ABSTRACT
The western sand darter, Ammocrypta clara, and the eastern sand darter, A. pellucida, are sand-dwelling fishes that have undergone range-wide population declines, presumably owing to habitat loss. Habitat use studies have been conducted for the eastern sand darter, but literature on the western sand darter remains sparse. To evaluate substrate selection and preference, western and eastern sand darters were collected from the Elk River, West Virginia, one of the few remaining rivers where both species occur sympatrically. In the laboratory, individuals were given the choice to bury into five equally available and randomly positioned substrates ranging from fine sand to granule gravel (0.12–4.0 mm). The western sand darter selected for coarse and medium sand, while the eastern sand darter was more of a generalist selecting for fine, medium, and coarse sand. Substrate selection was significantly different (p = 0.02) between species in the same environment, where the western sand darter preferred coarser substrate more often compared to the eastern sand darter. Habitat degradation is often a limiting factor for many species of rare freshwater fish, and results from this study suggest that western and eastern sand darters may respond differently to variations in benthic substrate composition.
Introduction
The western sand darter Ammocrypta clara and the eastern sand darter A. pellucida are the only sympatric species of the genus Ammocrypta (Near et al. Citation2000), and the Ohio River drainage is the sole region where both distributions are known to overlap (Cincotta & Welsh Citation2010). Over the years, the number of rivers where each species occurs has declined, presumably in response to degradation of physical stream habitat or water quality (Lachner Citation1956; Kuehne & Barbour Citation1983). Historically, the two species co-occurred in the Wabash (IN), Green (KY), Cumberland (KY), Kentucky (KY), Big Sandy (KY, WV), and Kanawha (WV) river systems (Williams Citation1975; Cincotta & Welsh Citation2010). However, the western sand darter is presumed to be extirpated from the Licking River in the Kentucky River drainage, the Big Sandy River drainage, and portions of the Wabash River drainage (Williams Citation1975; Burr & Warren Citation1986; Simon Citation2006). Several researchers have suggested that siltation of sand habitats has contributed to declines and local extirpations of sand darter populations, and as a result, the western and eastern sand darters have a threatened or critical status throughout most of their ranges (Holm & Mandrak Citation1996; Warren et al. Citation2000; Adams & Burr Citation2004; Grandmaison et al. Citation2004; Driver & Adams Citation2013).
Western and eastern sand darters are psammophiles, or organisms that occupy and thrive in sandy environments and possess a combination of specialized traits to flourish in a habitat of predominantly sand (Schaefer et al. Citation2005; Zuanon et al. Citation2006; Carvalho et al. Citation2014). Both species are slender, elongate, slightly translucent with cryptic pigmentation, and are known for burying in sand substrates (Williams Citation1975). Consequently, siltation of the stream bed may affect the ability of sand darters to bury into sand habitat (Daniels Citation1989; Holm & Mandrak Citation1996; Facey Citation1998). Sand darters are habitat specialists and thus sensitive to habitat alterations, which is why both species are often valued as indicators of ecosystem integrity (Grandmaison et al. Citation2004; Drake et al. Citation2008). Habitat use and sand grain size preference is well documented for the eastern sand darter (Daniels Citation1993; Facey & O'Brien Citation2004; Drake et al. Citation2008; O'Brien & Facey Citation2008; Tessler et al. Citation2012; Dextrase et al. Citation2014), but literature pertaining to the western sand darter is sparse, and little information is available from areas where the two species are sympatric.
Western and eastern sand darters inhabit medium to large rivers with a moderate current, loose sand, and gravel substrates, and spend the majority of the time buried just below the surface of sandy streambeds (Williams Citation1975; Daniels Citation1989). Daniels (Citation1993) regarded the eastern sand darter as one of the most habitat selective fishes of all freshwater species preferring areas of greater than 90% sand. In the field and laboratory setting, eastern sand darters exclusively associated with sand substrates, while water velocity, depth, and distance from the bank had little to no effect on the distribution of individuals (Daniels Citation1993). Laboratory studies that investigated eastern sand darter substrate use reported that individuals generally used medium (0.25–0.5 mm) sized particles (Daniels Citation1993; O'Brien & Facey Citation2008). Furthermore, field surveys revealed that the species was commonly detected in sand comprised of fine to medium (0.12–0.5 mm) particles, while few individuals were detected in areas where the particle size was larger than 1.0 mm (Facey & O'Brien Citation2004; Tessler et al. Citation2012; Dextrase et al. Citation2014). Pflieger (Citation1971) noted that in Missouri the western sand darter avoided strong currents, inhabiting shallow backwater areas, as well as quiet margins of a drainage canal at depths up to 1.5 m. Simon et al. (Citation1992) observed the western sand darter spawning and described the habitat as an area on the downstream side of an island with a slow current over coarse sand.
Although sand grain size appears to be an integral life history component of the western sand darter, little information is available on substrate size preference for this species. Additionally, little research exists documenting habitat selection behavior where both the western and eastern sand darters are sympatric. This study sought to examine western and eastern sand darter benthic habitat preferences in laboratory aquaria to (1) determine substrate size selection for the western sand darter, (2) evaluate substrate selection in an environment where the two species are sympatric, and (3) assess if substrate selection differs significantly between the two species.
Methods
Fish collection and aquaria setup
A total of 20 western sand darters and 20 eastern sand darters were collected from the lower Elk River during October 2015 and transported to the laboratory in an aerated cooler (). The laboratory trials took place over six weeks from October to early December 2015. The western and eastern sand darters mean total length was 52.7 mm (SD 2.30 mm) and 56.9 mm (3.24 mm), respectively. For collection, a straight 1.5 × 3 m seine with 3 mm mesh was used in wadable areas of the river, including upstream and downstream parallel seine hauls and perpendicular hauls pulled into shore (O'Brien & Facey Citation2008; Driver & Adams Citation2013). Sampling for the sand darters occurred during the fall because the Elk River experiences lower flows (i.e. <350 cfs) during this time. In the laboratory, fish were placed initially in a 473 L (183 cm × 46 cm × 56 cm) glass aquaria and allowed to acclimate prior to the start of the substrate selection trials. Water conditions within each tank were maintained by a sequence pump (2.6 L/min) recirculating water from a 379 L sump to the aquaria. Water quality was controlled with carbon filters, bio balls, and freshwater substitutions. The room temperature within the laboratory during late fall remained between 15 and 17 °C. Photoperiod was maintained with wide spectrum fluorescent plant bulbs and an electric timer (12 h light, 12 h dark). The fish were fed frozen bloodworms (chironomids) every other day throughout the experiment. On a day with a substrate trial, the fish were fed after the experiment.
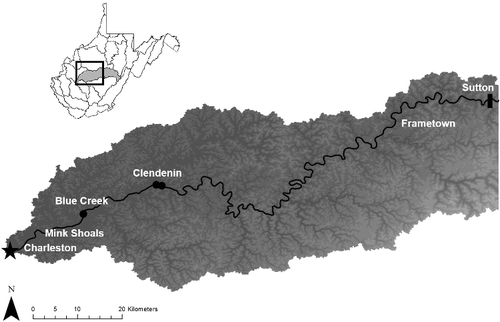
Figure 1. Map of the lower 190 rkm of the Elk River watershed shaded by elevation below the dam (black rectangle) in Sutton, WV, USA. Western sand darters have been detected from Mink Shoals to Clendenin and eastern sand darters have been detected from Mink Shoals to Frametown. The black circles represent the three sand darter collection locations.
Experimental design
The experimental design was similar to other aquaria-based fish burying behavior and habitat selection studies (Daniels Citation1989, Citation1993; Smith et al. Citation2011). The sand darters were allowed to acclimate in the laboratory aquaria for at least seven days prior to the start of the experiment. After the acclimation period, a subset of individuals were placed into the experimental aquarium sections. The purpose was to assess western sand darter habitat selection with and without the presence of the eastern sand darter. Two 473 L (183 cm × 46 cm × 56 cm) glass aquaria were divided in half by a mesh barrier, thus creating a total of four aquarium sections of equal size. For the first experiment, six western sand darters were placed in one aquarium section, and another six western sand darters were placed in a second aquarium section. For the second experiment, three western sand darters and three eastern sand darters were placed in the third aquarium section, and another three western sand darters and three eastern sand darters were placed in the fourth aquarium section. The same individuals were used in each section throughout the 15 trials.
Each aquarium section contained five plastic containers (24.9 × 15.7 × 5.3 cm) filled with approximately 5 cm of substrate: fine sand (0.125–0.25 mm), medium sand (0.25–0.5 mm), coarse sand (0.5–1.0 mm), very coarse sand (1.0–2.0 mm), and granule gravel (2.0–4.0 mm) (Wentworth Citation1922). Sand for this study was collected primarily from the Elk River (90%), as well as a later addition of aquaria sand (CaribSea Super Naturals Aquarium sand; 10%) to supplement the finer sand that was lost in suspension between trials. The sand for the substrate trials was sifted using a Gilson 20.3 cm (8 in) sieve Shaker (115 V/60 Hz) with US Standard brass sieves. Finer substrate was not used since particles smaller than fine sand may not remain settled due to fish activity and current from the water filter (O'Brien & Facey Citation2008).
At the start of each trial, fish were released into an aquarium section and given a choice of five equally available and randomly positioned substrate types. The random placement and the equal proportion of substrate types in a controlled environment allowed us to evaluate benthic habitat selection and preference (e.g. Garshelis Citation2000). A total of 15 trials were conducted with each trial lasting 48 h. At the end of each trial, the substrate containers were capped and transferred individually to a separate container, where the darters were gently removed from the sand, identified to species, and enumerated. We were concerned that an ideal-free distribution effect (i.e. Fretwell & Lucas Citation1969) could prevent six individuals in an aquarium section from using the same substrate container. A pilot study, however, found that 10 individual fish of both species would readily bury in an individual substrate container. In the Elk River, both species are syntopic and have been detected within the same sandbar (Cincotta & Welsh Citation2010). It is also possible that another density-dependent effect occurred, where an individual was attracted to a substrate container because it was being used by one or more individuals. Our study design, however, did not allow for measurement of this type of effect, and no known literature exists suggesting this type of social attraction. Overall, our sample size of fish was low because of difficulty of detecting the western sand darter and the conservation status associated with the western sand darter (WVDNR Citation2015).
Data analysis
To evaluate resource selection, the log-likelihood ratio test was used to determine if sand darter substrate selection was in proportion to its availability (i.e. random or nonrandom) within the experimental aquaria (Manly et al. Citation2002). Data for the western sand darter only aquaria and the combined species aquaria were pooled over the two tanks for each substrate size class and trial. Selection ratios were then calculated following Manly et al. (Citation2002) to evaluate substrate size preference. This method assumes that there is no unique identification of individuals, the proportion of resource categories are known, and a random sample of used resources is taken. The selection ratio for a given group is the proportion of used units in a category over the proportion of available categories (Manly et al. Citation2002). Selection ratio values greater than 1 indicate selection, and values less than 1 indicate avoidance (Manly et al. Citation2002). Bonferroni 95% confidence intervals were calculated for each selection ratio and were considered statistically significant when the interval did not contain the value of 1 (Manly et al. Citation2002). Furthermore, pairwise comparisons with Bonferroni 95% confidence intervals were generated to assess differences between grain size preferences. The substrate category granule gravel was removed from all statistical analyses because neither species utilized this habitat type.
A multinomial logistic regression model was used to compare if substrate size selection differed significantly between the two species of sand darters (Hosmer & Lemeshow Citation2000). Following Smith et al. (Citation2011), the response variable was substrate type and the explanatory variables were species and trial. Deviance statistics were used to determine if substrate selection significantly differed between the western and eastern sand darters. Furthermore, odds ratios were assessed to evaluate the effect of species on substrate selection. Odds are the ratio between the probability of using or not using a substrate type. The category ‘fine sand’ was designated as our reference category for the odds ratio. The reference category is an arbitrary designation and should be one which makes the subsequent inference the simplest or the most meaningful (Rogers & White Citation2007). Wald confidence intervals were estimated for the odds ratios to further examine significant differences between substrate size selections. Wald's confidence intervals that did not contain the value of 1 were statistically significant (Hosmer & Lemeshow Citation2000). The selection ratios, Bonferroni 95% confidence intervals, and pairwise comparisons were calculated using statistical software R (version 3.2.3), and the multi-logit model, odds ratios, and Wald's confidence intervals were generated using SAS (version 9.4).
Results
Western sand darter habitat use
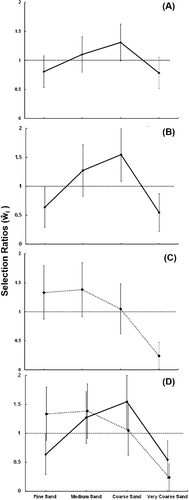
The log-likelihood ratio revealed that substrate selection was not in proportion to its availability (p = 0.04), indicating that sand grain size selection in the aquaria was nonrandom. Across the 15 trials, there were 174 instances of western sand darters found buried in the substrate, while there were 6 instances in which individuals were found above the surface of the sand. Western sand darters primarily buried in coarse (33%) and medium sand (28%), followed by fine (20%) and very coarse (19%) sand. No individuals were detected in granule gravel. Western sand darters selected for coarse (ŵi = 1.31) and medium (ŵi = 1.10) sand, and selected against fine (ŵi = 0.81) and very coarse (ŵi = 0.78) sand (). However, the Bonferroni 95% confidence intervals did not indicate significant selection for or against a specific substrate category (). The substrate selection ratios were further examined with a pairwise comparison with Bonferroni 95% confidence intervals, which revealed that coarse sand was selected with a higher probability compared to very coarse sand (p = 0.05) and coarse sand was selected over fine sand but not significantly (p = 0.08) (). Although the results were not all statistically significant, they suggest that western sand darters exhibited a tendency to select for sand habitat ranging in size from medium to coarse grains (0.25–1.0 mm) ().
Table 1. Substrate selection ratio estimates and the Bonferroni confidence intervals for the western sand darter only aquaria and combined species aquaria. Selection ratio values > 1 indicate selection, while values < 1 indicate avoidance. An asterisk * indicates significant selection or avoidance.
Table 2. Pairwise comparison with Bonferroni confidence intervals for the western sand darter only and combined species aquaria. An asterisk * indicates significant selection. The substrate categories used for the study included fine sand (FS), medium sand (MS), coarse sand (CS), and very coarse sand (VCS).
Figure 2. Western sand darter only experiment (A), western sand darters from the combined aquarium (B), eastern sand darters from the combined aquaria (C), and combined selection results (D). Values above the dashed line at 1 indicate selection and values below indicate avoidance. Bonferroni 95% confidence intervals that do not contain the value of 1 are statistically significant.
Sympatric microhabitat use
In the combined species aquaria, western and eastern sand darters each displayed nonrandom substrate selection (p <0.01). Across the 15 trials, western sand darters were found buried 88 times and eastern sand darters were found buried 84 times. Western sand darters were detected above the substrate just two times, and eastern sand darters were detected above the substrate six times. Western sand darters primarily buried in coarse (39%) and medium (32%) sand, while eastern sand darters primarily buried in medium (34%) and fine (33%) sand. To examine selection or avoidance, selections ratios were calculated for each species. Western sand darters significantly selected for coarse sand, while significantly selecting against fine and very coarse sand (). Eastern sand darters selected for fine, medium, and coarse sand, while significantly selecting against very coarse sand (). The pairwise comparisons revealed that western sand darters had a significantly higher probability of selecting coarse sand over fine and very coarse sand, as well as medium sand over very coarse sand (). In contrast, the eastern sand darter had a significantly higher probability of selecting fine, medium, and coarse sand over very coarse sand ().
Comparing the selection ratios, western and eastern sand darters each demonstrated a strong preference for medium sand. However, eastern sand darters selected for fine sand (ŵi = 1.33), while western sand darters selected against fine sand (ŵi = 0.64; ). The multinomial logistic regression demonstrated that substrate size selection differed significantly between the sand darter species for at least one substrate category (p = 0.02). The effect of sand darter species on substrate selection was further evaluated using odds ratios and Wald conference intervals, with the reference category fine sand. The odds ratios demonstrated a significant effect for species on coarse (odds ratio 3.26, CI [1.31, 8.13]) and very coarse (odds ratio 5.25, CI [1.50, 18.40]) sand. No species effect was found for medium sand (odds ratio 1.97, CI [0.81, 4.83]). Thus, western sand darters compared to eastern sand darters are 5.25 times more likely to select very coarse sand over fine sand and 3.26 times more likely to select coarse sand over fine sand. Overall, the western sand darter had a higher probability of selecting medium, coarse, and very coarse sand over fine sand compared to the eastern sand darter.
Discussion
Our aquaria-based study represents the first evaluation of sand grain size selection and preference for the western sand darter and the first investigation of substrate selection and preference in a region where the two species of sand darters are sympatric. The western and eastern sand darters are habitat specialists, occupying areas that consist of predominantly sand (Simon et al. Citation1992; Daniels Citation1993). Western sand darters preferred coarse to medium sand, while the eastern sand darters substrate preference mirrored previous studies, with the species preferring fine to medium sand grains and coarse sand to a lesser extent (e.g. Daniels Citation1993; Facey & O'Brien Citation2004). In general, western sand darters selected for a narrower range of substrate sizes compared to the eastern sand darter, and habitat use overlap occurred most often in the medium sand category. This study was contingent upon five designated substrate types that were similar to previous sand darter habitat use studies (Daniels Citation1993; O'Brien & Facey Citation2008) and represented a range of benthic habitats both species may encounter (Welsh & Perry Citation1998; Facey & O'Brien Citation2004; Tessler et al. Citation2012). Habitat use in the field could vary compared to the aquaria-based study, but our results demonstrated that each sand darter species exhibited nonrandom selection of substrate types, indicating that a certain benthic habitat was preferred compared to the other available sizes.
The western sand darter was recently discovered in the Elk River, where it was previously misidentified as the eastern sand darter (Cincotta & Welsh Citation2010). After the discovery, eastern sand darter museum specimens collected from 1986 to 2006 were reexamined, and a total of 17 western sand darters were documented in the lower 36 rkm of the Elk River, and all co-occurred with the eastern sand darter. Therefore, the two species are sympatric within the lower 36 rkm of the Elk River, whereas the eastern sand darter can be detected up to 135 rkm from the mouth (; Welsh & Perry Citation1998; Cincotta & Welsh Citation2010). The sand darters differences in substrate selection may influence the western sand darter's limited range in the Elk River. The restricted range of the western sand darter compared to the eastern sand darter in the Elk River is of conservation concern, since the Elk River is the only known location in West Virginia where the western sand darter persists, and represents the southeastern extent for both species (Cincotta & Welsh Citation2010).
Habitat availability below the Sutton Dam (lower 190 rkm) is potentially limited for the western sand darter compared to its more habitat generalist sister species. As a result, the quality of a certain sand habitat likely varies depending upon its position in the river, which is ultimately controlled by fluvial geomorphology, as well as soil development and vegetation (e.g. Vannote et al. Citation1980; Jackson et al. Citation2001; Wang et al. Citation2003). Given that western sand darters are restricted to the lower 36 rkm of the Elk River, this implies that this section of the river has more suitable habitat for the western sand darter (i.e. higher proportions of medium to coarse sand). Thus, larger more contiguous sand patches are presumably more available in the lower reaches of the Elk River, where additional sediment is added to the system from contributing tributaries. Furthermore, the maintenance of these sandy depositional areas is likewise influenced by the presence of the Sutton Dam, which impounds a large 6 km2 reservoir. The dam alters natural flow regimes and changes scouring and depositional patterns (e.g. Baxter Citation1977; Power et al. Citation1996; Poff & Hart Citation2002).
The aquaria-based experiment provided information on habitat use of two species of sand-dwelling darters, data that can be challenging to obtain in the field because of their burying behavior. Preference for larger substrate sizes may indicate that western sand darters are more sensitive to fine sediment deposition compared to eastern sand darters. Habitat selection study results indicate where a species is likely to find a set of conditions within their physiological tolerance (Rice Citation2005). Thus, the siltation of the sand darters preferred habitat is potentially more limiting for the western sand darter and may be a contributing factor to the western sand darters sporadic distribution in the Elk River and the Ohio River drainage. For instance, the eastern sand darter persists in the Licking (Kentucky), Tug (Kentucky, West Virginia), and Wabash (Indiana) rivers, whereas the western sand darter is presumed to be extirpated (Burr & Warren Citation1986; Simon Citation2006). Likewise, in the Wabash River, the eastern sand darter has been reported as increasing in distribution and abundance, while the western sand darter remains undetected or extirpated from the main channel (Simon Citation2006). Furthermore, in Indiana, the western sand darter is a species of special concern, while the eastern sand darter was delisted following a statewide survey of the species (INDNR Citation2004). Similar research could be conducted in other rivers that contain western sand darters, which would further document sand grain size preference across the range of this species.
The types of rivers that western and eastern sand darters occupy (i.e. moderately large with a low gradient) are often located in landscapes that attract urban development, industrialization, and agriculture. Therefore, these species face potential impacts from land use activities that increase the amount of siltation in the watershed; however, with improved land use practices, and other efforts to minimize impacts to watersheds, it is possible to protect and enhance sand darter populations (Grandmaison et al. Citation2004; COSEWIC Citation2009; Tessler et al. Citation2012). There are several places where the eastern sand darter was absent for more than 50 years and have since recolonized improved stretches of rivers (Tessler et al. Citation2012; Hopkins & Zimmerman Citation2014). Thus, understanding habitat use preferences can aid in the recovery of both species. Further information gained from sand darter habitat use studies may provide insight into the health and overall quality of an aquatic ecosystem, especially in large river systems that are impacted by urban development and intensive agriculture.
Acknowledgments
The authors appreciate the field and laboratory assistance provided by J. Aldinger, B. Crabill, K. Lambert, and B. Tierney. Additional thanks to M. Strager of West Virginia University and D. Cincotta from the West Virginia Division of Natural Resources. This study was performed under the auspices of West Virginia University IACUC protocol 15-0801. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors

P. A. Thompson
P. A. Thompson was a graduate student of Dr. Stuart A. Welsh at West Virginia University and is now a biological sciences technician with the U.S. Fish and Wildlife Service.

S. A. Welsh
S. A. Welsh is an adjunct Professor of Ichthyology at West Virginia University and Research Fisheries Biologist with the West Virginia Cooperative Fish and Wildlife Research Unit.

A. A. Rizzo
A. A. Rizzo is a graduate assistant and Ph.D. candidate in the fisheries laboratory of Dr. Stuart A. Welsh at West Virginia University.

D. M. Smith
D. M. Smith is a Fisheries Biologist with the West Virginia Division of Natural Resources and a PhD candidate at West Virginia University. Dustin's graduate adviser is Dr. Stuart Welsh.
References
- Adams GL, Burr BM. 2004. Conservation assessment for the eastern sand darter, Ammocrypta pellucida. Bedford (IN): USDA Forest Ser; p. 1–33 . (Report submitted to the Hoosier National Forest).
- Baxter RM. 1977. Environmental effects of dams and impoundments. Annu Rev Ecol Syst. 8:255–283.
- Burr BM, Warren ML. 1986. A distributional atlas of Kentucky fishes. Kentucky Nature Preserves Comm Sci Tech Ser. 4:398.
- Carvalho MS, Zuanon J, Ferreira EJ. 2014. Diving in the sand: the natural history of Pygidianops amphioxus (Siluriformes: Trichomycteridae), a miniature catfish of Central Amazonian streams in Brazil. Environ Biol Fish. 97:59–68.
- Cincotta DA, Welsh SA. 2010. Discovery of Ammocrypta clara (western sand darter) in the Upper Ohio River of West Virginia. Am Mid Nat. 163:318–325.
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC). 2009. Committee on the Status of Endangered Wildlife in Canada assessment and status report on the eastern sand darter Ammocrypta pellucida. Ottawa: Ontario populations and Quebec populations.
- Daniels RA. 1989. Significance of burying in Ammocrypta pellucida. Copeia. 1989:29–34.
- Daniels RA. 1993. Habitat of the eastern sand darter, Ammocrypta pellucida. J Freshw Ecol. 8:287–295.
- Dextrase AJ, Mandrak NE, Schaefer JA. 2014. Modelling occupancy of an imperiled stream fish at multiple scales while accounting for imperfect detection: implications for conservation. Freshw Biol. 59:1799–1815.
- Drake DAR, Power M, Koops MA, Doka SE, Mandrak NE. 2008. Environmental factors affecting growth of eastern sand darter (Ammocrypta pellucida). Can J Zool. 86:714–722.
- Driver LJ, Adams GL. 2013. Life history and spawning behavior of the western sand darter (Ammocrypta clara) in Northeast Arkansas. Am Mid Nat. 170:199–212.
- Facey DE. 1998. The status of the eastern sand darter, Ammocrypta pellucida, in Vermont. Can Field Nat. 112:596–601.
- Facey DE, O'Brien SM. 2004. Influence of substrate composition on distribution of eastern sand darters (Ammocrypta pellucida) in the Poultney River. In: Manley TO, Manley PL, Mihuc TB, editors. Lake Champlain: partnerships and research in the new millennium. New York (NY): Kluwer Academic/Plenum Publishers; p. 291–297.
- Fretwell SD, Lucas HJ Jr. 1969. On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheor. 19:37–44.
- Garshelis DL. 2000. Delusions in habitat evaluation: measuring use, selection, and importance. In: Boitani L, Fuller TK, editors. Research techniques in animal ecology: controversies and consequences. New York (NY): Columbia University Press; p. 111–164
- Grandmaison D, Mayasich J, Etnier D. 2004. Eastern sand darter status assessment. ( US Fish and Wildlife Service, Region, 3. NRRI Tech Rep; no. NRRI/TR-2003/40).
- Holm E, Mandrak NE. 1996. The status of the eastern sand darter, Ammocrypta pellucidia, in Canada. Can Field Nat. 110:462–469.
- Hopkins RL, Zimmerman B. 2014. First observation of the eastern sand darter (Ammocrypta pellucida) in Raccoon Creek (Ohio River Basin) in Southeastern Ohio in 57 Years. Northeastern Nat. 21:N13–N17.
- Hosmer DW, Lemeshow S. 2000. Applied logistic regression. Hoboken (NJ): Wiley.
- Indiana Department of Natural Resources (INDNR). 2004. Eastern sand darter is removed from state special concern list. Division of Fish and Wildlife, The Indiana Department of Natural Resources; p. 23. (Wildlife Diversity Sect Annu Rep).
- Jackson DA, Peres-Neto PR, Olden JD. 2001. What controls who is where in freshwater fish communities the roles of biotic, abiotic, and spatial factors. Can J Fish Aquat Sci. 58:157–170.
- Kuehne RA, Barbour RW. 1983. The American darters. Lexington (KY): University Press of Kentucky; p. 177.
- Lachner EA. 1956. The changing fish fauna of the upper Ohio River basin. In: Man and the waters of the upper Ohio basin. Pittsburgh (PA): University of Pittsburgh, Pymatuning Laboratory of Ecology; p. 64–78. Spec Pub 1.
- Manly BFJ, McDonald LL, Thomas DL, McDonald TL, Erickson WP. 2002. Resource selection by animals. Dordrecht: Kluwer Academic Publishers.
- Near TJ, Porterfield JC, Page LM. 2000. Evolution of cytochrome b and the molecular systematics of Ammocrypta (Percidae: Etheostomatinae). Copeia. 2000:701–711.
- O'Brien SM, Facey DE. 2008. Habitat use by the eastern sand darter, Ammocrypta pellucida, in two Lake Champlain tributaries. Can Field Nat. 122:239–246.
- Pflieger WL. 1971. A distributional study of Missouri fishes. Univ Kansas Publ, Mus Nat Hist. 20:225–570.
- Poff NL, Hart DD. 2002. How dams vary and why it matters for the emerging science of dam removal. Bio Sci. 52:659–668.
- Power ME, Dietrich WE, Finlay JC. 1996. Dams and downstream aquatic biodiversity: potential food web consequences of hydrologic and geomorphic change. Environ Manage. 20:887–895.
- Rice JC. 2005. Understanding fish habitat ecology to achieve conservation. J Fish Biol. 67:1–22.
- Rogers KB, White GC. 2007. Analysis of movement and habitat use from telemetry data. In: Guy, CS, Brown ML, editors. Analysis and interpretation of freshwater fisheries data. Bethesda (MD): American Fisheries Society; p. 625–676.
- Schaefer SA, Provenzano F, de Pinna M, Baskin JN. 2005. New and noteworthy Venezuelan Glanapterygine catfishes (Siluriformes, Trichomycteridae), with discussion of their biogeography and psammophily. Am Mus Novitates. 3496:1–27.
- Simon TP. 2006. Biodiversity of fishes in the Wabash River: status, indicators, and threats. Proc Indiana Acad Sci. 115:136–148.
- Simon TP, Tyberghein EJ, Scheidegger KJ, Johnston CE. 1992. Descriptions of protolarvae of the sand darters (Percidae: Ammocrypta and Crystallaria) with comments on systematic relationships. Ichthyol Explor Freshw. 3:347–358.
- Smith DM, Welsh SA, Turk PJ. 2011. Selection and preference of benthic habitat by small and large ammocoetes of the least brook lamprey (Lampetra aepyptera). Environ Bio Fish. 91:421–428.
- Tessler NR, Gottgens JF, Kibbey MR. 2012. The first observations of the eastern sand darter, Ammocrypta pellucida (Agassiz), in the Ohio portion of the Maumee River mainstem in sixty-five years. Am Mid Nat. 167:198–204.
- Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE. 1980. The river continuum concept. Can J Fish Aquat Sci. 37:130–137.
- Wang L, Lyons J, Rasmussen P, Seelbach P, Simon T, Wiley M, Kanehl P, Baker E, Niemela S, Stewart PM. 2003. Watershed, reach, and riparian influences on stream fish assemblages in the Northern Lakes and Forest Ecoregion, USA. Can J Fish Aquat Sci. 60:491–505.
- Warren Jr ML, Burr BM, Walsh SJ, Bart Jr HL, Cashner RC, Etnier DA, Ross ST. 2000. Diversity, distribution, and conservation status of the native freshwater fishes of the southern United States. Fish. 25:7–31.
- Welsh SA, Perry SA. 1998. Habitat partitioning in a community of darters in the Elk River, West Virginia. Environ Biol Fish. 51:411–419.
- Wentworth CK. 1922. A scale of grade and class terms for clastic sediments. J Geol. 30:377–392.
- Williams JD. 1975. Systematics of the percid fishes of the subgenus Ammocrypta, genus Ammocrypta, with descriptions of two new species. Bull Alabama Mus Nat Hist. 1:1–56.
- West Virginia Division of Natural Resources (WVDNR). 2015. West Virginia State Wildlife Action Plan. Elkin (WV): West Virginia Division of Natural Resources, Draft June 10, 2015.
- Zuanon J, Bockmann FA, Sazima I. 2006. A remarkable sand-dwelling fish assemblage from central Amazonia, with comments on the evolution of psammophily in South American freshwater fishes. Neotrop Ichthyol. 4:107–118.
