the ecology of Bosmina longirostris (OF Müller) (Cladocera). Crustaceana. 23:133–140.
- Brandl Z. 2005. Freshwater copepods and rotifers: predators and their prey. Hydrobiologia. 546:475–489.
- Brandl Z, Fernando CH. 1974. Feeding of the copepod Acanthocyclops vernalis on the cladoceran Ceriodaphnia reticulate under laboratory conditions. Can J Zool. 52:99–105.
- Burdis RM, Hirsch JK. 2004. Establishment of a viable population of Daphnia lumholtzi in Lake Pepin, Upper Mississippi River. J Freshwater Ecol. 20:205–207.
- Burdis RM, Hoxmeier RJH. 2011. Seasonal zooplankton dynamics in main channel and backwater habitats of the Upper Mississippi River. Hydrobiologia. 667:69–87.
- Charpentier F, Jamnick BH. 1994. ZCOUNT – a zoological counting program. Version 2.4. Gloucester: Voila Data Inc.
- Clarke KR. 1993. Non-parametric multivariate analysis of changes in community structure. Aust J Ecol. 18:117–143.
- Clarke KR, Ainsworth M. 1993. A method of linking multivariate community structure to environmental variables. Mar Ecol Prog Ser. 92:205–219.
- Clarke KR, Gorley RN. 2006. PRIMER v6: user manual/tutorial. Plymouth: PRIMER-E.
- Clarke KR, Warwick RM. 2001. Change in marine communities: an approach to statistical analysis and interpretation. 2nd ed. Plymouth: PRIMER-E.
- Comita GW. 1972. The seasonal zooplankton cycles, production and transformations of energy in Severson Lake, Minnesota. Arch Hydrobiol. 70:14–66.
- Culver DA, Boucherle MM, Bean DJ, Fletcher JW. 1985. Biomass of freshwater crustacean zooplankton form length-weight regressions. Can J Fish Aquat Sci. 42:1380–1390.
- Davidson Jr NL, Kelso WE, Rutherford DA. 1998. Relationships between environmental variables and the abundance of cladocerans and copepods in the Atchafalaya River Basin. Hydrobiologia. 379:175–181.
- Dejen E, Vijverberg J, Nagelkerke LAJ, Sibbing FA. 2004. Temporal and spatial distribution of microcrustacean zooplankton in relation to turbidity and other environmental factors in a large tropical lake (L. Tana, Ethiopia). Hydrobiologia. 513:39–49.
- DeMott WR, Kerfoot WC. 1982. Competition among cladocerans: nature of the interaction between Bosmina and Daphnia. Ecology. 63:1949–1966.
- Dettmers JM, Stein RA. 1996. Quantifying linkages among gizzard shad, zooplankton, and phytoplankton in reservoirs. Trans Am Fish Soc. 125:27–41.
- Dettmers JM, Wahl DH, Soluk DA, Gutreuter S. 2001. Life in the fast lane: fish and foodweb structure in the main channel of large rivers. J North Am Benthol Soc. 20:255–265.
- Dickerson KD, Medley KA, Havel JE. 2010. Spatial variation in zooplankton community structure is related to hydrologic flow units in the Missouri River, USA. River Res Appl. 26:605–618.
- Drenner RW, McComas SR. 1980. The roles of zooplankter escape ability and fish size selectivity in the selective feeding and impact of planktivorous fish. Evolution and ecology of zooplankton communities. Hanover (MA): University Press of New England; p. 587–593.
- Dumont HJ, Van de Velde I, Dumont S. 1975. The dry weight estimate of biomass in a selection of Cladocera, Copepoda, and Rotifera from the plankton, periphyton and benthos of continental waters. Oecologia. 19:75–97.
- Elser JJ, Hayakawa K, Urabe J. 2001. Nutrient limitation reduces food quality for zooplankton: Daphnia response to seston phosphorus enrichment. Ecology. 82:898–903.
- Gillooly JF, Dodson SI. 2000. Latitudinal patterns in the size distribution and seasonal dynamics of new world, freshwater cladocerans. Limnol Oceanogr. 45:22–30.
- Gliwicz ZM. 1990. Why do cladocerans fail to control algal blooms? Hydrobiologia. 200/201:98–97.
- Guelda DL, Koch RW, Jack JD, Bukaveckas PA. 2005. Experimental evidence for density‐dependent effects and the importance of algal production in determining population growth rates of riverine zooplankton. River Res Appl. 21:595–608.
- Hall DJ, Threlkeld ST, Burns CW, Crowley PH. 1976. The size-efficiency hypothesis and the size structure of zooplankton communities. Annu Rev Ecol Syst. 7:177–208.
- Hart RC. 1990. Zooplankton distribution in relation to turbidity and related environmental gradients in a large subtropical reservoir: patterns and implications. Freshwater Biol. 24:241–263.
- Hoxmeier RJH, DeVries DR. 1997. Habitat use, diet, and population structure of adult and juvenile paddlefish in the lower Alabama River. Trans Am Fish Soc. 126:288–301.
- Hirsch J. 2014. National Lakes assessment 2012: zooplankton communities in Minnesota Lakes (US). St. Paul (MN): Minnesota Department of Natural Resources and Minnesota Pollution Control Agency. Available from: https://www.pca.state.mn.us/sites/default/files/wq-nlap1-11.pdf
- Hynes HBN. 1970. The ecology of running waters. Toronto: University of Toronto Press.
- Jack JD, Thorp JH. 2002. Impacts of fish predation on an Ohio River zooplankton community. J Plankton Res. 24:119–127.
- Jha M, Pan Z, Takle ES, Gu R. 2004. Impacts of climate change on streamflow in the Upper Mississippi River Basin: a regional climate model perspective. J Geophys Res. 109:D09105. doi: 10.1029/2003JD003686.
- Junk WJ, Bayley PB, Sparks RE. 1989. The flood pulse concept in river-floodplain systems. Can Spec Publ Fish Aquat Sci. 106:110–127.
- Kerfoot CW. 1978. Combat between predatory copepods and their prey: Cyclops, Epischura, and Bosmina. Limnol Oceanogr. 23:1089–1102.
- Kirk KL, Gilbert JJ. 1990. Suspended clay and the population dynamics of planktonic rotifers and cladocerans. Ecology. 71:1741–1755.
- Lair N. 2006. A review of regulated mechanisms of metazoan plankton in riverine ecosystems: aquatic habitat versus biota. River Res Appl. 22:567–593.
- Lu M, Xie P, Tang H, Shao Z, Xie L. 2002. Experimental study of trophic cascade effect of silver carp (Hypophthalmichthys molitrixon) in a subtropical lake, Lake Donghu: on plankton community and underlying mechanisms of changes of crustacean community. Hydrobiologia. 487:19–31.
- Luecke C, Vanni MJ, Magnuson JJ, Kitchell JF, Jacobson PT. 1990. Seasonal regulation of Daphnia populations by planktivorous fish: implications for the spring clear-water phase. Limnol Oceanogr. 35:1718–1733.
- McCabe GD, O'Brien WJ. 1983. The effects of suspended silt on feeding and reproduction of Daphnia pulex. Am Midl Nat. 110:324–337.
- Meerbeek J. 2006. Large Lake monitoring program annual completion report: Lake Pepin, 2006. Lake City (MN): Minnesota Department of Natural Resources.
- Miller JR, Russell GL. 1992. The impact of global warming on river runoff. J Geophys Res. 97:2757–2764.
- Pace ML, Findlay SEG, Lints D. 1992. Zooplankton in advective environments: the Hudson River community and a comparative analysis. Can J Fish Aquat Sci. 49:1060–1069.
- Popp WA, Burdis RM, DeLain SA, Moore MJ. 2014. Temporal trends in water quality and biota in segments of Pool 4 above and below Lake Pepin, Upper Mississippi River: indications of a recent ecological shift. La Crosse (WI): Upper Mississippi River Restoration Long Term Resource Monitoring Program. (Completion Report; no. 2010D6).
- Porter KG, Orcutt JD. 1980. Nutritional adequacy, manageability, and toxicity as factors that determine the food quality of green and blue-green algae for Daphnia. Evolution and ecology of zooplankton communities. Hanover (MA): University Press of New England; p. 268–281.
- Richardson WB. 1992. Microcrustacea in flowing water: experimental analysis of washout times and a field test. Freshwater Biol. 28:217–230.
- Rosen RA, Hales DC. 1981. Feeding of paddlefish, Polyodon spathula. Copeia. 2:441–455.
- Simon TP. 1999. Assessing the sustainability and biological integrity of water resources using fish communities. Boca Raton (FL): CRC Press.
- Sluss TD, Jack JD. 2013. Ohio River zooplankton growth rates and community assemblages and their relationship to abiotic and biotic factors in navigation dam pools. River Syst. 21:55–70.
- Smith DG. 2001. Pennak's freshwater invertebrates of the United States: Rorifera to Crustacea. New York (NY): Wiley.
- Soballe DM, Fischer JR. 2004. Long Term Resource Monitoring Program procedures: water quality monitoring. La Crosse (WI): U.S. Geological Survey, Upper Midwest Environmental Sciences Center. (Technical Report; no. LTRMP 2004-T002-1). Available from: http://www.umesc.usgs.gov/documents/reports/2004/04t00201.pdf
- Sommer U, Gliwicz ZM, Lampert W, Duncan A. 1986. The PEG-model of seasonal succession of planktonic events in fresh waters. Arch Hydrobiol. 106:433–471.
- Spataru P, Gophen M. 1985. Feeding behaviour of silver carp Hypophthalmichthys molitrix Val. and its impact on the food web in Lake Kinneret, Israel. Hydrobiologia. 120:53–61.
- Sterner RW, Hagemeier DD, Smith WL, Smith RF. 1993. Phytoplankton nutrient limitation and food quality for Daphnia. Limnol Oceanogr. 38:857–871.
- Sterner RW, Schulz KL. 1998. Zooplankton nutrition: recent progress and a reality check. Aquat Ecol. 32:261–279.
- Torke B. 2001. The distribution of calanoid copepods in the plankton of Wisconsin Lakes. Hydrobiologia. 453/454:351–365.
- Thorp JH, Black AR, Haag KH, Wehr JD. 1994. Zooplankton assemblages in the Ohio River: seasonal, tributary, and navigation dam effects. Can J Fish Aquat Sci. 51:1634–1643.
- Thorp JH, Casper AF. 2003. Importance of biotic interactions in large rivers: an experiment with planktivorous fish, dreissenid mussels and zooplankton in the St. Lawrence River. River Res Appl. 19:265–279.
- Van Dijk GM, Van Zanten B. 1995. Seasonal changes in zooplankton abundance in the lower Rhine during 1987–1991. Hydrobiologia. 304:29–38.
- Van Egeren SJ, Dodson SI, Torke B, Maxted JT. 2011. The relative significance of environmental and anthropogenic factors affecting zooplankton community structure in Southeast Wisconsin Till Plain lakes. Hydrobiologia. 668:137–146.
- Vanni MJ. 1986. Competition in zooplankton communities: suppression of small species by Daphnia pulex. Limnol Oceanogr. 31:1039–1056.
- Vanni MJ. 1987. Effects of food availability and fish predation on a zooplankton community. Ecol Monogr. 57:61–88.
- Wahl DH, Goodrich J, Nannini MA, Dettmers JM, Soluk DA. 2008. Exploring riverine zooplankton in three habitats of the Illinois River ecosystem: where do they come from? Limnol Oceanogr. 53:2583–2593.
- Williamson CE. 1980. The predatory behavior of Mesocyclops edax: predator preferences, prey defenses, and starvation-induced changes. Limnol Oceanogr. 25:903–909.
- Williamson CE. 1984. Laboratory and field experiments on the feeding ecology of the cyclopoid copepod, Mesocyclops edax. Freshwater Biol. 14:575–585.
- Wu L, Culver DA. 1994. Daphnia population dynamics in western Lake Erie: regulation by food limitation and yellow perch predation. J Great Lakes Res. 20:537–545.
- Xie P, Yang Y. 2000. Long-term changes of Copepoda community (1957–1996) in a subtropical Chinese lake stocked densely with planktivorous filter-feeding silver and bighead carp. J Plankton Res. 22:1757–1778.