ABSTRACT
Information concerning turtle bycatch and its possible ramifications during biological sampling in freshwater systems is limited. Having such information will enable fisheries researchers and managers to modify fish sampling procedures to potentially minimize any impacts on turtle populations. Therefore, our objective was to utilize reflex response to characterize stress and mortality of western painted turtles (Chrysemys picta bellii) captured as bycatch in modified fyke nets during fish population assessments. Reflex-response-based condition classification (i.e. good, poor, unresponsive) data were collected from May to September during fish population assessments in 38 lakes and seven impoundments in northeast South Dakota from 2012 to 2014. We evaluated the relationship between observed condition and water temperature during two time periods, by turtle gender and size. Turtle bycatch mortality was assessed from June to September during fish population assessments in 14 lakes and three impoundments during 2013 and 2014. Delayed (i.e. overnight) mortality was determined for poor and unresponsive condition classifications and the relationship between observed mortality and water temperature for two time periods, by turtle gender and size was evaluated. Turtle condition decreased as water temperature increased. Condition was poorer during the May and June time period than August and September. Female turtles were in significantly worse condition than males. Condition was not related to turtle size. We observed varying mortality rates across condition classifications and the total estimated delayed mortality rate of known sex turtles was 36.4%. Mortality increased as temperature increased. Delayed mortality rates varied between the two sampling periods with higher mortality rates during the May and June time period. Female turtles had higher mortality rates than males. Delayed mortality did not vary by turtle size. Understanding the negative effects on bycatch during biological sampling is important to provide justification for the development and implementation of measures to protect non-target species.
Introduction
Information concerning bycatch and the ramifications for the species that make up the bycatch during freshwater biological sampling is limited. Most freshwater turtle bycatch research has focused on commercial fisheries utilizing hoop nets. The few studies pertaining to turtle bycatch during freshwater biological sampling are limited to bycatch and mortality rates during sampling for channel catfish (Ictaluris punctatus; Sullivan & Gale Citation1999; Michaletz & Sullivan Citation2002) and biological sampling of the Mississippi River (Barko et al. Citation2004). In a comparison of five passive fishing gears, fyke nets were found to capture the most turtles and caused the highest turtle mortality (Barko et al. Citation2004). They observed a bycatch rate for all turtles of 4.33 per net-night in large fyke nets, 1.78 per net-night in mini-fyke nets, and 0.28 per net-night in both large and small hoop nets. This is of particular concern during fish sampling, as fyke nets are recommended for sampling freshwater fish populations in lentic littoral areas (Miranda & Boxrucker Citation2009).
Turtle species diversity is low in northeast South Dakota with only two species present: western painted turtle (Chrysemys picta bellii) and common snapping turtle (Chelydra serpentina; Bandas Citation2003). Western painted turtles are the most common (Bandas Citation2003) and they are frequently captured during biological sampling. Recently, the bycatch of western painted turtles during fish population sampling with modified fyke nets in northeast South Dakota was quantified, with a mean catch rate of 2.56 turtles per net-night (Moos & Blackwell Citation2016).
Methods to assess the effects of prolonged submergence on freshwater turtle bycatch have varied among researchers. For example, Larocque et al. (Citation2012b) tested blood lactate levels to determine the effects of prolonged submergence in hoop nets. Bury (Citation2011) noted recovery time of a small sample of lethargic and non-responsive turtles captured in a submerged net and indicated further research was needed. LeDain et al. (Citation2013) observed seven reflex responses in painted turtles (Chrysemys picta) that were submerged for 12 h and compared these responses to blood lactate and blood pH to determine the effectiveness of using reflex response to assess the severity of anoxia. They determined that the use of reflex responses, especially tactile responses, was effective for identifying highly stressed turtles requiring assisted recovery.
Due to the potential to catch high numbers of turtles in fyke nets, it is important to understand the impact that fish population sampling can have on turtle bycatch. In addition, variables affecting stress of turtles in bycatch and associated mortality are not well known. Therefore, our objectives were to: (1) utilize a reflex response methodology similar to LeDain et al. (Citation2013) to classify the condition of western painted turtles collected as bycatch in modified fyke nets set overnight and determine how the identified condition is related to water temperature, time period, turtle sex and turtle size, (2) use condition classifications to quantify delayed mortality (i.e. after 24 h) of western painted turtles collected in modified fyke nets and determine if observed mortality is related to water temperature, time period, turtle sex and turtle size. Understanding the potential negative impacts on western painted turtles during fish population assessments provides researchers and fisheries managers with information necessary to take steps to potentially reduce bycatch mortality.
Methods
Study area
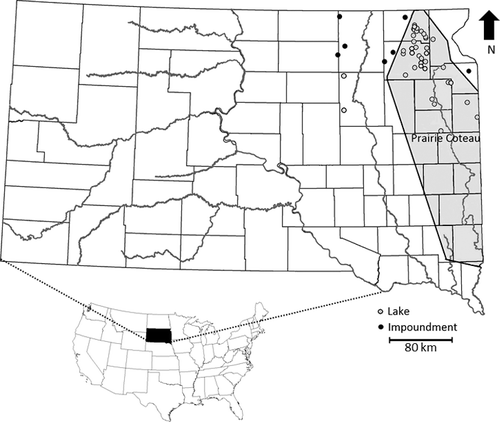
A total of 38 natural lakes and seven impoundments located in northeast South Dakota were sampled (). Among the lakes sampled, 36 are located within the Prairie Coteau. The Prairie Coteau is a 330 km long plateau running from northern Iowa to the North Dakota border and contains thousands of wetlands and lakes created during the Illinoian and Wisconsin glacial periods (Willis et al. Citation2007). Two lakes are located in the James River drainage along with five of the impoundments. One impoundment is located in the Red River drainage and one in the Minnesota River drainage. Lakes and impoundments in northeast South Dakota are relatively shallow, with maximum depths typically less than 8 m and the deepest being 12.5 m. Waters in northeast South Dakota are predominately eutrophic to hypereutrophic.
Figure 1. Locations of 38 lakes and seven impoundments where the condition and mortality of western painted turtles (Chrysemys picta bellii) captured as bycatch in modified fyke nets was assessed in northeast South Dakota during 2012–2014. The Prairie Coteau is highlighted in gray, and county boundaries and major rivers are shown.
Netting
Western painted turtle bycatch data from modified fyke nets were collected during fish population assessments in northeast South Dakota from May to September of 2012–2014 at 38 lakes and seven impoundments. Modified fyke nets differ from regular fyke nets in that they have rectangular frames to enhance their stability (Hubert Citation1996). Modified fyke nets were constructed of 1.9 cm mesh (bar measure), with a 15.2 m lead, two 0.9 × 1.5 m rectangular frames, and three 0.9 m diameter hoops (Moos & Blackwell Citation2016). The entrance was 7.6 cm wide × 0.9 m high, and had a maximum stretch width of approximately 17.8 cm. The throat was 18 cm in diameter, exhibited minimal stretch and included a crow's foot to reduce fish escapement.
Net locations used during fish population assessments were fixed at standardized locations and no net locations were sampled more than once annually. Nets were set in the morning, lifted the following day, and moved to a different standard location each day of the survey. Leads were staked on shore and stretched perpendicular to the shoreline. Depth and habitat at each net location varied; however, no depth or habitat data were recorded. Nets were not altered to allow turtles’ access to air (e.g. float, chimney, etc.) and the cod-end of the modified fyke net rested on the lake bottom. Nets that were set in shallow water, allowing turtles’ access to air, were excluded from the analysis.
Annual netting effort varied depending on waters sampled. Nine lakes and two impoundments are sampled annually and all other waters are sampled on a rotation ranging from 2 to 5 years. No waters were sampled more than once annually and assessments for individual waters occurred at approximately the same date each time they were surveyed.
Water temperature was recorded the first day of each fish population assessment. Fish population assessments generally occur over multiple days; therefore, data collected during each fish population assessment were assigned to the first day of the population assessment. The curved carapace length (CCL; mm) and sex of captured western painted turtles were recorded for all captured turtles. The CCL was measured with a flexible metal measuring tape and the sex of each turtle determined by observation of secondary sexual characteristics including foreclaw length and precloacal tail length (Cagle Citation1954; Gibbons Citation1968; Ernst Citation1971a; Gibbons & Lovich Citation1990; Mitchell Citation1994; Rowe Citation1997). Sex of western painted turtles <10 cm CCL is difficult to differentiate using secondary sexual characteristics; therefore, they were classified as undetermined. Sexual maturity of captured turtles was not determined.
Condition
We employed a method similar to LeDain et al. (Citation2013) that uses reflex responses to assess stress of turtles captured as bycatch in modified fyke nets during fish population sampling. Using tactile and escape responses, we classified turtle condition as good, poor, and unresponsive. Good condition was assigned to turtles exhibiting no adverse effects. When handled they were able to fully retract and hold appendages in their shell or actively clawed to escape. Poor condition turtles were lethargic, exhibiting an inability to retract and hold appendages in their shell and little to moderate movement of limbs, mouth, and eyes. Unresponsive condition was assigned to turtles that exhibited no movement when handled. The proportion of turtles exhibiting each classification was determined for each fish population assessment.
We used regression to test the proportion of each condition classification against water temperature; when the relationship was not linear, a non-linear equation was fit to the data. In addition, we compared data from two time periods, May–June and August–September, to determine if the timing (i.e. early or late summer) of fish population assessments influenced turtle condition. The May–June and August–September time periods were selected because they include the greatest temperature range and May–June encompasses increasing water temperatures and August–September contains decreasing water temperatures. July was excluded from this part of the analysis because water temperatures are consistently high. We used analysis of covariance (ANCOVA) to test if linear relationships between condition and water temperature, water temperature was the covariate, differed between the two time periods and to determine if the relationship between condition and CCL, CCL was the covariate, varied between males and females. We also tested whether a difference in the proportion of each gender by condition was observed within each time period using analysis of variance (ANOVA). Three time periods were tested in the ANOVA: May–June, July, and August–September. We included July in this analysis to determine if a difference in the condition between genders was observed during periods with high water temperature. Proportions used in the ANOVA analysis were logit transformed (Warton & Hui Citation2011). Assessments with less than 10 turtles (n = 10) were excluded from the analysis.
Mortality
Delayed mortality was assessed during June–September of 2013 and 2014. Good condition turtles were assumed to have high survival rates and were released after measuring. Subsamples of poor (n = 123) and unresponsive (n = 153) condition western painted turtles were retained during fish population assessments on 14 lakes and three impoundments. Sex and CCL were recorded for each turtle and they were separated by condition designation and placed on shore under inverted clear plastic containers. The clear plastic containers were 55.9 cm long × 40.6 cm wide × 17.8 cm high, with an opening cut in the long side that was 17.8 cm long × 7.6 cm high to allow turtles to escape. The containers were placed on shore near the waterline on gradual sloping shorelines with the escapement hole facing downhill. A 4.54 kg anchor was placed on top of each container to keeping it in place. The number of turtles placed under the container was ≤10, depending on size of turtles, to prevent overcrowding which could influence their ability to escape. Containers were left on shore overnight and checked the next day. Gender was determined and CCL was measured for turtle mortalities remaining in the containers. Data from containers that had been tampered with (n = 4) were not included in the analysis.
Observed mortality was evaluated for each sex by the condition classification, which was then applied to each fish population assessment to estimate the total mortality rate. We used linear regression to test the influence of water temperature on the estimated mortality rate. Assessments with less than 10 turtles captured (n = 10) were excluded from the analysis. We used the same time periods as in the condition analysis, May–June and August–September, and ANCOVA was used to test between time periods the influence the covariate water temperature had on observed mortality. Linear regression was used to determine if mortality varied by CCL for each sex. Male versus female variation in mortality across the covariate, CCL, was tested using ANCOVA. All statistical tests were completed using SYSTAT 13 (2009); alpha was set at 0.05.
Results
Condition
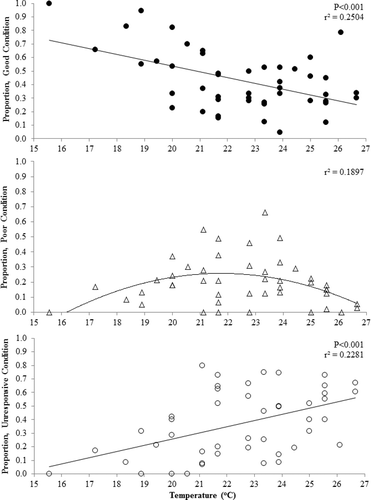
Condition was determined for 3435 western painted turtles collected during 1188 net-nights for a mean catch rate of 2.89 (SE = 0.17) turtles per net-night. Condition of collected turtles was 1484 (43.2%) in good condition, 792 (23.1%) in poor condition, and 1159 (33.7%) were unresponsive. As water temperature increased the proportion of turtles in good condition decreased (p < 0.001; r2 = 0.250) and the proportion of unresponsive turtles increased (p < 0.001; r2 = 0.228; ). A non-linear relationship was observed between poor condition turtles and water temperature with poor condition peaking between 20 and 24 °C ().
Figure 2. Proportion of good, poor, and unresponsive western painted turtles (Chrysemys picta bellii) by water temperature (°C). Turtles were captured during fish population assessments using modified fyke nets conducted from 2012 to 2014 in northeast South Dakota.
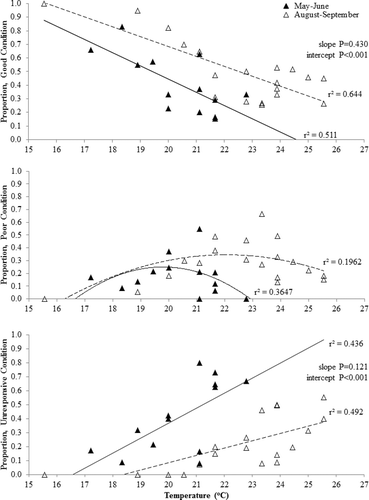
The proportion of good condition turtles was lower during the May–June time period than during August–September (). The slopes of the regression lines were not significantly different (p = 0.430); however, the intercepts were significantly different (p < 0.001). Comparison of the poor condition turtles from the two time periods indicated different non-linear trends, with the May–June peak between 20 and 21 °C and the August–September peak between 21 and 24 °C (). Similar to the relationships for good condition turtles, the slopes of the regressions for the two time periods for unresponsive condition turtles did not differ significantly (p = 0.121) but the intercepts (p < 0.001; ) were significantly different. The amount of variation in condition explained by temperature was higher during the May–June and August–September periods () than for the overall condition temperature relationship ().
Figure 3. Proportion of good, poor, and unresponsive western painted turtles (Chrysemys picta bellii) by water temperature (°C), for two time periods (May–June or August–September). Turtles were captured during fish population assessments using modified fyke nets conducted from 2012 to 2014 in northeast South Dakota.
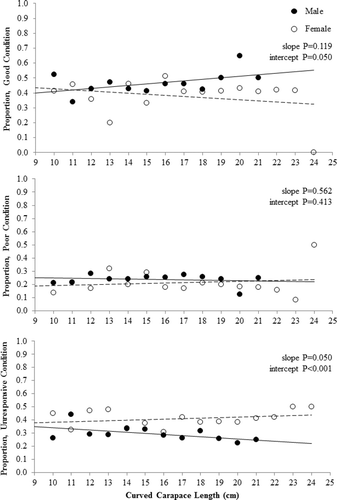
Condition was determined for 2160 male, 1184 female, and 91 undetermined sex turtles. The proportion of good (p = 0.104), poor (p = 0.638), or unresponsive condition (p = 0.080) males was not related to CCL (). Similarly, the proportion of good (p = 0.359), poor (p = 0.603), and unresponsive condition (p = 0.315) females did not change significantly across CCL (). The proportion of males in good condition tended to be higher than females across CCL; regression line slopes did not differ (p = 0.119) but the intercepts were significant (p = 0.050; ). The proportion of males and females in poor condition was similar across CCL as the slope (p = 0.562) and intercept (p = 0.413) of the two regression lines were similar (). The proportion of females classified as unresponsive was higher than males across CCL; both the slope (p = 0.050) and intercept (p < 0.001; ) of the regression lines differed significantly. No significant differences were observed in the proportion of males versus females in good condition (p = 0.705), poor condition (p = 0.368), and unresponsive condition (p = 0.321) across time periods.
Figure 4. Proportion of good, poor, and unresponsive western painted turtles (Chrysemys picta bellii) across curved carapace length (cm) for each gender (males solid circles, females open circles). Turtles were collected as bycatch during fish population assessments using modified fyke nets conducted from 2012 to 2014 in northeast South Dakota.
Mortality
Delayed mortality was evaluated for 123 poor condition and 153 unresponsive western painted turtles. Male mortality rates in the subsample were 16.5% (n = 85) for poor condition and 96.3% (n = 80) for unresponsive condition. Female mortality rates in the subsample were 24.3% (n = 37) for poor condition and 95.2% (n = 63) for unresponsive condition. Low numbers of poor condition (n = 1) and unresponsive condition (n = 10) undetermined sex turtles within the subsample preclude mortality analysis. Based on our delayed mortality estimates for each condition and sex, and assuming 100% survival of good condition turtles, we estimate that 33.2% (717 of 2160) of males and 42.1% (499 of 1184) of females collected as bycatch during fish population assessments with modified fyke nets perished. The overall estimated delayed mortality of known sex turtles is 36.4% (1216 of 3344).
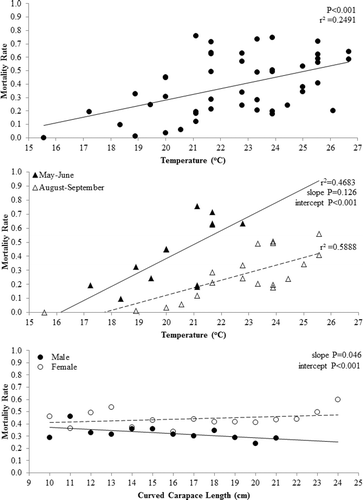
Estimated delayed mortality rates were related to water temperature (p < 0.001; r2 = 0.249) with an increasing trend in mortality as water temperature increased (). As with condition data, the relationship of mortality rate to water temperature varied by our defined time periods. Mortality rate was higher across temperatures during the May–June period than the August–September period as the intercepts of the regression lines differed significantly (p < 0.001) but the slopes were similar (p = 0.126; ). Similar to condition analysis, a higher proportion of the variability could be explained by changes in temperature during the May–June and August–September time periods (r2 = 0.468 and r2 = 0.588, respectively; ) when compared to the overall mortality and temperature relationship (r2 = 0.249; ). Delayed mortality did not change significantly across CCL for males (p = 0.072) or females (p = 0.274; ). Female mortality rates were higher across CCL than that observed for males; both the slopes (p = 0.046) and intercepts (p < 0.001; ) of the regression lines by sex differed significantly.
Figure 5. Relationship between estimated mortality rate and water temperature (°C; top), water temperature by time period (May–June = solid triangles and August–September = open triangles; middle) and curved carapace length (CCL; cm) for each gender (male = solid circle and female = open circle; bottom). Western painted turtles (Chrysemys picta bellii) were collected as bycatch during fish population assessments using modified fyke nets conducted from 2012 to 2014 in northeast South Dakota.
Discussion
We found that condition classification using reflex response was effective, easily incorporated into fish population assessments and provided new information concerning western painted turtle bycatch in modified fyke nets. Like LeDain et al. (Citation2013), we believe that the use of reflex response is a valuable method for assessing stress and condition of painted turtles captured as bycatch. All field personnel, including inexperienced interns, were able to classify the condition of captured turtles with reasonable confidence. Despite the subjective nature of the condition classifications, the only difficulty encountered was observing slight signs of stress and thereby determining the distinction between good and poor condition. The effectiveness of this classification technique for other species of turtles is unknown.
The time component of the standard fish population assessment protocol likely contributed to the variability observed in turtle condition and mortality in this study. Nets are set in the morning and checked the following morning and at any time during that interval turtles may be captured in the modified fyke net. A turtle that swims into a net five minutes before the net is pulled is likely to be classified as good condition even with factors present that increased stress and mortality rates, such as high water temperature and assessments conducted during the May–June period.
The scope of bycatch mortality was limited during this study as we only measured delayed mortality of turtles placed on shore. Long-term impacts and the effects of the study design on mortality are not known and may vary based on treatment of affected turtles. LeDain et al. (Citation2013) noted mixed results when testing the recovery of turtles placed in water versus on a platform. No significant difference was observed among blood lactate, blood pH, or reflex responses between the two treatments. However, reflex response values were lower for turtles recovering on the platform compared to those recovering in water.
Water temperature is an important factor in the condition and mortality rates of western painted turtles captured as bycatch during fish population assessments conducted with modified fyke nets. Western painted turtles appear to be able to cope with prolonged (i.e. up to 24 h) submergence at water temperatures below 20 °C. Stress increased as water temperature increased, as was evidenced by increasing proportions of poor and unresponsive condition turtles and increasing mortality rates. Temperature has been previously identified as an important factor in turtle bycatch mortality. Barko et al. (Citation2004) noted that the greatest mortality of turtle bycatch was correlated with higher water temperature in the upper Mississippi River. Gatten (Citation1981) noted that whole-body lactate levels in painted turtles increased to 300 mg% after two days forced submergence at 25 °C; while an increase to the same whole-body lactate level required submergence for two weeks at 5 °C. Ultsch et al. (Citation1984) observed painted turtle mortality if blood pH decreased by one full unit.
The observed non-linear relationship in poor condition turtles may indicate that there is a critical temperature threshold range of 20–24 °C for western painted turtles submerged for up to 24 h. Herbert and Jackson (Citation1985) studied recovery of western painted turtles after prolonged anoxic submergence and they determined the duration of submergence at each tested temperature by a decrease in blood pH to 7.0–7.1. At 15 °C, blood pH decreased to 7.1 after 3 days submerged and full recovery of blood pH took 2 days. At 20 °C, blood pH decreased to 7.2 after only 12 h submerged and required 36 h for full recovery.
We observed that western painted turtles are better able to survive up to 24 h submergence during the August and September assessments with higher condition ratings, lower mortality rates and higher temperature threshold range than during May and June assessments. The mechanism causing the observed variation in the condition and mortality of turtle bycatch during the two time periods is unknown. One possible factor may be seasonal acclimation. Olson and Crawford (Citation1989) in a comparison between all four seasons observed lower protein content, greater non-bicarbonate buffering capacity, and higher lactate dehydrogenase activity in the heart muscle of painted turtles during autumn. However, the timeframe of October and November tested by Olson & Crawford (Citation1989) was later than what we tested. Another possible factor is that turtles captured during the May and June time period may be more stressed due to natural seasonal patterns. Waters in northeast South Dakota are typically not ice-free until early to mid-April; thus, spring courtship and mating likely occurs from late-April to early-June (Gibbons Citation1968; Ernst Citation1971b). It is important to note, courtship and mating has been observed during autumn among painted turtles (reviewed in Ernst Citation1971c). Nesting likely takes place between late-May and early-July (Cagle Citation1954: Ernst Citation1971b: Moll Citation1973: Iverson & Smith Citation1993). During courtship and mating, increased blood lactate levels have been observed in both male and female red-sided garter snakes (Thamnophis sirtalis parietalis; Shine et al. Citation2004) and increased corticosterone has been observed in male and female red-spotted newts (Notophthalmus viridescens; Reedy et al. Citation2014), indicating increased stress. Romero (Citation2002) noted that glucocorticoid levels were greatest during breeding periods for most reptile species tested, including some turtle species; however, no data for painted turtles were presented. In addition, among turtle species that have not shown increased glucocorticoid levels during breeding periods, effects of stress may not be apparent due to downregulation of the adrenocortical response (Moore & Jessop Citation2003). The researchers noted that, although mortality may increase, it may be necessary to downregulate adrenocortical response during reproduction to maximize reproductive success. Female painted turtles that had just completed nesting were found to have increased whole-body lactate levels (Congdon & Gatten Citation1989). However, females were not in significantly worse condition compared to males during any time periods in our study. It appears that differences in condition by time period were similar for both genders. It is unclear whether any of these factors or an unknown variable accounts for the variation in stress level in western painted turtles between May–June and August–September assessments.
The increased mortality rate we observed for female western painted turtles is cause for concern. Increased adult female mortality could have drastic implications for turtle populations. Reed et al. (Citation2002) noted the annual removal of two adult female alligator snapping turtles (Macrochelys temminckii) from a total population of 200 would result in a 50% reduction in the population within 50 years. Midwood et al. (Citation2015), using population viability analysis to look at long-term effects of commercial bycatch mortality, indicated that an increase in annual mortality exceeding two adult female painted turtles was sufficient to extirpate the population from Lake Opinicon, Ontario, Canada, within 350 years.
Mortality rates did not vary by CCL for either gender. However, because turtles ≥10 cm in CCL comprise >97% of the western painted turtle bycatch in modified fyke nets (Moos & Blackwell, Citation2016), most of the observed mortality occurs among larger, likely adult, western painted turtles. Painted turtles are long-lived and have high mortality rates at the egg and juvenile life stages with mortality rates decreasing substantially once they reach sexual maturity (Wilbur Citation1975; Mitchell Citation1988; Frazer et al. Citation1991). Brooks et al. (Citation1991), studying mortality of common snapping turtles, noted that increased mortality of adults, among species that have long life spans, late sexual maturity and high juvenile mortality, results in prolonged population recovery time or extirpation unless immigration, fecundity, growth rate, or juvenile survivorship increase.
It is evident that biological sampling with modified fyke nets has the potential to capture and kill large numbers of western painted turtles. However, it is not well known how the bycatch mortality impacts turtle populations. Midwood et al. (Citation2015) indicated that population extirpation, resultant of commercial bycatch, could occur among the four turtle species they examined, including painted turtles. Further research modeling bycatch mortality is needed to better understand potential effects on turtle populations.
The results of this study provide information necessary to increase awareness of bycatch during biological sampling. This is important because fisheries researchers and resource managers need to be cognizant of the potential negative impacts on non-target species that can occur during standard sampling and hopefully will subsequently try to minimize the impacts on bycatch. The differences in condition and mortality by time period observed in this study indicate that researchers should avoid using modified fyke nets during May and June; however, in a previous study (Moos & Blackwell Citation2016) observed turtle bycatch rates were higher during the August and September time period, offsetting the differences in mortality rate. To reduce bycatch mortality, we recommend fisheries researchers and managers avoid overnight sets of modified fyke nets when water temperatures are >20 °C. Also, setting nets in shallow water providing turtles’ access to air would reduce bycatch mortality (Bury Citation2011). Finally, net alterations, including exclusion devices (Lowry et al. Citation2005; Fratto, Barko, and Scheibe Citation2008; Fratto, Barko, Pitts, et al. Citation2008; Larocque et al. Citation2012a), escapement devices (Lowry et al. Citation2005; Fratto, Barko, Pitts, et al. Citation2008; Larocque et al. Citation2012a), or floats (Larocque et al. Citation2012b), could also reduce turtle bycatch mortality. However, net alteration designs in most of the aforementioned studies resulted in altered catch dynamics, including size structure or catch rates among some species of fish. Further refinement of net alteration designs is needed to provide mortality reduction methodology for a wide range of sampling conditions.
Acknowledgments
We want to thank all the permanent and seasonal South Dakota Department of Game, Fish and Parks employees that assisted with the collection of data, especially R. Braun and S. Kennedy. In addition, substantial improvement to the manuscript was made thanks to the critiques of two anonymous reviewers and the editor.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors

Tyrel S. Moos
Tyrel S. Moos is a fisheries biologist with the South Dakota Department of Game, Fish and Parks (Webster, South Dakota). In addition to monitoring and managing angling resources in northeast South Dakota, he has assisted with research focused on determining effective aging structures for multiple fish species and understanding and mitigating mortality of freshwater turtle by catch during fish population sampling.

Brian G. Blackwell
Brian G. Blackwell is a fisheries biologist with the South Dakota Department of Game, Fish and Parks (Webster, South Dakota). Brian completed his BS from South Dakota State University (SDSU), MS from Texas A&M University and PhD from SDSU. Primary research interests are applied fisheries management and fisheries ecology.
References
- Bandas SJ. 2003. Geographical distribution and morphometrics of South Dakota turtles [master's thesis]. Brookings (SD): South Dakota State University. Available from: pubstorage.sdstate.edu/wfs/thesis/Bandas-Sarah-J-MS-2003.pdf
- Barko VA, Briggler JT, Ostendorf DE. 2004. Passive fishing techniques: a cause of turtle mortality in the Mississippi River. J Wildl Manage. 68:1145–1150.
- Brooks RJ, Brown GP, Galbraith DA. 1991. Effects of a sudden increase in natural mortality of adults on a population of the common snapping turtle (Chelydra serpentina). Can J Zool. 69:1314–1320.
- Bury RB. 2011. Modifications of traps to reduce bycatch of freshwater turtles. J Wildl Manage. 75:3–5.
- Cagle FR. 1954. Observations on the life cycle of painted turtles (genus Chrysemys). Am MidlNat. 52:225–235.
- Congdon JD, Gatten RE. 1989. Movements and energetics of the nesting Chrysemys picta. Herpetologica. 45:94–100.
- Ernst CH. 1971a. Sexual cycles and maturity of the turtle Chrysemys picta. Biol Bull. 140:191–200.
- Ernst CH. 1971b. Population dynamics and activity cycles of Chrysemys picta in southeastern Pennsylvania. J Herpetol. 5:151–160.
- Ernst CH. 1971c. Observations of the painted turtle, Chrysemys picta. J Herpetol. 5:216–220.
- Fratto ZW, Barko VA, Scheibe JS. 2008. Development and efficacy of a bycatch reduction device for Wisconsin-type fyke nets deployed in freshwater systems. Chelonian Conserv Biol. 7:205–212.
- Fratto ZW, Barko VA, Pitts PR, Sheriff SL, Briggler JT, Sullivan KP, McKeage BL, Johnson TR. 2008. Evaluation of turtle exclusion and escapement devices for hoop-nets. J Wildl Manage. 72:1628–1633.
- Frazer NB, Gibbons JW, Greene JL. 1991. Growth, survivorship and longevity of painted turtles Chrysemsy picta in a southwestern Michigan marsh. Am Midl Nat. 125:245–258.
- Gatten RE. 1981. Anaerobic metabolism in freely diving painted turtles (Chrysemys picta). J Exp Zool. 216:377–385.
- Gibbons JW. 1968. Reproductive potential, activity, and cycles in the painted turtle, Chrysemys picta. Ecology. 49:399–409.
- Gibbons JW, Lovich JE. 1990. Sexual dimorphism in turtles with the emphasis on the slider turtle (Trachemys scripta). Herpetol Monogr. 4:1–29.
- Herbert CV, Jackson DC. 1985. Temperature effects on the responses to prolonged submergence in the turtle Chrysemys picta bellii. I. Blood acid-base and ionic changes during and following anoxic submergence. Physiol Zool. 58:655–669.
- Hubert WA. 1996. Passive capture techniques. In: Murphy BR, Willis DW, editors. Fisheries techniques. 2nd ed. Bethesda (MD): American Fisheries Society; p. 169.
- Iverson JB, Smith GR. 1993. Reproductive ecology of the painted turtle (Chrysmeys picta) in the Nebraska Sandhills and across its range. Copeia. 1993:1–21.
- Larocque SM, Cooke SJ, Blouin-Demers G. 2012a. Mitigating bycatch of freshwater turtles in passively fished fyke nets through the use of exclusion and escape modifications. Fish Res. 125–126:149–155.
- Larocque SM, Cooke SJ, Blouin-Demers G. 2012b. A breath of fresh air: avoiding anoxia and mortality of freshwater turtles in fyke nets by the use of floats. Aquat Conserv. 22:198–205.
- LeDain MRK, Larocque SM, Stoot LJ, Cairns NA, Blouin-Demers G, Cooke SJ. 2013. Assisted recovery following prolonged submergence in fishing nets can be beneficial to turtles: an assessment with blood physiology and reflex impairment. Chelonian Conserv Biol. 12:172–177.
- Lowry MB, Pease BC, Graham K, Walford TR. 2005. Reducing the mortality of freshwater turtles in commercial fish traps. Aquat Conserv. 15:7–21.
- Michaletz PH, KP Sullivan. 2002. Sampling channel catfish with tandem hoop nets in small impoundments. N Am J Fish Manage. 22:870–878.
- Midwood JD, Cairns NA, Stoot LJ, Cooke SJ, Blouin-Demers G. 2015. Bycatch mortality can cause extirpation in four freshwater turtle species. Aquat Conserv 25:71–80.
- Miranda LE, J Boxrucker. 2009. Warmwater fish in standing waters. In: Bonar SA, Hubert WA, Willis DW, editors. Standard methods for sampling North American freshwater fishes. Bethesda (MD): American Fisheries Society; p. 36–38.
- Mitchell JC. 1988. Population ecology and life histories of the freshwater turtles Chyrsemys picta and Sternotherus odoratus in an urban lake. Herpetol Monogr. 2:40–61.
- Mitchell JC. 1994. The reptiles of Virginia. Washington (DC): Smithsonian Inst. Press.
- Moll EO. 1973. Latitudinal and intersubspecific variation in reproduction of the painted turtle, Chrysmeys picta. Herpetologica. 29:307–318.
- Moore IT, Jessop TS. 2003. Stress, reproduction, and adrenocortical modulation in amphibians and reptiles. Horm Behav. 43:39–47.
- Moos TM, Blackwell BG. 2016. Characterization of western painted turtle bycatch in fyke nets during freshwater fish population assessments. J Fish Wildl Manage. 7:222–230; e1944-687X. doi: 10.3996/102014-JFWM-077
- Olson JM, Crawford KM. 1989. The effect of seasonal acclimatization on the buffering capacity and lactate dehydrogenase activity in tissues of the freshwater turtle Chrysemys picta marginata. J Exp Biol. 145:471–476.
- Reed RN, Congdon JD, Gibbons JW. 2002. The alligator snapping turtle [Macrochelys(Macrochelys) temminckii]: a review of ecology, life history, and conservation, with demographic analyses of the sustainability of take from wild populations. Aiken (SC): Savannah River Ecology Laboratory; p. 17. Available from: http://srelherp.uga.edu/projects/BobReedAlligatorSnapper-02.pdf
- Reedy AM, Edwards A, Pendlebury C, Murdaugh L, Avery R, Seidenberg J, Aspbury AS, Gabor CR. 2014. An acute increase in the stress hormone corticosterone is associated with mating behavior in both male and female red-spotted newts, Notophthalmus viridescens. Gen Comp Endocrinol. 208:57–63.
- Romero LM. 2002. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen Comp Endocrinol. 128:1–24.
- Rowe JW. 1997. Growth rate, body size, sexual dimorphism and morphometric variation in four populations of painted turtles (Chrysemys picta bellii) from Nebraska. Am Midl Nat. 138:174–188.
- Shine R, Phillips B, Langkilde T, Lutterschmidt DI, Waye H, Mason RT. 2004. Mechanisms and consequences of sexual conflict in garter snakes (Thamnophis sirtalis, Colubridae). Behav Ecol. 15:654–660.
- Sullivan KP, Gale CM. 1999. A comparison of channel catfish (Ictalurus punctatus) catch rates, size distributions, and mortalities using three different gears in a Missouri impoundment. Am Fish Soc Symp. 24:293–300.
- Ultsch GR, Herbert CV, Jackson DC. 1984. The comparative physiology of diving in North American freshwater turtles. I. Submergence tolerance, gas exchange, and acid-based balance. Physiol Zool. 57:620–631.
- Warton DI, Hui FK. 2011. The arcsine is asinine: the analysis of proportions in ecology. Ecology. 92:3–10.
- Willis DW, Stukel SM, Brown ML. 2007. Eastern natural lakes. In: Berry CR, Higgins KF, Willis DW, Chipps SR, editors. History of fisheries and fishing in South Dakota. Pierre (SD): South Dakota Department of Game, Fish and Parks; p. 124–127.
- Wilbur HM. 1975. The evolutionary and mathematical demography of the turtle Chrysemys picta. Ecology. 56:64–77.
