ABSTRACT
Dynamic changes in body color are commonly used as a means of intraspecific communication and for crypsis. It is well established that subordinate fish signal to dominant fish by darkening their body color. Similarly, salmonids can adapt their body color to match their environment. What is not known is whether social interactions or environment plays a larger role in driving dynamic changes in body color, as these factors rarely occur in isolation. Experiments conducted in artificial stream channels with wild brook trout tested for the potential of color matching by varying light and dark substrates and included treatments with individuals as well paired cohorts to test for the effect of social hierarchies. Fish displayed a broad ability to adapt their coloration to match a range of substrates, with a strong preference for dark substrate. In paired trials, dominant fish matched light substrate more effectively while subordinates displayed darker coloration, resulting in subordinates being poorly matched to their surroundings. Mismatching the environment in order to send appropriate social signals could have negative consequences that further reduce fitness in subordinate fish.
Introduction
Visual displays allow territorial salmonids to communicate information about social status and to minimize costs of dangerous fighting (e.g. Keenleyside and Yamamoto Citation1962; Grosenick et al. Citation2007). Body color darkening is closely associated with submission in contests between juvenile salmonids undertaking aggressive interactions (O'Conner et al. Citation1999). Losers of territorial disputes display submissive darkening to avoid conflict with familiar dominants (Höglund et al. Citation2000; O'Connor et al. Citation2000) and thus allowing for stable groups to form social hierarchies through cooperative signaling (Hurd Citation1997). Previous research indicates that the basis of color change is morphologic plasticity with very little genetic control (Whiteley et al. Citation2009; Westley et al. Citation2013), and that skin darkening is positively correlated with stress hormones (Boddingius Citation1976; Höglund et al. Citation2000).
In addition to dynamic changes in body color for social signaling, salmonids can also alter their body color to better match their habitat. Juvenile salmonids can rapidly alter their body color in response to light conditions and become more mottled when holding position close to the streambed (Jenkins Citation1969). In addition, their bodies lighten at night (Hafeez and Quay Citation1970), and preference has been found for positioning over low reflectance (Donnelly and Dill Citation1984) and dark surfaces (Gibson and Keenleyside Citation1966; Hafeez and Quay Citation1970), especially in brook trout (Salvelinus fontinalis). Overall skin coloration can also change in response to environmental conditions, with darker skin observed with darker substrate and vice versa (Westley et al. Citation2013).
While social hierarchies and habitat clearly influence body color of salmonids, it is unclear how these two factors interact and which is more influential. By subjecting brook trout in artificial stream channels to a variety of substrate compositions, both individually and in familiar cohort pairs with an established dominant and subordinate fish, we are able to infer whether social hierarchies or the environment are more likely to determine body coloration. Specifically, we hypothesize that if it is advantageous for an individual to have a wide range of color change potential, then individual brook trout will match body coloration to a broad range of substrate intensities. We further hypothesize that darker substrate will be preferred because fish darken in response to stress. Finally, we hypothesize that in a social hierarchy, dominant individuals will maintain the selective advantage and match the substrate more effectively than subordinate individuals when over light colored substrate.
Methods
Eight wild-caught eastern brook trout (total length 100–135 mm) were divided into four cohort pairs (one dominant and one subordinate) that were maintained throughout the study. An individual's place within the hierarchy was determined through behavioral observations as well as size discrepancies, with the largest fish being the most dominant in competitive interactions (Snow Citation2014). Dominant fish were on average 12 mm longer than subordinate fish and a t-test indicated a significant difference in size between the dominant and subordinate fish (p = 0.002). Each fish experienced each experimental treatment in the same sequence, with individual trials of color matching and substrate preference followed by paired cohort trials.
Four artificial stream channels (1.30 m long, 0.36 m wide × 0.40 m deep) were used for experimental trials. Channels had one lengthwise viewing window and the other three walls were light blue. Low-velocity circulation was kept constant and temperature ranged from 13 to 15 °C. While not conducting trials, fish were housed in individual tanks without substrate and with visual barriers between enclosures. The evening before experimental trials, brook trout were moved to their respective channels to allow for a 12-h acclimation period.
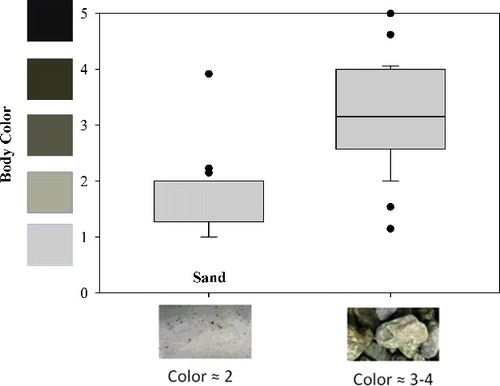
A color intensity scale was derived from the spectrum of colors present in the substrate used during trials (). The number that most closely resembled the substrate and body color was recorded as a whole integer. All observations of fish coloration were made behind a blind in order to reduce external stress.
Figure 1. Box plots showing the distribution of body color for individual brook trout as they appeared over light sand and darker rock substrates. The mean body color for the individuals over sand was 1.76, which was significantly lighter than when presiding over rocks, with a mean of 3.30. The color intensity scale ranged from the lightest shade (1) to the darkest shade (5) found in the substrate used for experimentation. Middle values were interpolated from the end members using Photoshop (Adobe Systems, Inc.).
The first experiment tested the extent of color matching possible for individual brook trout using a paired t-test. Two artificial channels contained dark rocks (46 mm median grain size) from the natal stream with a 3–5 rating on the color scale. Two channels contained a uniform light sandy substrate (0.4–1.0 mm) with a 1–2 color rating. Body color of each individual fish was recorded over each substrate every hour from 7:30 am to 2:30 pm for one day.
The second experiment tested whether individual brook trout had a preference for substrate color using a Chi-squared test. Light and dark substrates were placed in the channels in a randomized 50:50 configuration. Initially a light sandy substrate was used because it was more uniform in color, which was tested against the natal darks rocks to determine preference. Additional trials utilized rounded, light-colored quartzite rocks (median grain size 32 mm) in order to eliminate preference for a ‘type’ of substrate (i.e. gravel vs. sand). Observations occurred hourly from 7:30 am to 2:30 pm for one day per fish for each substrate type, and the individual's position within the tank (light or dark side) and body color was recorded.
The third experiment tested color matching within a familiar dominance hierarchy by placing the paired subordinate and dominant fish in a channel with light-colored sand and a few rocks as hiding cover to reduce aggressive interactions. This utilized the assumption that subordinate fish would darken (e.g. O'Connor et al. Citation1999, Citation2000; Höglund et al. Citation2000) and therefore additional cohort trials over dark substrate were not tested. Body color for both individuals was recorded every 90 minutes from 7:30 am to 3:00 pm for one day per cohort pair.
Results
All fish displayed the ability to match body color to the substrate (). The mean body color (1.76 on the color intensity scale) of all fish while presiding over lightly colored sand (1–2) was significantly lighter (p < 0.001) than the mean body color (3.30) of the same fish presiding over darker colored river rock (3–5).
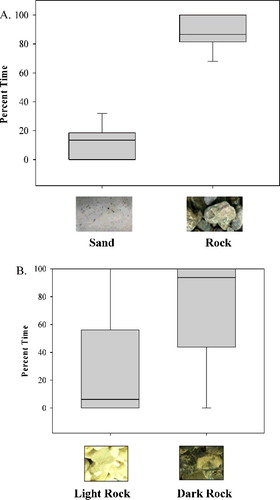
In trials where both substrates were available, a preference for the darker substrate was more often displayed (). Preference remained regardless of the light substrate being sand (χ2 = 27; p < 0.001) or gravel (χ2 = 16; p < 0.001), with preference defined as a relative percentage of time spent over a particular substrate.
Figure 2. Box plots illustrating the average percent time for each individual brook trout in trials with both light and dark substrates available. (A) Trials conducted with light sand and dark rock; (B) trials conducted with light and dark rocks.
When paired over light substrate, dominant fish displayed more effective color matching and resulted in mismatched coloration for the subordinate fish that displayed a dark body near a light substrate (). In the presence of a dominant fish, subordinates were darker than when alone (p = 0.02) and in comparison to the dominant fish (p = 0.01); and when individually observed, subordinate fish maintained a similar baseline color as dominant fish (). By testing the difference between dominant and subordinate fish in individual versus paired trials, the interaction was significant (p = 0.001). Our observations also indicated that dominant fish chased the subordinate until they retreated to the opposite end of the channel, where they remained darkened despite being out of direct contact. Although darker intensity rocks were provided as hiding cover in order to reduce injury to subordinate fish, it is unlikely that subordinate fish could match these small habitat patches (<20% of the channel bed).
Figure 3. Picture depicting the social hierarchy present in brook trout. The dominant fish pictured on the right is lightly colored and better matching the predominantly sandy substrate than subordinate fish displaying a dark body and seeking refuge near hiding cover (Photo credit: Kyle Snow).

Table 1. Pooled means (and standard deviations) of body color for dominant and subordinate fish during individual (n = 8) and paired trials (n = 4) over lightly colored sand.
Discussion
Although this study was limited to a small sample size with a repeated measures design, our findings on the effect of body color changes due to social interactions and environment were consistent with previous findings. The novel aspect of our study was investigating the interaction between social interactions and the environment on body coloration. During individual trials, the tendency to match the substrate was equal regardless of social status. This suggests that all fish are equally able to change body color; however, subordinate fish were inhibited by the presence of dominant fish, resulting in a mismatch when the environment was light colored.
Color variation for social signaling or for crypsis can have important fitness implications and mismatching could have detrimental consequences. Because coloration is a display that signals submission and reduces aggressive interactions and injuries (Keenleyside and Yamamoto Citation1962; Boddingius Citation1976; O'Conner et al. Citation1999; Höglund et al. Citation2000; O'Conner et al. 2000; Grosenick et al. Citation2007), mismatched coloration can send an incorrect signal. Results of our study indicate that subordinate fish may not match their environment, which could result in reduced survival by being more obvious to predators (Donnelly and Whoriskey Citation1991, Citation1993).
Acknowledgments
The authors would like to thank the Department of Biology at James Madison University for supporting undergraduate research. The authors would especially like to thank colleague Kyle Snow for initial observations that led to the development of this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Charlie Watt
Charlie Watt participated in 1.5 years of undergraduate research and gained a BS degree in biology from James Madison University.
Corey Swanson
Corey Swanson assisted with animal care and participated in undergraduate research while earning a BS degree in biology from James Madison University.
Dillon Miller
Dillon Miller participated in undergraduate research and earned a BS degree in biology from James Madison University.
Lihua Chen
Lihua Chen is an associate professor of statistics at James Madison University. She develops statistical methods and provides advice on statistical methodology to subject matter experts by collaborating with faculty members and mentoring undergraduate research.
Christine May
Christine May is an associate professor of biology at James Madison University. She engages undergraduates in research through traditional one-on-one mentoring and through course-embedded research in freshwater ecology.
References
- Boddingius J. 1976. The influence of social rank on adenohypophysial cell activity in Salmo irideus. Cell Tissue Res. 170:383–414.
- Donnelly WA, Dill LM. 1984. Evidence for crypsis in coho salmon, Oncorhynchus kisutch (Walbaum), parr; substrate colour preference and achromatic reflectance. J Fish Biol. 25(2):183–195.
- Donnelly WA, Whoriskey FG Jr. 1991. Background-color acclimation of brook trout for crypsis reduces risk of predation by hooded mergansers Lophodytes cucullatus. North Am J Fish Manage. 11:206–211.
- Donnelly WA, Whoriskey FG. 1993. Transplantation of Atlantic salmon (Salmo salar) and crypsis breakdown. Ottawa (ON): Canadian Special Publication of Fisheries and Aquatic Sciences; p. 25–34.
- Gibson RJ, Keenleyside MHA. 1966. Responses to light of young Atlantic Salmon (Salmo salar) and Brook Trout (Salvelinus fontinalis). J Fish Res Board Can. 23(7):1007–1024.
- Grosenick L, Clement TS, Fernald RD. 2007. Fish can infer social rank by observation alone. Nature. 445:429–432.
- Hafeez MA, Quay WB. 1970. The role of the pineal organ in the control of phototaxis and body coloration in rainbow trout (Salmo gairdneri, Richardson). Zeitsch Physiol Chem. 68:403. doi:10.1007/BF00297738
- Höglund E, Balm PHM, Winberg S. 2000. Skin darkening, a potential social signal in subordinate arctic charr (salvelinus alpinus): the regulatory role of brain monoamines and pro-opiomelanocortin-derived peptides. J Exp Biol. 203(11):1711–1721.
- Hurd PL. 1997. Cooperative signaling between opponents in fish fights. Anim Behav. 54:1309–1315.
- Jenkins TM Jr. 1969. Observations on color of brown and rainbow trout (Salmo trutta and S. gairdneri) in stream habitats, with description of unusual color pattern in Brown Trout. Trans Am Fish Soc. 98:517–519.
- Keenleyside MHA, Yamamoto FT. 1962. Territorial behavior of juvenile Atlantic salmon (Salmo salar L.). Behaviour. 19(1):139–168.
- O'Connor KI, Metcalife NB, Taylor AC. 1999. Does darkening signal submission in territorial contests between juvenile Atlantic salmon, Salmo salar? Anim Behav. 58:1269–1276.
- O'Connor KI, Metcalfe NB, Taylor AC. 2000. Familiarity influences body darkening in territorial disputes between juvenile salmon. Anim Behav. 59(6):1095–1101.
- Snow K. 2014. Effects of fine sediment on juvenile brook trout habitat use and social interactions [master's thesis]. Harrisonburg (VA): James Madison University; 49 p.
- Westley PAH, Stanley R, Fleming IA. 2013. Experimental tests for heritable morphological color plasticity in non-native brown trout (Salmo trutta) populations. PLoS One. 8(11):e80401.
- Whiteley AR, Gende SM, Gharrett AJ, Tallmon DA. 2009. Background matching and color-change plasticity in colonizing freshwater sculpin populations following rapid deglaciation. Evolution. 63(6):1519–1529.
