ABSTRACT
Accurately ageing fish is essential for fisheries managers when calculating population dynamics for a particular species. Despite being a popular sport fish in its native range with a large expanding non-native range, otoliths of white perch Morone americana have not been validated. White perch were collected monthly during 2015–2016 from Sooner Lake, Oklahoma to remove sagittal otoliths to validate annulus formation. Marginal increment analysis verified a single annulus formed once yearly in otoliths of white perch and formation was complete by April or June, depending on fish age. Because descriptions of population characteristics of invasive white perch in Oklahoma are limited, the dynamics of the Sooner Lake population was described. The Sooner Lake white perch population is characterized by rapid growth rates, average longevity and mortality rates, high proportional size distribution and below average condition. The Sooner Lake population is a non-stunted population, which has not previously been documented in Oklahoma. White perch form a single opaque band once annually in sagittal otoliths, validating this structure as an ageing method for this species. This study expands on the existing literature on invasive white perch population characteristics and serves as a reference to which other Oklahoma populations can be compared.
Introduction
White perch Morone americana are native to the Atlantic slope drainages of North America from Quebec, Canada south to South Carolina, USA (Scott and Crossman Citation1973). Throughout its native range, the white perch is commercially and recreationally valuable (Mansueti Citation1961). Furthermore, white perch have a large and expanding non-native range. White perch have invaded or were introduced into the Great Lakes (Larsen Citation1954; Busch et al. Citation1977), the Illinois Waterway and throughout the Upper Mississippi River system (Irons et al. Citation2002). White perch populations have also become established in inland lakes and reservoirs in several other states through intentional (Zuerlein Citation1981) or accidental introduction through contaminated fish stockings (Wong et al. Citation1999; Harris Citation2006; Kuklinski Citation2007). White perch have invaded 21 states and 1 province outside of their native range (Fuller et al. Citation2014), and it can only be expected that this number will increase as populations continue to spread through the Arkansas and Mississippi River systems (Irons et al. Citation2002).
As white perch populations continue to expand in their non-native range, there is an increasing need to understand population dynamics in order to make appropriate management decisions. The ability to accurately age fish is essential for fisheries managers when estimating growth, mortality rates, and assigning year classes to understand recruitment for a particular species. For many years, fishery managers relied on scales to estimate ages of white perch to understand population demographics (Sheri and Power Citation1969; Wallace Citation1971; St. Pierre and Davis Citation1972; Marcy and Richards Citation1974; Busch and Heinrich Citation1982; Schaeffer and Margraf Citation1986). Otoliths have recently been used as the preferred structure for estimating ages of white perch (Harris Citation2006; Kuklinski Citation2007; Feiner et al. Citation2012; Bethke et al. Citation2014). Despite the current use as an ageing structure, annulus formation on sagittal otoliths has not been validated for white perch.
Validation of otoliths as an ageing structure has been completed for many species (Hales and Belk Citation1992; Secor et al. Citation1995; Clayton and Maceina Citation1999; Bettinger and Crane Citation2011; Blackwell and Kaufman Citation2012). Although no study has evaluated ageing accuracy for white perch, otoliths have been validated for the congeneric striped bass (Heidinger and Clodfelter Citation1987; Secor et al. Citation1995). Furthermore, Morone hybrid Morone chrysops x M. saxatilis otoliths were validated by evaluating annulus formation and comparing age estimates for known aged fish (Snyder et al. Citation1983). Kuklinski (Citation2007) suggested that white perch ages estimated from sectioned otoliths were more precise than those observed in whole otoliths. However, due to difficulties encountered during the ageing of white perch in that study, further research validating otoliths as an ageing structure is necessary (K. Kuklinski, personal communication).
Because white perch are an important commercial and sport fish species in their native range (Mansueti Citation1961), are rapidly expanding into other systems (Irons et al. Citation2002), and there is a need to verify ageing methodology for a particular calcified structure (Beamish and McFarland Citation1987) our primary objective was to validate that a single annulus is formed yearly in otoliths of white perch using marginal increment analysis and determine the timing of annulus deposition. Second, because studies characterizing white perch populations in Oklahoma are limited (but see Kuklinski Citation2007) we described population characteristics of an invasive population in Sooner Lake, Oklahoma from which we validated otolith annulus formation.
Methods
Study area
Sooner Lake is a 2185 ha reservoir located in north central, Oklahoma. The reservoir serves as a cooling water source for a coal-fired power generation facility operated by Oklahoma Gas and Electric Company. Sooner Lake is in the Arkansas River basin, and although not directly connected, Sooner Lake water level is maintained by pumping water from the Arkansas River. White perch were first documented in the Arkansas River within Oklahoma in 2000 (Kuklinski Citation2007), and were found in Sooner Lake in 2006 (Oklahoma Department of Wildlife Conservation (ODWC) unpublished data). White perch abundance in Sooner Lake has been increasing since establishment (Copeland Citation2016).
Study design
White perch were collected from Sooner Lake, monthly from April 2015 to June 2016, using boat-mounted electrofishing (pulsed DC, high voltage, Smith Root 7.5 GPP) and experimental gillnets. A white perch sample was not collected in May 2016 because of equipment breakdown. Because each of these gear types has size-selective biases, a multiple gear approach was implemented to ensure that all size and age classes of white perch were represented in the sample (Kuklinski Citation2007; Feiner et al. Citation2012; Bethke et al. Citation2014). Furthermore, day and night sampling was conducted monthly to ensure no diel differences in size or age class representation in the sample. Each monthly sample consisted of twelve 600 s electrofishing transects (7200 s of pedal time) and four experimental gill nets set perpendicular to the shoreline in inshore and offshore locations. Sites were chosen at random by laying a 300 m2 grid over the map of the lake in ArcGIS, individually numbering each grid square and using a random number generator to select the grid to be sampled. New sites were selected at random each month for electrofishing and gillnet sampling. The experimental gill nets used were 61 m long × 1.8 m deep and constructed of eight 7.6 m panels (12.7, 15.9, 19.1, 25.4, 38.1, 50.8, 63.5, and 76.2 mm bar mesh).
White perch from both sampling methods were combined for marginal increment analysis and age estimation and population assessment. For marginal increment analysis, we sought to collect 25 white perch monthly. Whereas, our goal for population assessment purposes was to collect 10 white perch per 10 mm length group to ensure that all size and age classes were represented in the sample.
Following capture, each fish was measured for total length (TL; mm) and weight (g), and sagittal otoliths were removed for age estimation. Otoliths were sectioned in a transverse plane, polished using 1600 grit wet/dry sandpaper, stood polished side up in modeling clay and submerged with water, and viewed with a dissecting microscope (4–45×) by shining light from a fiber optic light source through the otolith to illuminate annuli. Annuli, which appeared as dark rings on a light background, were counted to assign an age estimate to each fish. Each otolith was evaluated in random order by two independent readers (Hoff et al. Citation1997). When there was a disagreement on an estimated age, a concert reading was conducted by both readers and a final age estimate was determined.
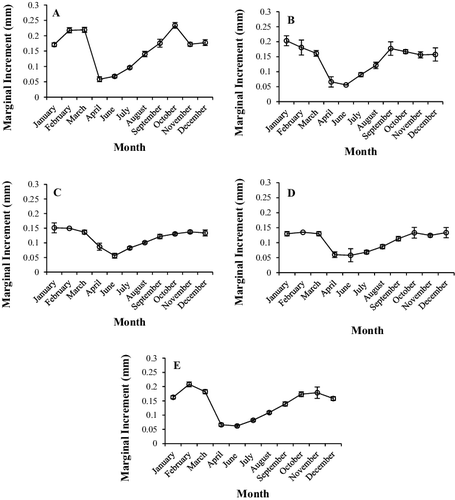
Marginal increment analysis was performed by measuring the width of the hyaline zone following the last opaque band on the distal edge of the otolith to validate that a single opaque band (annulus) was formed annually and determine the timing of annulus formation (Clayton and Maceina Citation1999; Blackwell and Kaufman Citation2012). Measurements were taken on the dorsal side of the sulcus in transverse plane, as this provided the clearest view of annuli. Otoliths were measured (mm) using ToupCam (Toup Tek Photonic, Zhejieng, P. R. China) computer software and camera attached to a dissecting microscope. Marginal increment data were separated into age classes (age 1, ages 2–3, ages 4–5, ages 6–8, and all ages combined), and mean increment distance was graphed by month. Marginal increment data were analyzed by age class to determine if annulus formation timing varied with age. Age 0 fish were not used in the marginal increment analysis because they were not measurable (no opaque band present). Age classes were combined to account for small sample size.
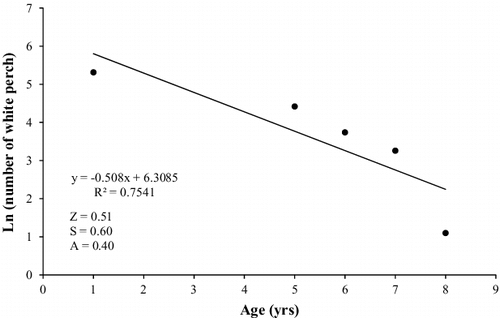
A length-frequency histogram and proportional size distribution (PSD) of quality (200 mm; PSDq), preferred (250 mm; PSDp) and memorable (300 mm; PSDm) sized fish (Gabelhouse Citation1984) was used to visualize and quantify white perch size structure. A log10 weight to log10 TL length–weight regression was used to describe the weight to length relationship of the population. White perch condition was evaluated by calculating relative weight (Wr) using standard weight equations presented by Bister et al. (Citation2000). A von Bertalanffy growth model was used to describe growth of white perch. Catch-curve-regression was used to assess total mortality of white perch. Age 0 white perch were not considered to be fully recruited to the sampling gear, so they were removed from the catch-curve analysis. Furthermore, because of severe drought in Oklahoma from 2012 to 2014, white perch recruitment was inconsistent in those years, therefore age 2 to age 4 fish were removed from catch-curve analysis. Total mortality was calculated by regressing the loge number of fish caught on age to estimate instantaneous total mortality (Z) and total annual mortality (A = 1 − e−z; Ricker Citation1975).
Results
A total of 571 white perch were aged and ages ranged from 1 to 8 years old (). The marginal increment measurements indicated that a single opaque band was formed once annually between April and June in white perch otoliths from Sooner Lake (). This was true when evaluating fish by age class or when all age classes were pooled. However, the timing of annulus formation varied by age class ((A–E)). Age 1 white perch completed annulus formation by late April ((A)), however formation occurred by early June for all other age classes (ages 2–8; (B–E)).
Table 1. Distribution of white perch ages collected monthly from Sooner Lake, Oklahoma during 2015–2016.
Figure 1. Mean marginal increment measurement by month from white perch otoliths for (A) age 1, (B) ages 2–3, (C) ages 4–5, (D) ages 6–8, and (E) pooled ages. Error bars represent the standard error of the mean.
White perch collected from Sooner Lake ranged from 56 to 308 mm TL (). This population was dominated by sub-stock and stock sized fish, although quality (PSDq = 58) and preferred (PSDp = 31) sized fish were also abundant (). The abundance of memorable sized fish was low (PSDm = 1). Growth of white perch was rapid to age 2, but slowed dramatically through age 8. The modelled von Bertalanffy growth curve indicates that white perch approach maximum length steadily (K = 0.39), with individuals in the population growing to 74% of their expected TL (L∞ = 294) by age 2 (). The total annual mortality estimate for the white perch population in Sooner Lake was 40% ().
Figure 2. Length-frequency distribution (by 10 mm TL increments) of white perch collected from Sooner Lake, Oklahoma during 2015–2016.
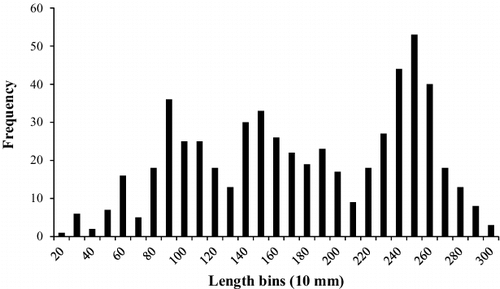
Table 2. Proportional size distribution (PSD) and mean relative weight (Wr; standard deviation) by length categories, and length–weight relationship for white perch collected from Sooner Lake, Oklahoma during 2015–2016.
Figure 3. Von Bertalanffy growth curve calculated from otolith age estimates for white perch collected from Sooner Lake, Oklahoma during 2015–2016.
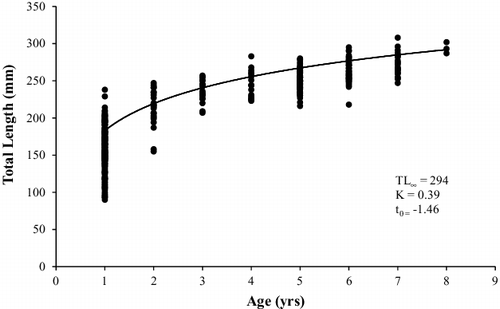
Figure 4. Catch-curve regression and total annual mortality (A) for white perch collected from Sooner Lake, Oklahoma during 2015–2016.
The weight–length relationship of white perch was log (W) = −5.36 + 3.22 log (TL) (R2 = 0.99; ). This weight–length relationship results in Wr values that are below average for all length categories. Wr values were poorest for sub-stock (85), stock (84), and memorable (84) size classes. Whereas, Wr values were slightly below average for quality and preferred size fish (92 and 93, respectively).
Discussion
Marginal increment analysis of white perch otoliths indicated that a single opaque band was formed once annually. Formation of a single annulus in the sagittal otoliths of white perch validates this structure for ageing this species. Although scales have been validated as an ageing structure for white perch (Sheri and Power Citation1969; Wallace Citation1971), this is the first validation of otoliths for ageing white perch. Otoliths have been validated for other Morone species. Snyder et al. (Citation1983) verified annulus formation and timing, and ageing accuracy for hybrid striped bass in Florida. Secor et al. (Citation1995) validated accuracy of striped bass ages 3–7 for Chesapeake Bay striped bass. Furthermore, Heidinger and Clodfelter (Citation1987) accurately assigned ages for 0–4-year-old known-aged striped bass from northern Illinois cooling ponds.
The timing of annulus formation in otoliths of white perch from Sooner Lake was dependent on age. Age 1 fish typically formed an annulus by late April, whereas older fish (ages 2–8) formed an annulus by early June. Because a sample was not able to be collected in May 2016, it is possible that annulus formation occurred for some fish in May. Nonetheless, a single annulus formed by June for adult white perch. In a study evaluating annulus formation in yellow perch otoliths collected from South Dakota, Blackwell and Kaufman (Citation2012) determined that annulus formation timing varied by fish age, with young fish completing annulus formation earlier than older fish. Similarly, Crawford et al. (Citation1989) found that young Florida largemouth bass Micropterus salmoides floridanus formed an annulus sooner than older bass in several populations.
Differences in the timing of annulus formation are driven by timing of annual growing seasons (Wallace Citation1971). White perch collected from Sooner Lake completed annulus formation between April and June depending on age. Similarly, Wallace (Citation1971) determined that annulus formation in white perch scales formed between April and June. However, an annulus formed on scales of white perch collected from the Bay of Quinte, Lake Ontario in July (Sheri and Power Citation1969). Furthermore, Secor et al. (Citation1995) observed new annulus formation in striped bass otoliths collected in Chesapeake Bay during February–April. Conversely, Snyder et al. (Citation1983) determined that annulus formation occurred in otoliths of Morone hybrids from central Florida in April–May, similar to timing in Sooner Lake white perch. Understanding when annulus completion occurs allows fisheries managers to collect fish for ageing purposes at the appropriate time to obtain the most accurate age estimates.
Growth of white perch from Sooner Lake was rapid, reaching quality size (>200 mm TL) by age 2, comparable to white perch collected from Lake Erie where fish reached the commercially acceptable size of 200 mm TL by age 2 (Schaeffer and Margraf Citation1986). White perch growth was rapid in the first two years of life, but slowed considerably after age 2, similar to growth rates from the Lower Connecticut River (Marcy and Richards Citation1974), Lake Ontario (Busch and Heinrich Citation1982), and Browning Oxbow, Kansas (Stein Citation2001). Fast growth rates equated to a high abundance of white perch reaching quality (PSDq = 60) and preferred (PSDp = 32) size classes. PSD of quality size fish were comparable to values calculated for white perch from Browning Oxbow, Kansas (RSDq = 51), but were considerably higher for preferred sized fish (RSDp = 9; Stein Citation2001). Despite fast growth rates and high PSD, the mean relative weight of the Sooner Lake white perch population was below average for all size classes. Relative weight values from Sooner Lake were comparable to those calculated for white perch from Browning Oxbow, Kansas for quality and preferred sized fish (93 and 98, respectively), however relative weight for stocked sized fish was considerably higher (113; Stein Citation2001). The disparity between mean relative weights for small and large fish is likely attributed to intraspecific competition for forage resources. Mansueti (Citation1961) suggested that intraspecific competition for food resources drives growth of white perch. The larger fish may be able to better compete for these resources than smaller fish when white perch densities are high, leading to the larger fish being in better overall condition.
Annual mortality of white perch in Sooner Lake is 40%. Similarly, Stein (Citation2001) estimated total annual mortality for a non-native population of white perch in a Missouri River oxbow at 45%. However, these mortality estimates are considerably lower than those calculated for native populations. Annual mortality estimates for white perch from the James River averaged 69%, whereas mortality was 58% from the York River (St. Pierre and Davis Citation1972). Similarly, annual mortality rates calculated for white perch from the Delaware River ranged from 54% to 58% for males and females, respectively (Wallace Citation1971). The differences in annual mortality estimates between native and non-native populations may be due to high exploitation rates in native populations where white perch are commercially and recreationally valuable (Mansueti Citation1961). Angler exploitation of white perch in Sooner Lake appears minimal, but when harvest of white perch does occur, they are usually used as bait to target blue catfish Ictalurus furcatus (N. Copeland, ODWC, personal communication). Natural mortality in the form of predation likely contributes the greatest to the total annual mortality rate in Sooner Lake. Copeland (Citation2016) found that most predators in Sooner Lake consumed white perch at minimal levels, and only saugeye Sander vitreus x Sander canadensis consumed them in appreciable amounts. In Oklahoma, saugeye have been used as a biological control of overcrowded, stunted white crappie Pomoxis annularis populations (Summers et al. Citation1994; Boxrucker Citation2002). Saugeye predation may be keeping the Sooner Lake white perch population from overpopulating and stunting.
White Perch are prone to overpopulating inland waterbodies where they are non-native, resulting in populations of stunted individuals (Zuerlein Citation1981; Kuklinski Citation2007; Gosch et al. Citation2010; Feiner et al. Citation2012; Bethke et al. Citation2014). Stunted white perch populations are characterized by a high abundance of slow growing fish that rarely exceed 300 mm TL or attain 5 years of age (Busch et al. Citation1977; Zuerlein Citation1981). Stunted and non-stunted white perch populations have been documented in Kansas (Zuerlein Citation1981), Nebraska (Zuerlein Citation1981; Gosch et al. Citation2010), North Carolina (Feiner et al. Citation2012; Bethke et al. Citation2014), and now in Oklahoma. Kuklinski (Citation2007) found that white perch in Kaw Lake, Oklahoma rarely exceeded 200 mm TL and age 3. Age and growth of white perch from Sooner Lake was markedly different with many fish in the 5–8 age classes and fish attaining 300 mm TL.
Our results are the first to validate that an annulus forms in the sagittal otolith of white perch, making this structure suitable for estimating age for this species. Due to the possible differences in annulus formation timing based on location (due to differences in growing seasons) and fish age, it is critical that fisheries managers understand when a new annulus is formed. This will determine when fish should be collected for age determination, as assigning inaccurate age estimates could affect population parameters calculated from those ages. This study also describes population characteristics for invasive white perch in Sooner Lake. Our results suggest a non-stunted population, and provide parameters that future studies in Oklahoma can be compared. Future evaluations should assess white perch throughout their non-native range in Oklahoma to determine their impacts to Oklahoma reservoir fisheries and to determine factors leading to stunted and non-stunted populations. Furthermore, the use of saugeye as a management tool to control or prevent white perch stunting is in need of further study.
Acknowledgments
The authors thank those individuals that assisted with labor-intensive field work including Clayton Porter, Jeff Tibbits, Amie Robison, Ashley Nealis, Nate Copeland, Bill Wentroth, Michael Hollie, and Steve O'Donnell. A special thanks to Amie Robison, Michael Hollie, Brandon Chien, and Shelby Jeter for preparing ageing structures to be aged and measured. We thank K. Kuklinski (ODWC) and D.R. Stewart (USFWS) for reviewing an earlier draft of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Michael J. Porta
Michael J. Porta is a fisheries research biologist for the Oklahoma Department of Wildlife Conservation, Oklahoma Fishery Research Laboratory. He received a B.S. in Wildlife and Fisheries Management from Frostburg State University in 2006 and a M.S. in Natural Resource Ecology and Management from Oklahoma State University in 2011. His current research addresses reservoir sport fish management questions in Oklahoma, but his primary interests are black bass population dynamics and sunfish management.
Richard A. Snow
Richard A. Snow is a fisheries research biologist for the Oklahoma Department of Wildlife Conservation, Oklahoma Fishery Research Laboratory. He received a B.S. in Conservation Biology from St. Gregory's University in 2007 and a M.S. in Natural Resource Ecology and Management from Oklahoma State University in 2014. His current research addresses reservoir sport fish management questions in Oklahoma, however he is particularly interested in studying the four gar species found in Oklahoma.
References
- Beamish RJ, McFarlane GA. 1987. Current trends in age determination methodology. In: Summerfelt RC, Hall GE, editors. The age and growth of fish. Ames (IA): The Iowa State University Press; p. 15–42.
- Bethke BJ, Rice JA, Aday DD. 2014. White perch in small North Carolina reservoirs: what explains variation in population structure? Trans Am Fish Soc. 143:77–84.
- Bettinger JM, Crane JS. 2011. Validation of annulus formation in otoliths of notchlip redhorse (Moxostoma collapsum) and brassy jumprock (Moxostoma sp.) in Broad River, South Carolina, with observations on their growth and mortality. Southeastern Naturalist. 10:443–458.
- Bister TJ, Willis DW, Brown ML, Jordan SM, Neuman RM, Quist ME, Guy CS. 2000. Proposed standard weight (Ws) equations and standard length categories for 18 warmwater nongame and riverine fish species. North Am J Fish Manage. 20:570–574.
- Blackwell BG, Kaufman TM. 2012. Timing of yellow perch otolith annulus formation and relationship between fish and otolith lengths. North Am J Fish Manage. 32:239–248.
- Boxrucker, J. 2002. Improved growth of a white crappie population following stocking of saugeye: a top-down density-dependent growth response. North Am J Fish Manage. 22:1425–1437.
- Busch WDN, Davies DH, Nepszy SJ. 1977. Establishment of white perch Morone americana, in Lake Erie. J Fish Res Board Can. 34:1039–1041.
- Busch WDN, Heinrich JW. 1982. Growth and maturity of white perch in Lake Ontario. NY Fish Game J. 29:206–208.
- Clayton DL, Maceina MJ. 1999. Validation of annulus formation in gizzard shad otoliths. North Am J Fish Manage. 19:1099–1102.
- Copeland N. 2016. Predation of white perch in Sooner Reservoir: is a biological control possible? (Final Report: Project No. F15AF00550). Norman.
- Crawford S, Coleman WS, Porak WF. 1989. Time of annulus formation in otoliths of Florida largemouth bass. North Am J Fish Manage. 9:231–233.
- Feiner ZS, Aday DD, Rice JA. 2012. Phenotypic shifts in white perch life history strategy across stages of invasion. Biol Inv. 14:2315–2329.
- Fuller P, Maynard E, Raikow D, Larson J, Fusaro A, Neilson M. 2014. Morone americana. Gainesville (FL): USGS Nonindigenous Aquatic Species Database. Available from: http://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=777 Revision Date: 11/4/2013.
- Gabelhouse DW Jr. 1984. A length-categorization system to assess fish stocks. North Am J Fish Manage. 4:273–285.
- Gosch NJC, Stittle JR, Pope KL. 2010. Food habits of stunted and non-stunted white perch (Morone americana). J Freshwater Ecol. 25:31–39.
- Hales LS Jr., Belk MC. 1992. Validation of otolith annuli of bluegills in a southeastern thermal reservoir. Trans Am Fish Soc. 121:823–830.
- Harris JL. 2006. Impacts of the invasive white perch on the fish assemblage of Kerr Reservoir, Virginia [MSc thesis]. Blacksburg (VA): Virginia Polytechnic Institute and State University.
- Heidinger RC, Clodfelter K. 1987. Validity of the otolith for determining age and growth of walleye, striped bass, and smallmouth bass in power plant cooling ponds. In: Summerfelt RC, Hall GE, editors. The age and growth of fish. Ames (IA): The Iowa State University Press; p. 15–42.
- Hoff GR, Logen DJ, Douglas MF. 1997. Otolith morphology and increment validation in young lost river and shortnose suckers. Trans Am Fish Soc. 126:488–494.
- Irons KS, O'Hara TM, McClelland MA, Pegg MA. 2002. White perch occurrence, spread, and hybridization in the middle Illinois River, upper Mississippi River system. Trans Ill State Acad Sci. 95:207–214.
- Kuklinski KE. 2007. Ecological investigation of the invasive white perch in Kaw Lake, Oklahoma. Proc Okla Acad Sci. 87:77–84.
- Larsen A. 1954. First record of the white perch (Morone americana) in Lake Erie. Copeia. 1954:154.
- Mansueti R. 1961. Movements, reproduction and mortality of the white perch (Roccus americanus) in the Patuxent Estuary. Chesap Sci. 2:142–205.
- Marcy BC, Richards FP. 1974. Age and growth of the white perch Morone americana in the lower Connecticut River. Trans Am Fish Soc. 103:117–120.
- Ricker WE. 1975. Computation and interpretation of biological statistics in fish populations. Ottawa: Fisheries Research Board of Canada Bulletin 191.
- Schaeffer JS, Margraf FJ. 1986. Population characteristics of the invading white perch (Morone Americana), in western Lake Erie. Int Assoc Gt Lakes Res. 12:127–131.
- Scott WB, Crossman EJ. 1973. Freshwater fishes of Canada. Ottawa: Fisheries Research Board of Canada Bulletin 184.
- Secor DH, Trice TM, Hornick HT. 1995. Validation of otolith-based ageing and a comparison of otolith and scale-based ageing in mark-recaptured Chesapeake Bay striped bass, Morone saxatilis. Fish Bull. 93:186–190.
- Sheri AN, Power G. 1969. Annulus formation on scales of the white perch Morone americanus (Gmelin), in the Bay of Quinte, Lake Ontario. Trans Am Fish Soc. 98:322–326.
- Snyder LE, Borkowski WK, McKinney SP. 1983. The use of otoliths for ageing Morone hybrids. Proc Annu Southeast Assoc Fish Wildl Agencies. 37:252–256.
- Pierre RASt., Davis J. 1972. Age, growth, and mortality of the white perch, Morone americana, in the James and York Rivers, Virginia. Chesap Sci. 13:272–281.
- Stein JE. 2001. Biology of nonindigenous white perch and yellow bass in an oxbow of the Missouri River [MSc thesis]. Emporia (KS): Emporia State University.
- Summers GL, Boxrucker J, Gabelhouse DW Jr. 1994. Introduction of saugeye to control overcrowded crappie populations. Trans North Am Wildl Nat Res Conf. 59:274–280.
- Wallace DC. 1971. Age, growth, year class strength, and survival rates of the white perch, Morone americana (Gmelin), in the Delaware River in the vicinity of artificial Island. Chesap Sci. 12:205–218.
- Wong RK, Noble RL, Jackson JR, Van Horn S. 1999. White perch invasion of B. Everett Jordan Reservoir, North Carolina. Proc Annu Southeast Assoc Fish Wildl Agencies. 53:162–169.
- Zuerlein G. 1981. The white perch, Morone americana (Gmelin) in Nebraska (Nebraska Technical Series No. 8). Lincoln (NE): Nebraska Game and Parks Commission.
