ABSTRACT
The growth model of Pistia stratiotes L. (Pistia-model) was verified using two independent sets of published field data (07° 26' N, 03° 53' E Nigeria; 27° 30' N, 80° 30' W USA) to answer the following question: Is the Pistia-model, originally designed for P. stratiotes under natural conditions in Egypt, suitable to simulate the growth of P. stratiotes in tropical and subtropical regions? The Pistia-model simulates the growth dynamics and biomass production of well-established, monospecific stands of P. stratiotes based on fixed carbon gain and loss using first-order differential equations. General trends for shoots biomass, such as the slow initial growth rate followed by a high growth rate, the peak biomass, and the decline of biomass due to senescence, were successfully reproduced by the Pistia-model. Many characteristics typical for the roots biomass, such as the increase in the roots biomass during the early growing season because of the translocation of materials from shoots, and the reduction of roots biomass during the later period of the season, were also reproduced. The level of agreement between the simulated values and actual field data indicated that the Pistia-model can simulate the biomass of P. stratiotes over a wide range of latitudes.
1. Introduction
Water lettuce (Pistia stratiotes L.) is a perennial free-floating aquatic macrophyte of the family Araceae (Eid et al. Citation2016). It has a worldwide distribution in tropical and sub-tropical waters, and is absent only in Antarctica (Parsons & Cuthbertson Citation2001). Holm et al. (Citation1977) described this plant ‘as one of the most widely distributed of all the hydrophytes’ occurring in most of the warmer regions of the world. P. stratiotes is one among the world's worst weeds (Holm et al. Citation1977) and it was considered as invasive species (Yang et al. Citation2014). P. stratiotes has adverse effects on biodiversity and the environment, due to the ability of developing dense mats, impeding fishing and boat transport, as well as constituting a health hazard by sheltering disease carrying insects and snails (Abbasi et al. Citation1991; Mbati & Neuenschwander Citation2005; Yang et al. Citation2014). On the other hand, P. stratiotes has been shown to have great potential for the management of water quality, due to its capacity for the accumulation of heavy metals from wastewater (Srivastava et al. Citation2008; Galal & Farahat Citation2015).
A simulation model for growth dynamics and biomass productions of P. stratiotes has been developed and called Pistia-model (Eid et al. Citation2016). This model is based on fixed carbon gain and loss (photosynthesis, respiration, mortality, and translocation of materials from shoots to roots) using first-order differential equations. Pistia-model distinguishes two state variables, shoots and roots, and it is driven by solar radiation and air temperature and depends on initial shoots and roots biomass and the nutrient (TN, total nitrogen and TP, total phosphorus) status of the growing medium. In the present study, the growth model of P. stratiotes (Pistia-model) was verified using two independent sets of published field data (07° 26′ N, 03° 53′ E Nigeria; 27° 30′ N, 80° 30′ W USA) to answer the following question: Is the Pistia-model, originally designed for P. stratiotes under natural conditions in Egypt, suitable to simulate the growth of P. stratiotes in tropical and subtropical regions?
2. Materials and methods
2.1. Outline of the Pistia-model
The Pistia-model is a dynamic numerical model developed by Eid et al. (Citation2016) to simulate the growth dynamics and biomass production of P. stratiotes under natural conditions in Egypt based on fixed carbon gain and loss using first-order differential equations. The biomass of P. stratiotes (g DM m−2) was divided into two fractions (shoots and roots) which were considered as the state variables. The biomass of each fraction was reproduced by the Pistia-model by integrating over time the net growth of the plant as a function of photosynthesis, respiration, mortality, and translocation. Equations, process descriptions, and associated parameters are listed in Appendices 1 and 2. The Pistia-model simulates the seasonal variation of the shoot and root biomasses (g DM m−2) and calculates the gross production, total losses (total metabolic losses due to respiration and mortality), translocation to the roots (translocation of photosynthetic assimilates from shoots to the roots), and net production (difference between gross production and total losses). In the Pistia-model, solar radiation, air temperature, TN, TP, and the initial biomass of the shoots and roots were considered forcing functions. The Pistia-model is written in FORTRAN and consists of a main program and three subroutines with one-day time steps used in computation.
2.2. Input data subroutine
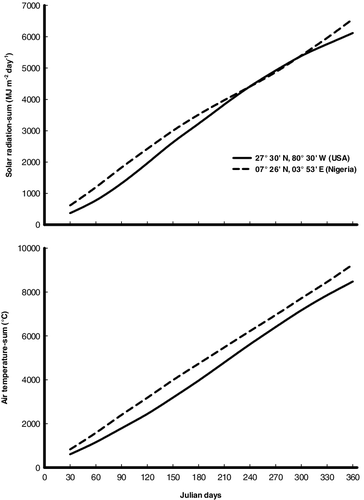
This subroutine allows the user to interact with the Pistia-model to establish the simulation conditions. For the simulation, the input data were the daily solar radiation (MJ m−2 day−1) (), the daily averaged air temperature (°C) (), the initial biomass of shoots and roots (g DM m−2), and the TN (mg L−1) and TP (mg L−1) of water supporting P. stratiotes populations (). This subroutine converts daily solar radiation to photosynthetic active radiation (PAR) using a conversion factor of 0.45 (Asaeda & Karunaratne Citation2000). In the Pistia-model, air temperature influenced the rates of many processes, but PAR only drove photosynthesis (Eid et al. Citation2016).
Figure 1. Meteorological characteristics (NASA-POWER Citation2016) driving the simulations at different latitudes: 07° 26′ N, 03° 53′ E (Nigeria) and 27° 30′ N, 80° 30′ W (USA). (A) Daily solar radiation (MJ m−2 day−1), (B) daily average air temperature (°C).
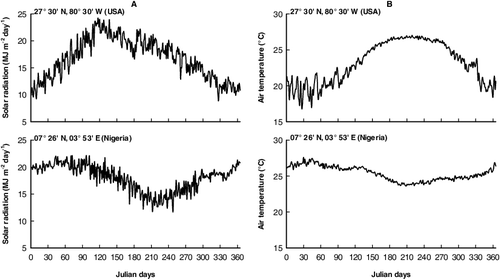
Table 1. Characteristics of sampling sites supporting Pistia stratiotes populations at different latitudes.
2.3. Shoots subroutine
This subroutine addresses the daily change in shoots biomass and the interactions with the roots. Photosynthesis is the source supporting shoots growth.
2.4. Roots subroutine
This subroutine calculates the daily change in roots biomass. The translocation of photosynthesized materials is the source supporting roots growth.
2.5. Model implementation
The main program first calls the input data subroutine to establish the initial conditions of the simulation run. Once the model is initialized, the program, on each simulation day, reads the input data and calls the shoots subroutine and roots subroutine to simulate P. stratiotes growth. The growth equations for shoots and roots were solved simultaneously using the fourth-order Runge–Kutta method (Butcher Citation2008). The time scale was in Julian days and at the end of each simulation day, the program outputs the daily results.
2.6. Description of the two field studies
2.6.1. Awba Lake, Nigeria (07° 26′ N, 03° 53′ E)
The study was conducted in the period from February 1979 to May 1979 at the eastern, center and western tip of Awba Lake, Nigeria (Sharma & Sridhar Citation1981). It is a man-made lake and has a volume of 227 million L of water. The whole lake was covered with a carpet of P. stratiotes which in the shallow areas, especially near the banks, occurs in association with Nymphaea species. The plant samples were collected from three different points in the lake at weekly intervals over a period of four months, covering two seasons peculiar to Nigeria, one the dry season and the other the wet season. The individual plant method of Newbould (Citation1967) was used to estimate the biomass and productivity of P. stratiotes. The collected plant samples were counted and dried at 105 °C for 24 h and the dry weights were determined. Because meteorological data for the year 1979 were not available, 10-year data from 1984 to 1993 (NASA-POWER Citation2016) for daily solar radiation and daily air temperature were averaged to estimate the appropriate values for 1979.
2.6.2. Florida, USA (27° 30′ N, 80° 30′ W)
The field study was carried out in the period from November 1985 to April 1987 at an unused aquaculture pond and a road side drainage ditch in Florida, (USA) by DeWald and Lounibos (Citation1990) to examine the seasonal trends in leaf area, leaf and plant densities, leaf and root biomasses, and flowering. The methods used for the plant sampling were modifications of those described by Hall and Okali (Citation1974) for P. stratiotes in Lake Volta (Ghana). At 30 days intervals, five random quadrats were collected from each site using a stainless steel sampling tool 30 × 30 × 70 cm, with serrated teeth around the perimeter of the bottom to penetrate the P. stratiotes mat. The number of plants was counted as well as the number of leaves and flowers. The surface area of leaves in each sample was measured using a Licor leaf area-meter. Dry weights of shoots and roots were measured after oven drying at 80 °C for 48 h. The daily means of air temperature and solar radiation for Florida sites were obtained from the website of NASA-POWER (Citation2016).
2.7. Verification of the Pistia-model
The Pistia-model was verified using two independent sets of published field data: 07° 26′ N, 03° 53′ E (Nigeria) and 27° 30′ N, 80° 30′ W (USA), for which field data were available. In the present study, the biomass and leaf area index (LAI) data collected from the two sites in Florida (aquaculture pond and a road side drainage ditch) were averaged and the means were used in the verification. The daily means of air temperature and solar radiation were obtained for these two latitudes from the website of NASA-POWER (Citation2016) (), and TN and TP were obtained from literature (). Because no initial shoots or roots biomass was reported, a series of simulations were conducted using different initial shoots and roots biomass values, and the value that provided the best fit of the simulated biomass to the actual biomass (from the two published field studies) was then used (). The deviations of the simulated results from the observed field data were assessed in three ways: standard deviation as a percent, regression of observed (y-axis) vs. simulated data (x-axis) and Wilcoxon signed-rank test (Pineiro et al. Citation2008; Eid et al. Citation2012). All the statistical analyses were carried out using Statistica 7.1 (Statsoft Citation2007).
3. The simulation results
In Awba Lake, the shoots started to grow at the beginning of February (142.6 g DM m−2), reached its maximum biomass of 371.8 g DM m−2 in mid of March, and then decreased and reached the lowest value at the end of May (195.6 g DM m−2) when the plants moved into the senescence stage (). The roots biomass increased from 26.4 g DM m−2 at the beginning of February to 67.0 g DM m−2 in mid-March and decreased to a minimum of 14.1 g DM m−2 in early May. In Florida, the shoots started to grow at the beginning of March (19.9 g DM m−2), reached its maximum biomass of 335.9 g DM m−2 at the beginning of October, and then decreased and reached the lowest value in next February (200.4 g DM m−2) when the plants moved into the senescence stage (). The roots biomass increased from 33.1 g DM m−2 at the beginning of March to 62.0 g DM m−2 at the beginning of September and decreased to a minimum of 39.5 g DM m−2 in next February. The maximum shoots biomass was 10.7% higher in Awba Lake compared to Florida site, while maximal biomass of roots was 1.1 times in Awba Lake that in Florida.
Figure 2. Simulated results (lines) and measured data (symbols; data from Sharma & Sridhar Citation1981) of the biomass and leaf area index (LAI) of Pistia stratiotes populations at 07° 26′ N, 03° 53′ E. Total biomass = shoots biomass + roots biomass.
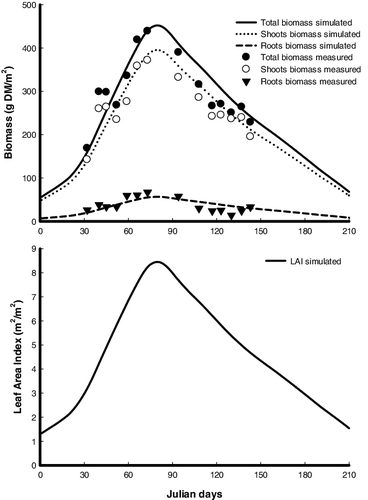
Figure 3. Simulated results (lines) and measured data (symbols; data from DeWald & Lounibos Citation1990) of the biomass and leaf area index (LAI) of Pistia stratiotes populations at 27° 30′ N, 80° 30′ W. Total biomass = shoots biomass + roots biomass.
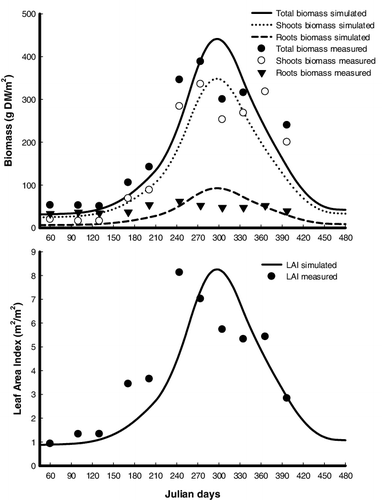
The capability of the Pistia-model to predict the seasonal variation of shoots and roots biomass as well as LAI of P. stratiotes was verified using published field data from two different populations of P. stratiotes growing in Awba Lake (Nigeria) and Florida (USA). General trends for the shoots biomass and LAI, including a slow initial absolute growth rate followed by a high absolute growth rate, peak of biomass and LAI, end of growth, and decline of biomass and LAI, were successfully reproduced by the Pistia-model ( and ). Many characteristics typical to the roots biomass, such as the increase in the biomass during the early growing season due to the translocation of materials from the shoots and the reduction of biomass during the later period of the season, were also reproduced. The level of agreement between the simulated values and the actual field data indicated that although the simulated results were a little different from the observations, the Pistia-model is capable of simulating seasonal changes in biomass of shoots and roots and LAI for P. stratiotes populations in tropical (Awba Lake) and subtropical (Florida) regions ( and ). The results of the Wilcoxon signed-rank test indicated that there was no significant difference between the estimated and actual means of the biomass and LAI (no field data for comparison existed for Awba Lake) ().
Table 2. The deviations of simulated results reproduced by Pistia-model from observed field data at different latitudes.
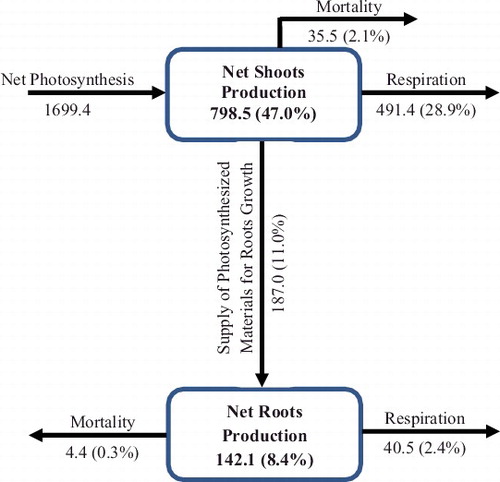
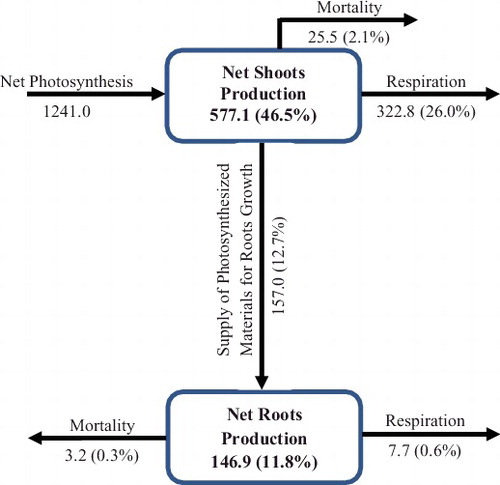
The production and seasonal fluxes of dry matter of P. stratiotes in Awba Lake were estimated using the modeled quantities (). The annual gross production and net shoots production was 1699.4 and 798.5 g DM m−2, respectively. The supply of photosynthesized materials for roots growth showed 11.0% to the gross production. The respiration of the shoots and roots consumes a considerable amount of net photosynthetic materials 28.9% and 2.4%, respectively. The mortality of the shoots and roots consumes a considerable amount of net photosynthetic materials 2.1% and 0.3%, respectively. The production and seasonal fluxes of dry matter of P. stratiotes in Florida were estimated using the modeled quantities (). The annual gross production and net shoots production were 1241.0 and 577.1 g DM m−2, respectively. The supply of photosynthesized materials for roots growth showed 12.7% to the gross production. The respiration of the shoots and roots consumes a considerable amount of net photosynthetic materials 26.0% and 0.6%, respectively. The mortality of the shoots and roots consumes a considerable amount of net photosynthetic materials 2.1% and 0.3%, respectively.
4. Discussion
The objective of the present study was to verify the Pistia-model (a mathematical model developed to simulate the growth pattern of a well-established, monospecific stand of P. stratiotes under natural conditions) using two independent sets of published field data. The difficulty in finding good quality, suitable data-sets (which present the seasonal growth pattern of both shoots and roots biomass of P. stratiotes) poses a considerable impediment in such verifications. Perdomo et al. (Citation2008) have tried using a cyclic function and double monod equations to determine the growth dynamics of P. stratiotes under natural conditions in temperate climate, where this study depended mainly on empirical relations, and thus its scope for further understanding of the dynamics of growth was limited. According to the authors’ knowledge, the study of Eid et al. (Citation2016) is the first study that have been carried out to analyze the growth dynamics of P. stratiotes using numerical simulation models. A special feature of the Pistia-model verified in the present study is its ability to predict the growth pattern of monospecific stands of P. stratiotes from varying locations. Predicting the growth and production of P. stratiotes is necessary in aquatic habitat management and making such tools commercially available for such purposes is long delayed (Eid et al. Citation2016). Generally, the quantitative estimation of parameters related to plant growth such as translocation of photosynthesized materials between plant organs, respiration, and mortality of plant organs, is difficult by direct measurement. In contrast, the Pistia-model which was tested in this paper can synthesize quantitative information about the physiological processes and thus deliver estimates of ecological and physiological responses that may be hard to measure directly.
Daily average air temperature- and daily solar radiation-sum values in Awba Lake were about 9.2% and 7.3%, respectively, higher than the Florida site (). P. stratiotes in Awba Lake experiences higher temperature and more daily solar radiation throughout the growing season than it does in Florida site. Photosynthetic production and net production were higher in Awba Lake (1699.4 and 940.6 g DM m−2 yr−1, respectively) than in the populations in Florida (1241.0 and 724.0 g DM m−2 yr−1, respectively). The more productive nature of P. stratiotes in Awba Lake seems to be mainly due to greater solar radiation as well as warmer temperatures. Moreover, Awba Lake receives considerable amounts of organic pollution, which promote the growth of P. stratiotes (Sharma & Sridhar Citation1981). However, in Awba Lake, the larger biomass and higher temperature resulted in larger respiration and mortality losses where the respiration and mortality were 60.9% and 39.0% higher for P. stratiotes populations in Awba Lake compared to Florida site. In the present study, shoots respiration consumes a considerable amount of photosynthetic production (26.0%–28.9%). Therefore, respiration of shoots is an unavoidable component when constructing the carbon budget of P. stratiotes. Irrespective of the variation in the climatic conditions, the percentage of annual fluxes remained more or less equal, illustrating the adaptation of this species to different climatic conditions.
Figure 6. Daily solar radiation (MJ m−2 day−1) and daily average air temperature (°C) sums at 07° 26′ N, 03° 53′ E (Nigeria) and 27° 30′ N, 80° 30′ W (USA).
The growth trends of P. stratiotes in Awba Lake and Florida were like that of our previous study on the same species in Egypt (Eid et al. Citation2016). The differences in the specific time of the growing stages between these studies were related to the effects of local environmental factors, such as climate and nutrient conditions. The maximum total biomass of P. stratiotes in Awba Lake (43.9 t DM ha−1) and Florida (38.8 t DM ha−1) is higher than the maximum total biomass recorded in Mediterranean region (35.8 t DM ha−1; Eid et al. Citation2016). This finding is supported by our previous study on Eichhornia crassipes (Eid & Shaltout Citation2017), which indicated that the gross and net production of E. crassipes populations were higher in the tropical and sub-tropical regions than in the Nile Delta (Mediterranean region). Since biomass is often largely determined by specific habitat conditions (Luther Citation1983), the high biomass in the tropical and sub-tropical regions could be due to environmental factors that vary with latitude such as temperature, day length, solar irradiance, and length of growing season (Sand-Jensen Citation1989; Eid et al. Citation2010, Citation2013). Variation may also be partly attributed to biotic factors such as mutualists/facilitators or predators/pathogens. The growth and maximum photosynthesis of P. stratiotes are driven by air temperature and solar radiation (Eid et al. Citation2016) and depend on the nutrient status of the growing medium (Hall & Okali Citation1974). This finding means that the growth and total biomass of P. stratiotes correlate with latitudinal changes in air temperature and solar radiation. A similar finding was reported by Asaeda et al. (Citation2005) and Hai et al. (Citation2006) for the emergent macrophytes Typha angustifolia and Typha latifolia and Eid and Shaltout (Citation2017) for E. crassipes.
5. Conclusion
In conclusion, the Pistia-model was applied to Awba Lake (Nigeria) and Florida (USA). Generally, a good fit was found between simulated and measured biomass and LAI. Pistia-model can be used to assess growth of P. stratiotes under various site specific and climatological conditions and thus used as a predictive tool for assessing the potential growth of P. stratiotes over a wide range of latitudes. It might be useful for practical applications, such as the management of irrigation and drainage canals, and to derive management recommendations for P. stratiotes control in regions where the species is considered invasive by assessing the critical growth stages of this plant.
Acknowledgments
The author thanks two anonymous reviewers for their useful comments on an earlier version. The author extends his appreciation to the Deanship of Scientific Research at King Khalid University for funding this work.
This is to acknowledge any financial interest or benefit that has arisen from the direct applications of my research.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
Notes on contributors
Ebrahem M. Eid
Ebrahem M. Eid is an associate professor of Plant Ecology, Botany Department, Faculty of Science, Kafr El-Sheikh University, Kafr El-Sheikh, Egypt. He obtained his PhD degree in plant ecology from Faculty of Science, Tanta University, Tanta, Egypt (2009). His fields of research experience include plant population ecology, aquatic plant biology, ecological modeling, water pollution, nutrient budget, and carbon sequestration. He has attended many training courses and scholarships in Egypt, Germany, the Netherlands, Hungary, Finland, Estonia, Japan, and Czech Republic; as well as several national and international symposia and conferences in Egypt and abroad. He published 34 publications in national and international specialized journals, covering many aspects of plant ecology.
References
- Abbasi SA, Nipaney PC, Panholzer MB. 1991. Biogas production from the aquatic weed Pistia (Pistia stratiotes). Bioresour Technol. 37:211–214.
- Asaeda T, Hai DN, Manatunge J, Williams D, Roberts J. 2005. Latitudinal characteristics of below and above-ground biomass of Typha: a modeling approach. Ann Bot. 96:299–312.
- Asaeda T, Karunaratne S. 2000. Dynamic modeling of the growth of Phragmites australis: model description. Aquat Bot. 67:301–318.
- Butcher JC. 2008. Numerical methods for ordinary differential equations. West Sussex: Wiley.
- DeWald LB, Lounibos LP. 1990. Seasonal growth of Pistia stratiotes L. in South Florida. Aquat Bot. 36:263–275.
- Eid EM, Galal TM, Dakhil MA, Hassan LM. 2016. Modeling the growth dynamics of Pistia stratiotes L. populations along the water courses of south Nile Delta, Egypt. Rend Fis Acc Lincei. 27:375–382.
- Eid EM, Shaltout KH. 2017. Growth dynamics of water hyacinth (Eichhornia crassipes): a modelling approach. Rend Fis Acc Lincei. 28:169–181.
- Eid EM, Shaltout KH, Al-Sodany YM, Soetaert K, Jensen K. 2010. Modeling growth, carbon allocation and nutrient budget of Phragmites australis in Lake Burullus, Egypt. Wetlands. 30:240–251.
- Eid EM, Shaltout KH, Asaeda T. 2012. Modeling growth dynamics of Typha domingensis (Pers.) Poir. ex Steud. in Lake Burullus, Egypt. Ecol Mod. 243:63–72.
- Eid EM, Shaltout KH, Asaeda T. 2013. Growth dynamics of Potamogeton pectinatus L. in Lake Burullus, Egypt: a modeling approach. Afr J Ecol. 52:414–426.
- Galal TM, Farahat EA. 2015. The invasive macrophyte Pistia stratiotes L. as a bioindicator for water pollution in Lake Mariut, Egypt. Environ Monit Assess. 187:701–710.
- Gamage NPD, Asaeda T. 2004. Population dynamics of water hyacinth (Eichhornia crassipes). Rep Res Educ Ctr Inlandwat Environ. 2:35–40.
- Hai DN, Asaeda T, Manatunge J. 2006. Latitudinal effect on the growth dynamics of harvested stands of Typha: a modeling aaproach. Estuarine Coast Shelf Sci. 70:613–620.
- Hall JB, Okali DUU. 1974. Phenology and productivity of Pistia stratiotes L. on the Volta Lake, Ghana. J Appl Ecol. 11:709–725.
- Holm LG, Plucknett DL, Pancho JV, Herberger JP. 1977. The world's worst weeds: distribution and biology. Honolulu (HI): The University Press of Hawaii.
- Hu W, Salomonsen J, Xu F-L, Pu P. 1998. A model for the effects of water hyacinth on water quality in an experiment of physic-biological engineering in Lake Taihu, China. Ecol Mod. 107:171–188.
- Jørgensen SE, Jørgensen LA, Nielsen LK, Mejer HF. 1981. Parameter estimation in eutrophication modeling. Ecol Mod. 13:111–129.
- Lorber MN, Mishoe JW, Reddy PR. 1984. Modeling and analysis of waterhyacinth biomass. Ecol Mod. 24:61–77.
- Luther H. 1983. On life forms, and above-ground and underground biomass of aquatic macrophytes. Acta Bot Fenn. 123:1–23.
- Mbati G, Neuenschwander P. 2005. Biological control of three floating water weeds, Eichhornia crassipes, Pistia stratiotes, and Salvinia molesta in the Republic of Congo. BioControl. 50:635–645.
- NASA-POWER. 2016. Climatology resource for agroclimatology. Washington (DC): NASA Prediction of Worldwide Energy Resource. Available from: http://power.larc.nasa.gov/cgi-bin/cgiwrap/solar/agro.cgi
- Newbould PJ. 1967. Methods for estimating the primary production of forests. Oxford: Blackwell.
- Parsons WT, Cuthbertson EG. 2001. Noxious weeds of Australia. Canberra: CSIRO Publishing.
- Perdomo S, Fujita M, Ike M, Tateda M. 2008. Growth dynamics of Pistia stratiotes in temperate climate. In: Vymazal J, editor. Wastewater treatment, plant dynamics and management in constructed and natural wetlands. The Netherlands: Springer Science + Business Media B.V.; p. 277–287.
- Pineiro G, Perelman S, Guerschman JP, Paruelo JM. 2008. How to evaluate models: observed vs. predicted or predicted vs. observed? Ecol Mod. 216:316–322.
- Sale PJM, Orr PT, Shell GS, Erskine DJC. 1985. Photosynthesis and growth rates in Salvinia molesta and Eichhornia crassipes. J Appl Ecol. 22:125–137.
- Sand-Jensen K. 1989. Environmental variables and their effect on photosynthesis of aquatic plant communities. Aquat Bot. 34:5–25.
- Sharma BM, Sridhar MKC. 1981. The productivity of Pistia stratiotes L. in a eutrophic lake. Environ Pollut. 24:277–289.
- Soetaert K, Hoffmann H, Meire P, Starink M, van Oevelen D, van Regenmortel S, Cox T. 2004. Modeling growth and carbon allocation in two reed beds (Phragmites australis) in the Scheldt estuary. Aquat Bot. 79:211–234.
- Srivastava J, Gupta A, Chandra H. 2008. Managing water quality with aquatic macrophytes. Rev Environ Sci Biotechnol. 7:255–266.
- Statsoft. 2007. Statistica version 7.1. Tulsa (OK): Statsoft Inc.
- Tucker CS. 1983. Culture density and productivity of Pistia stratiotes. J Aquat Plant Manage. 21:40–41.
- Yang X, Chen S, Zhang R. 2014. Utilization of two invasive free-floating aquatic plants (Pistia stratiotes and Eichhornia crassipes) as sorbents for oil removal. Environ Sci Pollut Res. 21:781–786.
Appendices
Appendix 1. List of equations used in Pistia-model to simulate the growth of Pistia stratiotes populations at different latitudes
Table
Appendix 2. List of parameters used in Pistia-model to simulate the growth of Pistia stratiotes populations at different latitudes