ABSTRACT
In this study, we evaluated the effects of two dominant microfilamentous algae (i.e. Melosira granulata and Oscillatoria sp.), collected from the West Lake, on growth and metabolism of Daphnia magna. Our experiment utilized 13C and 15N dual labeling to calculate the carbon and nitrogen isotopic turnover rates and half-life times in D. magna. The two labeled types of filaments were offered to D. magna as sole food sources or as paired mixtures with the unlabeled Scenedesmus obliquus. Labeled S. obliquus served as the control. Combined results showed that D. magna had a higher grazing rate on Oscillatoria sp. than on M. granulate and a small percentage of unlabeled S. obliquus addition could improve the grazing rate in both filamentous algae, especially for Oscillatoria sp., which had the highest carbon and nitrogen isotopic turnover rates and the lowest half times, even superior to the sole S. obliquus treatment. Our study revealed that D. magna could utilize the two dominant filamentous algae as a food source for their growth and metabolism, and a small percentage addition of S. obliquus could ameliorate the negative impact of these two filamentous algae on D. magna.
Introduction
Cladocerans, especially Daphnia, form a key link in energy and nutrient mobilization between primary producers and higher trophic levels in most aquatic ecosystems (Gliwicz & Lamper Citation1990; Sterner & Hessen Citation1994; Jansson et al. Citation2007; Lampert Citation2011; Sanni et al. Citation2013; Bednarska et al. Citation2014). Burns et al. (Citation1989) provided evidence that Daphnia carinata was able to collect trichomes of Anabaena minutissima var. attenuate with average length 77–180 μm. Also, some studies demonstrated that Daphnia would be unable to control cyanobacteria blooms once fully developed (Schoenberg & Carlson Citation1984; Danielsdottir et al. Citation2007), but intense herbivory by Daphnia could prevent blooms from initiating (Sarnelle Citation2007). Besides, Meijer (Citation2000) summarized the results of 18 case studies in the Netherlands and found that Daphnia was able to limit phytoplankton biomass in spring but not in summer. And adult Daphnia magna were less sensitive to poor quality food than juveniles (Vanni et al. Citation1992). Because of the significant role of cladocerans in the aquatic food webs, studies on cladoceran–filament interaction could provide important information for a better understanding of aquatic ecosystem function and could contribute to forecasting or potentially preventing algal imbalance due to anthropogenic alterations of the environment. In this paper, the cladoceran we studied was D. magna, the most common freshwater invertebrate model species (Smutná et al. Citation2014).
Filamentous algae blooms have become prevalent in numerous freshwaters as climate change generally benefits the growth of many species of harmful algae (Paerl & Huisman Citation2009; Kosten et al. Citation2012). Reports about the reciprocal response of filamentous algae defending against zooplankton grazing are very limited (Ger et al. Citation2014). It was reported that the coexistence of some larger-bodied Daphnia spp. with filamentous cyanobacteria in some lakes may be facilitated by undeterred consumption of some groups of in situ plankton (diatoms, flagellates and chlorococcales) and avoidance of cyanobacterial filamentous (Epp Citation1996). Filaments like Oscillatoria and Melosira may be important for daphnids as a source of nutrition, but may interfere with the process of food collection, related to the filamentous algae concentration, length and challenging ingestion (Fulton Citation1988; Gliwicz & Lampert Citation1990; Gulati et al. Citation2001; Kâ et al. Citation2012). Other research showed that the weight-specific ingestion rates of D. magna were higher on the shorter Oscillatoria filaments than on the longer ones (Gulati et al. Citation2001). Furthermore, cyanobacteria can shape microevolutionary changes in Daphnia populations (Bednarska et al. Citation2014). It was also observed that the filaments of both Melosira and Oscillatoria were inadequate food source for Daphnia because they could not sustain growth and reproduction of planktonic animals as well as unicellular algae could not sustain growth and reproduction of planktonic animals as well as unicellular algae, such as Scenedesmus (Porter & Orcutt Citation1980; Haney Citation1987; Lampert Citation1987; DeBernardi & Giussani Citation1990; Bednarska et al. Citation2014).
The main effect of algae proliferation is the imbalance in the base of the food web that depends on various environmental factors such as nutrients, light, temperature and water movement (Xu et al. Citation2007; Yang et al. Citation2012). In this study, 13C and 15N dual labeling was used for carbon and nitrogen stable isotopic turnover modeling to estimate the grazing rate of labeled food in D. magna. After a consumer is provided with an isotopically different diet, the tissues may come to reach a new isotopic equilibrium with the diet after a time lag. Then the turnover process can be mathematically predictable, reflecting rates of both growth and catabolic tissue replacement of animals (Carleton et al. Citation2008; Carleton & Rio Citation2010; Heady & Moore Citation2013). Meanwhile, stable isotopic signatures can be effectively used to trace food sources, elucidate trophic interactions and presumably reflect food web structure (Woodland et al. Citation2012; Xia et al. Citation2013). The objective of this study was to evaluate the effects of two filamentous algae species, Melosira granulata and Oscillatoria sp., on growth and metabolism of D. magna, after their coexistence in vivo for three months. The two filaments were given as food to six clones of D. magna either alone or together with the unlabeled green alga Scenedesmus obliquus. M. granulata and Oscillatoria sp., the two filaments employed in these experiments, were dominant phytoplankton species in the West Lake, Hangzhou, China. The green algae, S. obliquus, contains sterols but is deficient in C-20 polyunsaturated fatty acids; thus, it is an intermediate quality food source among the phytoplankton algal foods (Freese & Martin-Creuzburg Citation2013) and was used as a reference food in these D. magna-feeding experiments. The purpose of the present experiment was to test the hypothesis that the food mixture that included S. obliquus would significantly increase D. magna grazing rates on the two dominant filaments collected from the West Lake.
Methods
Sampling filamentous algal source
The West Lake is a typical shallow lake located in the central city of Hangzhou, on the southeastern coastline of China (120°16′E, 30°15′N). It is about 6.5 km2 in surface area with an average depth of 2.27 m, and a high annual average nitrogen level of 2.34 mg/L (>1.5 mg/L) (Jin et al. Citation2015). The experimental algae M. granulata and Oscillatoria sp. were collected from Xiaonanhu (one sub-lake of the West Lake) in March 2014.
Culture isolation and purification
The collected wild samples of M. granulata and Oscillatoria sp. were isolated by Pasteur pipet and cultured in bacteria-free nutrient solutions at the Freshwater Algae Culture Collection of the Institute of Hydrobiology. M. granulata was cultured in Conway-silicate medium at 22.5 ± 1 °C, while Oscillatoria sp. was cultured in Blue-Green-11 medium at 25 °C. Both algae were cultivated in sterile bottles with breathable sealing film under a 12L:12D photoperiod and inoculated once every two weeks to maintain their exponential growth.
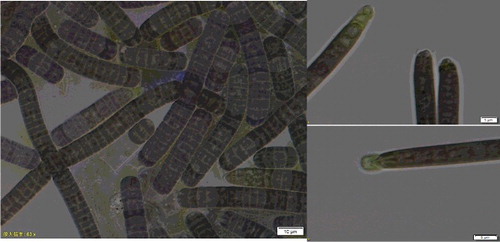
Trichomes of Oscillatoria sp. in liquid medium were smooth and tended to grow solitary cells first. Cells were wider than they were long (2.65–3.2 μm long by 5.23--6.69 μm wide), contained phycoerythrin and were usually without sheaths. Apical cells had calyptras. It was a novel taxon ().
Total genomic DNA was obtained using a cetyl-trimethyl ammonium bromide method adapted for cyanobacteria (Vaz et al. Citation2015). The 16S-23S rRNA ITS sequences from the remaining Oscillatoria sp. strains were amplified by polymerase chain reaction (PCR) using the primers pA (5΄-AGAGTTTGATCCTGGCTCAG-3΄) (Edwards et al. Citation1989) and B23s (5΄-CTTCGCCTCTGTGTGCCTAGGT-3΄) (Lepère et al. Citation2000). PCR amplification, cloning and sequencing were performed as described by Liu et al. (Citation2014). The nucleotide sequences obtained in this study and related sequences retrieved from Gen Bank were aligned (16S/ITS rRNA matrix with length of 2022 bp).
Daphnia culture
The experiment was performed with a clone of D. magna, which was stored in the Institute of Hydrobiology, Chinese Academy of Sciences. The experimental animals were maintained for months at a temperature of 24 ± 1 °C in laboratory batch cultures with a photoperiod of 12L:12D (dim light). Fifty milliliters of tap water per individual and sufficient food mixtures of Oscillatoria sp., M. granulata and S. obliquus were provided simultaneously. The tap water was first aerated for 24 h in a large container and pre-filtered through a 25-μm filter before use and it was changed once every two days. Moreover, previous research (Strathman Citation1967) indicated that algal carbon content of approximately 2.5 mg C/Lin a given volume of zooplankton medium would be sufficient for zooplankton survival and population growth. Thus, the same food concentration was set for these experimental animals.
Additionally, after three months of observation, there were no symptoms of an acute toxicity strain (Zhang et al. Citation2011) appearing in the M. granulata and Oscillatoria sp. cultures. However, clones fed with the mixed filamentous algae showed a longer maturity time at the beginning, when comparing with the sole feeding of S. obliquus (Repka Citation1998). Thus, a non-toxic strain allowed us to exclude any toxic effect of the two filaments on D. magna.
Preparation of tracer algal foods
Filaments of Oscillatoria sp., M. granulata and S. obliquus in exponential growth phase were harvested and incubated under the original light, temperature and culture conditions in a shaking table at constant temperature for 4 h (Walsh & O'Neil Citation2014). Additionally, uniformly dispersed suspensions of Oscillatoria sp. were obtained before algae labeling by filtering them through a plankton net (mesh size: 25 μm). Before the algae incubation, about 0.007 g NaH13CO3 (98 at % 13C) and 0.007 g 15NH4Cl (98 at % 15N) (Sigma) per liter of medium were added. After labeling, the algal cells were collected and washed on a pre-combusted (450 °C for 5 h) 25-mm diameter glass fiber filter paper: Whatman type GF/C. Meanwhile, the algae were washed repeatedly with deionized water to remove unassimilated 13C and 15N isotopes. The labeled algae were then resuspended in a suitable volume of distilled water for the feeding experiment. Fresh food suspensions were made every 24 h.
Dual labeling feeding experiment
As shows, five feeding treatments were designed in this experiment and each treatment included three replicate beakers each with 6 animals (18 animals per treatment). Samples were taken 19 times during each experimental period, so 57 beakers (500 mL) were prepared for every feeding treatment as the experiment began. Three hundred milliliters of tap water that was aerated for 24 h in a large barrel and pre-filtered using a net (mesh size of 25 μm) before use, was added to each beaker. Similar-sized (9-days-old) and healthy individuals (n = 6) of D. magna were added to each beaker next. The aforementioned conditions for D. magna were maintained throughout the feeding experiment (24 ± 1 °C; 12L:12D light–dark cycle). The experiment was then started immediately by adding 1 mL of isotopically labeled algae to each beaker. Here, we also tested whether the addition of good quality food (S. obliquus) could ameliorate the negative impact of these two filamentous algae on Daphnia. Thus, in the experimental system, treatment groups with or without unlabeled green algae were designed ().
Table 1. Daphnia-algae feeding treatments. The ratio of unlabeled S. obliquus to labeled filaments was 1:10.
Each D. magna-algae treatment group was incubated for 96 h. Algae, the animal's food source, were added to the system every 8:00 am during the experimental period and the aerated tap water was changed once every two days before providing the food algae to maintain the supply of algae at 2.5 mg C/L.
Sample collection and growth rates
Three beakers of D. magna were randomly sampled for analysis of carbon and nitrogen stable isotopes at hours 0, 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48, 56, 64, 72, 80, 88 and 96. Time 0 values of the isotopes were treated as background values. Each beaker of animals was transferred to a 100-μm sieve, washed and then rinsed with distilled water and left for 16 h for gut evacuation (Taipale et al. Citation2008). All adults were then individually transferred to pre-weighed tin cups and dried at 60 °C yielding 0.2–1.2 mg dry mass for stable isotope analysis.
The growth rate of D. magna was calculated using the following exponential growth model:(1)
where Mf is the mass at time t (mg), Mo is the mean initial mass (mg), k is the growth rate of each beaker's total D. magna (per hour, n = 6), t is time (h).
Stable isotope analysis
The stable C (13C) and N (
15N) isotope ratio values were generated after analysis of samples on a continuous-flow isotope ratio mass spectrometer (Delta Plus; Finnigan, Bremen, Germany) coupled to a Carlo Erba NA 2500 elemental analyzer (Carlo Erba Reagenti, Milan, Italy). Stable isotope ratios were expressed in
notation as parts per thousand (‰) relative to the international standards according to the equation:
, where X is 13C or 15N and R is the corresponding ratio of 13C/12C or 15N/14N.
value is the measure of heavy to light isotope in the sample, whereby higher
values denote a greater proportion of the heavy isotope. The standard for N was atmospheric N and for C, Vienna Pee Dee belemnite. The reference material for
15N was ammonium sulfate (IAEAUSGS25), and that for
13C was carbonate (IAEA–NBS18), supplied by the US Geological Survey (Denver, CO, USA) and certified by the International Atomic Energy Agency (Vienna, Austria). On a daily basis, an internal working standard, urea (
15N = −1.53‰,
13C = −49.44‰), was employed. The average standard deviations of replicate measurements for
13C and
15N were both
.
The raw data from the stable isotope analysis were normalized between 0 and 1 by the equation: , due to the different amount and absorption rates of isotopic compound additions in these three algae: S. obliquus, M. granulata and Oscillatoria sp.
The software package Origin 8.0 was applied to estimating isotopic turnover rates in D. magna tissue, using iterative least-squares fitting of the regression to the normalized data for the following equation, which has two components (): growth (tissue addition, k) and metabolic tissue replacement (m) (Hesslein et al. Citation1993; Gamboa-Degado et al. Citation2011; Rojas-Casas et al. Citation2013):
(2)
In this model, where is the
value of animal at any time after the labeling,
is the initial delta value of the animal with unlabeled food (‰),
is the final delta value in equilibrium after the labeling (‰) and
is the coefficient determining the rate of change over time t (h). The value
is equivalent to the sum of the isotopic turnover due to growth (k) and non-growth metabolic processes, i.e. the organism's metabolism (m). In addition, k% and m% are defined by the following equation (Hesslein et al. Citation1993; Xu et al. Citation2011):
(3)
In addition, coefficient provides an indicator of the time period necessary for half of the tissue carbon or nitrogen isotope to be replaced by new carbon or nitrogen after animals consume a new diet (half time,
) (MacAvoy et al. Citation2006; Gamboa-Delgado et al. Citation2014).
(4)
Statistical analysis
For statistical evaluation of the treatment groups, we applied one-way analysis of variance (ANOVA) to compare the growth rates and survival rates of D. magna, and least significant difference (LSD) was carried out using the SPSS package. LSD values were reported at the 5% level of significance. As for the isotopic turnover rates () estimated from EquationEquation (2)
(2) , if the 95% confidence intervals for the two groups did not overlap, it can be inferred that the percentage difference between groups was statistically significant at the 5% level (Sedgwick Citation2012).
Results
D. magna growth and survival rates
The ratio fluctuations of sampled weight/original weight of D. magna in the five feeding treatments are shown in . At the end of this experiment, D. magna reared under the five diet treatments showed significantly different mean growth rates (p < 0.001, ). D. magna in the sole S. obliquus treatment (S) had the fastest growth rate (0.0422 ± 0.007 d−1), which was much higher than other treatments (p < 0.001, ). There were similar growth rates between O1 and O2 (p > 0.05), and a significantly lower growth rate was observed in the D. magna which fed solely on M. granulata (M1, 0.026 ± 0.005 d−1, p < 0.01).
Figure 2. Growth of D. magna in each beaker, which fed by sole S. obliquus (S, as control), sole M. granulata (M1), M. granulata mixed with S. obliquus (M2), sole Oscillatoria sp. (O1) and Oscillatoria sp. mixed with S. obliquus (O2) during the 96-h experiment. Each symbol represents the mean value in the D. magna-algae feeding treatment, with the error bar representing the standard deviation.
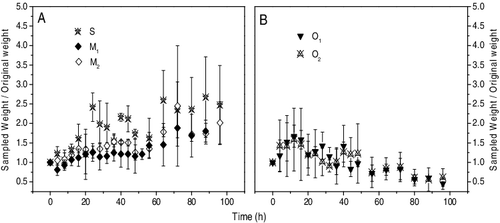
Table 2. One-way ANOVA for the mean of growth rate (k) of the five different D. magna-algae feeding treatments.
Table 3. The results of LSD post hoc for comparing the mean of growth rate (k) of the five different D. magna-algae feeding treatments.
Because the survival rates showed no variation in the treatment S (100% ± 0%), the remaining group's data were analyzed by one-way ANOVA and LSD post hoc tests ( and ). Results showed that there were significant differences in the remaining four treatments (p < 0.01) except for between the treatment M1 and M2 (98% ± 1.5% and 97% ± 1.7%, p > 0.05). Otherwise, D. magna that were fed with Oscillatoria sp. (O1 and O2) had much lower survival rates (50% ± 7.6% and 66.7% ± 6.1%, p < 0.01).
Table 4. One-way ANOVA for the mean of survival rate of the rest four different D. magna-algae feeding treatments.
Table 5. The results of LSD post hoc test for comparing the mean survival rate of the remaining four different D. magna-algae feeding treatments.
Additionally, the estimated percent contributions of growth to isotopic tissue turnover (according to EquationEquation (3(3) )) for each feeding treatment group were: S (42.3%
), M2 (23.9%
), O1 (19.3%
), O2 (17.9%
) and M1 (2.7%
) (). And there should be higher k% values of O1 and O2, for 33.3%–50% mortality rate occurred during the last 48 h of the whole 96 h experimental animal feeding period.
Table 6. Growth rate (k), final survival rate and catabolic tissue turnover rate (m) estimated for the five different D. magna-algae feeding treatments.
Isotopic turnover modeling
Isotope turnover in each feeding treatment was well described by the exponential, one-pool model (EquationEquation (2(2) )) (r2
0.914 or better, p < 0.005). The model estimation revealed that different algae feeding treatments were characterized by different isotope turnover rates (). The expected changes in carbon and nitrogen stable isotope turnovers for D. magna of five diet treatments estimated with the time-based models are illustrated in . For all of the five diet treatments, the carbon isotopic turnover rates, driven by both growth and metabolism (
), varied substantially among different diets, with the highest value for O2 (0.0903 ± 0.0193 h−1), followed by M2, S, O1, and the lowest value for M1 (0.0072 ± 0.0015 h−1). On the other hand, for all five diet treatments, the nitrogen isotopic turnover rates (
) displayed the highest and lowest values for O2 (0.0516 ± 0.0159 h−1) and M1 (0.0091 ± 0.0008 h−1), respectively. But the value of the remaining three feeding treatments in sequence from high to low was S, O1 and M2. Thus,
15N showed a different pattern from
13C in the three treatments: S, O1 and M2.
Table 7. Isotopic turnover modeling parameters estimated of the five different D. magna-algae feeding treatments.
Figure 3. Changes in 13C and
15N values of D. magna fed by sole S. obliquus (S, as control), sole M. granulata (M1), M. granulata mixed with S. obliquus (M2), sole Oscillatoria sp. (O1) and Oscillatoria sp. mixed with S. obliquus (O2) during the 96-h experiment. Each symbol represents the mean value in the D. magna-algae feeding treatment, with the error bar representing the standard deviation.
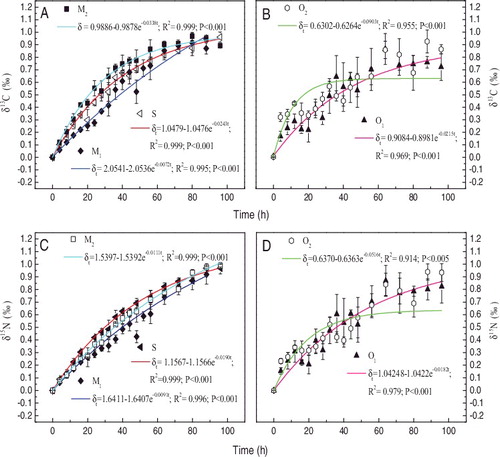
During the 96 h experimental feeding treatments, most D. magna completed their isotopic turnover. According to EquationEquation (4)(4) , estimated half times of carbon and nitrogen in D. magna ranged from 7.679 to 95.74 h () and the differences were attributed to diet type. The diet of M. granulata alone (M1) yielded the longest carbon and nitrogen half times in tissue (95.74 and 75.88 h, respectively), while diets consisting of higher nutrient levels, such as treatment S (S. obliquus alone) elicited shorter half times (28.53 and 36.40 h, respectively). In addition, mixed diet treatments containing unlabeled S. obliquus had relatively fast carbon and nitrogen half times: M2, 20.54 and 62.30 h, respectively; O2, 7.679 and 13.44 h, respectively.
Discussion
Although some previous studies have mentioned several kinds of filamentous algae species being grazed by Daphnia (King & Shiel Citation1993; Farzaneh et al. Citation2013; Sikora & Dawidowicz Citation2014), few studies have examined M. granulata and Oscillatoria sp. as food sources for D. magna.
The main objective of this study was to evaluate the different growth and metabolic rates of D. magna, when they were fed by filamentous algae. A laboratory D. magna-algae feeding experiment with a period of 96 h was performed to illuminate the different carbon and nitrogen isotopic turnover rates in D. magna which fed on filamentous algae alone or in mixtures. This research demonstrated that D. magna does indeed have a certain feeding rate on filamentous algae such as M. granulata and Oscillatoria sp., supporting the view that daphnids can grow and reproduce on a diet dominated by filamentous cyanobacteria (Repka Citation1998). Furthermore, the treatment groups which contained a small quantity of good quality food (S. obliquus) showed higher carbon and nitrogen isotopic turnover rates driven by both growth and metabolism () relatively, demonstrating that the poor nutritional value of filamentous algae could be completely overcome by supplementation (Bednarska et al. Citation2014).
Filamentous cyanobacteria have been reported to have many negative effects on Daphnia spp., including their lower nutritional value or poor manageability (Bednarska et al. Citation2014; Brzeziński Citation2015). Brzeziński (Citation2015) demonstrated that Daphnia hyaline × galeata hybrids were, on average, superior to parental species in the absence of filaments, but in the presence of filaments, one of the parental species (D. hyalina) was superior. Additionally, It was also reported that most herbivorous zooplankton (such as Bosmina longirostris) were capable of consuming some filamentous algae (e.g. the diatom Melosira granulata angustissima) at rates similar to or higher than those of unicellular algae (Fulton Citation1988). Only the feeding of Diaphanosoma brachyurum and Moina micrura seemed to be primarily limited by the filamentous morphology (Fulton Citation1988). Furthermore, some observations by King and Shiel (Citation1993) showed that D. carinata would feed on M. granulata too. Nevertheless, the combination of siliceous covering and long filamentous form (up to ∼200 μm long) suggested that M. granulata might be a difficult food item for D. carinata to handle and that interference with feeding might occur when the diatom was present in high concentrations.
The performance of D. magna in the different experimental treatment groups varied from best to poorest in the following order: Oscillatoria sp. mixed with S. obliquus (O2), S. obliquus (S) alone, Oscillatoria sp. (O1) alone, M. granulata mixed with S. obliquus (M2) and M. granulata (M1) alone. The results from O1 and M2 were very similar. Thus, the performance of D. magna was most negatively affected by M. granulata alone (M1), perhaps because the alga is harder to handle and ingest due to its physiological characters (Schwartz & Ballinger Citation1980). In the M. granulate alone treatment, half time () increased to 95.74 h, which was three times the control treatment, S (around 28 h). In addition, the nutritive value of Oscillatoria sp. often appeared to be higher than M. granulata. Therefore, in the Oscillatoria sp. plus S. obliquus (O2) treatment group, the highest isotopic turnover rates and the lowest half time (
) was observed. Thus, the negative effects caused by the poor manageability of Oscillatoria sp. could be completely overcome by supplementation with the common good quality food (S. obliquus). Nevertheless, the M. gralulata mixed with S. obliquus (M2) treatment had turnover rates and half time (
) similar to the Oscillatoria sp. alone (O1) treatment. Here for M. granulata, addition of the common good quality food only partially diminished the effects of their poor nutritional quality.
In spite of the lower nutritional value of Melosira and Oscillatoria as food source leading to a negative influence on Daphnia fitness when compared with Scenedesmus, the effect is not general for all clones or life history parameters (Bednarska et al. Citation2014). There always exist better adapted individuals (Bernatowicz & Pijanowska Citation2011), and this phenomenon had been also observed during the daily culture of D. magna. Thus, a higher isotopic turnover rate coupled to growth and metabolic rates could be still achieved after the coexistence of Daphnia and filaments for a period of time, which allowed for some adaptation to occur.
According to our investigations, abundance of short filaments of Oscillatoria sp. (average length: 36 μm or less) were suspended and mixed with a small amount of Melosira spp. in the south small lake (one sub-lake of the West Lake, Hangzhou) in late March 2014. And it has been shown that Daphnia consume food particles not exceeding 50–60 μm in size (Gliwicz Citation1969; Bloem & Vijverberg Citation1984). Moreover, the two species of filamentous algae are often appeared with an abundance of zooplankton such as Daphnia galeata, Bosmina, Bosminopsis deitersi, Sinocalanus and D. magna also appeared with a lower biomass.
This study did not measure Daphnia feeding on algal detritus and the mixture of different filamentous algae together labeled by different stable isotopes, respectively, or set up an in situ experiment. Thus, the long-term effects of growth and metabolic turnover rates in filamentous algae or the combination of some in situ dominant planktonic animal species (D. galeata, B. longirostris, Sinocalanus dorrii, etc.) remained to be evaluated in the future.
Conclusion
This study has affirmed that D. magna can utilize filamentous algae M. granulata and Oscillatoria sp. as food sources for their growth and metabolism. Furthermore, a significantly higher isotopic turnover rate appeared when a small percentage of S. obliquus was added, and the mixture of a few S. obliquus added could even increase the fitness of animals. Thus, a certain concentration of cladoceran such as Daphnia may have the potential ability to decrease populations of Oscillatoria mixed with Melosira, when combining with some other in situ dominant planktonic animal species.
Acknowledgments
The authors thank Tianli Li for isolation and laboratory incubation technical guidance on filamentous algae, Xianfen Xiang is thanked for culturing experience shared of D. magna, and Ming Yang is acknowledged for sample collection assistant during the experimental period.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Hong Fu
Hong Fu, majoring in environmental science, is a graduate student of Institute of Hydrobiology, Chinese Academy of Sciences, the University of Chinese Academy of Science.
Jun Xu
Jun Xu, focusing on the researches about trophic interactions in food webs, is a researcher and master tutor in Institute of Hydrobiology, Chinese Academy of Sciences.
Enrong Xiao
Enrong Xiao, focusing on the researches about ecological restoration for water bodies, is a researcher and master's supervisor in Institute of Hydrobiology, Chinese Academy of Sciences.
Feng He
Feng He, focusing on the field of scientific research and engineering application of constructed wetlands and water restoration, is a professor and doctoral supervisor in Institute of Hydrobiology, Chinese Academy of Sciences.
Peng Xu
Peng Xu, majoring in environmental science, is a doctoral candidate of Institute of Hydrobiology, Chinese Academy of Sciences, the University of Chinese Academy of Science.
Qiaohong Zhou
Qiaohong Zhou, focusing on the interactions of environmental microbiology and contaminated waters, is a researcher and master's supervisor in Institute of Hydrobiology, Chinese Academy of Sciences.
Zhenbin Wu
Zhenbin Wu, focusing on the field of scientific research and engineering application of constructed wetlands and water restoration, is a professor and doctoral supervisor in Institute of Hydrobiology, Chinese Academy of Sciences.
References
- Bednarska AB, Pietrzak B, Pijanowska J. 2014. Effect of poor manageability and low nutritional value of cyanobacteria on Daphnia magna life history performance. J Plankton Res. 0:1–10.
- Bernatowicz P, Pijanowska J. 2011. Daphnia response to biotic stress is modified by PCBs. Ecotoxicol Environ Saf. 74:711–718.
- Bloem J, Vijverberg J. 1984. Some observations on the diet and food selection of Daphnia hyalina (Cladocera) in an eutrophic lake. Hydrobiol Bull. 18:39–45.
- Brzeziński T. 2015. Filamentous cyanobacteria alter the relative fitness in a Daphnia hybrid species complex. Freshwater Biol. 60:101–110.
- Burns CW, Forsyth DJ, Haney JF, James MR, Lampert W, Pridmore RD. 1989. Coexistence and exclusion of zooplankton by Anabaena rninutissima var. attenuata in Lake Rotongaio. Archiv fuer Hydrobiologie Ergebnisse Limnologie. 32:63–82.
- Carleton SA, Kelly L, Anderson-Sprecher R, Martı´nez RC. 2008. Should we use one-, or multi-compartment models to describe 13C incorporation into animal tissues? Rapid Commun Mass Spectrom. 22:3008–3014.
- Carleton SA, Rio CM. 2010. Growth and catabolism in isotopic incorporation: a new formulation and experimental data. Funct Ecol. 24:805–812.
- Danielsdottir MG, Brett MT, Arhonditsis GB. 2007. Phytoplankton food quality control of planktonic food web processes. Hydrobiologia. 589:29–41.
- DeBernardi R, Giussani G. 1990. Are blue-green algae a suitable food for zooplankton? An overview. Hydrobiologia. 200/201:29–41.
- Edwards U, Rogall T, Blöcker H, Emde M, Böttger EC. 1989. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 17:7843–7853.
- Epp GT. 1996. Grazing on filamentous bacteria by Daphnia pulicaria. Limnol Oceanogr. 41:560–567.
- Farzaneh H, Fatemeh MA, Anita K. 2013. The influence of Lyngbya sp. and Oscillatoria Sp2 isolated from paddy fields in Mazandaran Province, Iran by bioassay. World Appl Sci J. 24:240–246.
- Freese HM, Martin-Creuzburg D. 2013. Food quality of mixed bacteria-algae diets for Daphnia magna. Hydrobiologia. 715:63–76.
- Fulton RS. 1988. Grazing on filamentous algae by herbivorous zooplankton. Freshwater Biol. 20:263–271.
- Gamboa-Delgado J, Castañeda-Solís JD, Nieto-López MG, Villarreal-Cavazos D, Cruz-Suárez LE. 2014. Isotopic evaluation of the nutritional contribution of poultry by-product meal and fish meal to the growth of pacific white shrimp, Litopenaeus vannamei. J World Aquaculture Soc. 45:430–438.
- Gamboa-Delgado J, Peña-Rodríguez A, Cruz-Suárez LE, Ricque D. 2011. Assessment of nutrient allocation and metabolic turnover rate in Pacific white shrimp Litopenaeus vannamei co-fed live macroalgae Ulvaclathrata and inert feed: dual stable isotope analysis. J Shellfish Res. 30:969–978.
- Ger KA, Hansson LA, Lürling M. 2014. Understanding cyanobacteria-zooplankton interactions in a more eutrophic world. Freshwater Biol. 59:1783–1798.
- Gliwicz ZM. 1969. Studies of the feeding of pelagic zooplankton in lakes with varying trophy. PaĹ, stwoweWydawnictwo Naukowe. OddziaĹ. 17:663–708.
- Gliwicz WR, Lampert W. 1990. Food thresholds in Daphnia species in the absence and presence of blue-green filaments. Ecology. 71:691–702.
- Gulati RD, Bronkhorst M, Donk EV. 2001. Feeding in Daphnia galeata on Oscillatoria limnetica and on detritus derived from it. J Plankton Res. 23:705–718.
- Haney JF. 1987. Field studies on zooplankton–cyanobacteria interactions. NZ J Mar Freshwater Res. 21:467–475.
- Heady WN, Moore JW. 2013. Tissue turnover and stable isotope clocks to quantify resource shifts in anadromous rainbow trout. Oecologia. 172:21–34.
- Hesslein RH, Hallard KA, Ramlal P. 1993. Replacement of sulfur, carbon, and nitrogen in tissue of growing broad whitefish (Coregonusnasus) in response to a change in diet traced by δ34S, δ13C and δ15N. Can J Fish Aquat Sci. 50:2071–2076.
- Jansson M, Persson L, De Roos AM, Jones RI, Tranvik LJ. 2007. Terrestrial carbon and intraspecific size-variation shape lake ecosystems. Trends Ecol Evol. 22:316–322.
- Jin ZF, Chen LX, Li FL, Pan ZY, Jin MT. 2015. Effects of water transfer on water quality and estimation of the pollutant fluxes from different sources into West Lake, Hangzhou City, China. Environ Earth Sci. 73:1091–1101.
- Kâ S, Mendoza-Vera JM, Champalbert G, Pagano M, Bouvy M, N'Gom-Ka R. 2012. Can tropical freshwater zooplankton graze efficiently on cyanobacteria? Hydrobiologia. 679:119–138.
- King CR, Shiel RJ. 1993. Functional response to Daphnia carinata King when feeding on the Filamentous Diatom Melosira granulata. Freshwater Res. 44:761–768.
- Kosten S, Huszar VLM, Bécares E, Costa LS, Donk E, Hansson LA, Jeppesen E, Kruk C, Lacerot G, Mazzeo N et al., 2012. Warmer climates boost cyanobacterial dominance in shallow lakes. Glob Change Biol. 18:118–126.
- Lampert W. 1987. Laboratory studies on zooplankton–cyanobacteria interactions. NZ J Mar Freshwater Res. 21:483–490.
- Lampert W. 2011. Daphnia: development of a model organism in ecology and evolution. Oldendorf/Luhe: International Ecology Institute.
- Lepère C, Wilmotte A, Meyer B. 2000. Molecular diversity of Microcystis strains (Cyanophyceae, Chroococcales) based on 16S rDNA sequences. Syst Geogr Plants. 70:275–283.
- Liu Y, Xu Y, Xiao P, Pan Q, Yu G, Li R. 2014. Genetic analysis on Dolichospermum (Cyanobacteria; sensu Anabaena) populations based on the culture-independent clone libraries revealed the dominant genotypes existing in Lake Taihu, China. Harmful Algae. 31:76–81.
- MacAvoy SE, Arneson LS, Bassett E. 2006. Correlation of metabolism with tissue carbon and nitrogen turnover rate in small mammals. Oecologia. 150:190–201.
- Meijer ML. 2000. Biomanipulation in the Netherlands: 15 years of experience [dissertation]. Wageningen (The Netherlands): Wageningen Agricultural University.
- Paerl HW, Huisman J. 2009. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environ Microbiol Rep. 1:27–37.
- Porter KG, Orcutt JD. 1980. Nutritional adequacy, manageability, and toxicity as factors that determine the food quality of green and blue-green algae for Daphnia. In: Evolution and ecology of zooplankton communities. Hanover, New Hampshire: p. 268–281.
- Repka S. 1998. Effects of food type on the life history of Daphnia clones from lakes differing in trophic state. Ⅱ. Daphnia cucullata feeding on mixed diets. Freshwater Biol. 38:685–692.
- Rojas-Casas MG, Nieto-LópezLucía MG, Cruz-Suárez E. 2013. Simultaneous estimation of the nutritional contribution of fish meal, soy protein isolate and corn gluten to the growth of Pacific white shrimp (Litopenaeusvannamei) using dual stable isotope analysis. Aquaculture. 380–383:33–40.
- Sanni LA, Kaski O, Salonen K, Pulkkinen K. 2013. Responses of algae, bacteria, Daphnia and natural parasite fauna of Daphnia to nutrient enrichment in mesocosms. Hydrobiologia. 715:5–18.
- Sarnelle O. 2007. Initial conditions mediate the interaction between Daphnia and bloom-forming cyanobacteria. Limnol Oceanogr. 52:2120–2127.
- Schoenberg SA, Carlson RE. 1984. Direct and indirect effects of zooplankton grazing on phytoplankton in a hypereutrophic lake. Oikos. 42:291–302.
- Schwartz SS, Ballinger RE. 1980. Variations in life history characteristics of Daphnia pulex fed different algal species. Oecologia. 44:181–184.
- Sedgwick P. 2012. Confidence intervals and statistical significance: rules of thumb. BMJ. 345:e4960.
- Sikora A, Dawidowicz P. 2014. Do the presence of filamentous cyanobacteria and an elevated temperature favor small-bodied Daphnia in interspecific competitive interactions? Fundam Appl Limnol/Arch Hydrobiol. 185(3–4):307–314.
- Smutná M, Babica P, Jarque S et al., 2014. Acute, chronic and reproductive toxicity of complex cyanobacterial blooms in Daphnia magna and the role of microcystins. Toxicon. 79:11–18.
- Sterner RW, Hessen DO. 1994. Algal nutrient limitation and the nutrition of aquatic herbivores. Annu Rev Ecol Syst. 25:1–29.
- Strathman RR. 1967. Estimating the organic carbon content of phytoplankton from cell volume or plasma volume. Limnol Oceanogr. 12:411–418.
- Taipale S, Kankaala P, Tiirola M, Jonse RI. 2008. Whole-lake dissolved inorganic 13C additions reveal seasonal shifts in zooplankton diet. Ecology. 89:463–474.
- Vanni MJ, Lampert W. 1992. Food quality effects on life history traits and fitness in the generalist herbivore Daphnia. Oecologia. 92:48–57.
- Vaz MGMV, Genuário DB, Andreote APD, Malone CFS, Anna CLS, Barbiero L, Fiore ML. 2015. Pantanalinema gen. nov. and Alkalinemagen. nov.: novel pseudanabaenacean genera (Cyanobacteria) isolated from saline–alkaline lakes. Int J Syst Evol Microbiol. 65:298–308.
- Walsh BM, O'Neil JM. 2014. Zooplankton community composition and copepod grazing on the West Florida Shelf in relation to blooms of Kareniabrevis. Harmful Algae. 38:63–72.
- Woodland RJ, Rodríguez MA, Magnan P, Glémet H, Cabana G. 2012. Incorporating temporally dynamic baselines in isotopic mixing models. Ecology. 93:131–144.
- Xia B, Gao QF, Dong SL, Wang F. 2013. Carbon stable isotope turnover and fractionation in grass carp Ctenopharyngodonidella tissues. Aquat Biol. 19:207–216.
- Xu J, Cao T, Zhang M, Li ZQ, Zhang M, Ni L, Xie P. 2011. Isotopic turnover of a submersed macrophyte following transplant: the roles of growth and metabolism in eutrophic conditions. Rapid Commun Mass Spectrom. 25:3267–3273.
- Xu J, Zhang M, Xie P. 2007. Stable carbon isotope variations in surface bloom scum and subsurface seston among shallow eutrophic lakes. Harmful Algae. 6:679–685.
- Yang Q, Xie P, Shen H, Xu J, Wang PL, Zhang B. 2012. A novel flushing strategy for diatom bloom prevention in the lower-middle Hanjiang River. Water Res. 46:2525–2534.
- Zhang X, Hu HY, Warming TP, Christoffersen KS. 2011. Life history response of Daphnia magna to a mixotrophic golden alga, Poterioochromonas sp., at different for food levels. Bull Environ Contam Toxicol. 87:117–123.