ABSTRACT
We investigated the breeding season, growth rate and dispersal of the dark chub, Candidia sieboldii, an endangered species in Japan. A mark–release–recapture survey was performed in three branches of the Ishizu River system in Osaka Prefecture, Japan, between May 2012 and August 2013. A total of 963 individuals were marked in one river branch and 275 (29%) were recaptured at least once in the same river section. The breeding season was determined to be June–August, and the size at maturity for both males and females was estimated at about 70 mm standard length. Growth rates calculated from standard lengths of recaptured individuals were higher between April and September than between October and March. Among 963 marked individuals, only a single fish was found 500 m downstream from the release point. Moreover, although we released 409 more marked individuals in the three branches to follow inter-branch dispersal between September and December 2013, we observed none. These results indicate that this species has low dispersal and a short lifespan of 1–2 years. This short lifespan could increase the risk of breeding failure, and the low frequency of dispersal, restricted by weirs, could make population re-establishment and recovery difficult.
Introduction
Recently, a number of freshwater fishes have shown population declines because of deteriorating river environments (e.g. Dudgeon et al. Citation2006; Helfman Citation2007), and there is a need to establish conservation programs for such species (e.g. Maitland Citation1995; Secretariat of the Conservation on Biological Diversity Citation2010). Molecular analyses in Japan have revealed some cryptic species that have now been described as new species (e.g. Okazaki et al. Citation1991; Takehana et al. Citation2003). However, in the case of two cryptic species of dark chub, ecological information has been confused and there have been few studies of life-history traits to help conserve these species.
The dark chub, Candidia sieboldii (Cyprinidae), is a Japanese endemic freshwater fish found in the middle and lower courses of rivers, lakes and ponds (Hosoya et al. Citation2003). This species was originally distributed in the western parts of Honshu, Shikoku and Kyushu, Japan (Hosoya Citation2013). Additionally, it is now distributed in the Kanto district of eastern Japan, after being artificially introduced (Hosoya Citation2013). Previously, this species was treated as type A of Candidia temminckii and the present C. temminckii was treated as type B (Hosoya Citation1993, using the former name of Zacco temminckii types A and B). Watanabe and Mizuguchi (Citation1988) and Okazaki et al. (Citation1991) found morphologic and genetic differences between these two types. Hosoya et al. (Citation2003) described C. sieboldii as a new species by using morphological differences in the branched rays of the anal fin, lateral line scales and the presence of bands on both sides of the body.
This species is declining in many parts of its natural distribution because of deterioration of river environments and the influence of invasive alien species (e.g. Yamaguchi Prefecture Citation2002, using the former name of Z. temminckii type A; Osaka Prefecture Citation2014). It has been placed on the Red Data List in 10 prefectures in Japan, including the Osaka Prefecture; however, there have been few studies of natural population dynamics to conserve it. Moreover, most life-history information in the literature preceding the work of Hosoya et al. (Citation2003) is from the observations of C. temminckii, which is a more common species in Japan.
This study was designed to elucidate the seasonal growth and population dynamics of C. sieboldii. Kondo et al. (Citation2012) investigated the movement of this species in an artificial still-water canal without weirs and found that individuals sometimes travelled more than 2000 m in 5 days. We performed a mark–release–recapture survey in three branches of the Ishizu River in Osaka Prefecture, central Japan, to investigate this fish's life cycle and dispersal ability.
Methods
Study sites
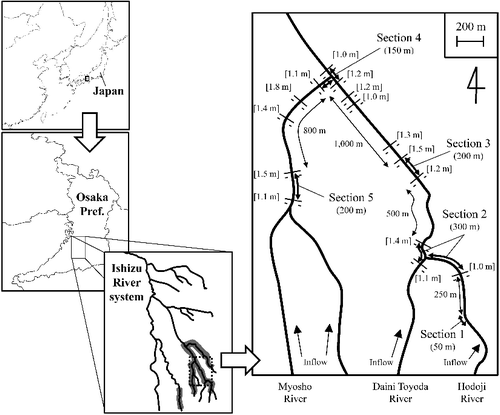
Study sites were located in three branches of the Ishizu River system in the city of Sakai, Osaka Prefecture, Japan, namely the Hodoji, Daini Toyoda and Myosho rivers (). The sites were at elevations of 60–80 m above sea level and 12–14 km from the river mouth. The surface widths of the river branches were 1–3 m. According to the annual report on water conditions of rivers, river discharge near the study site was 0.04–0.59 m3/s between 2012 and 2016 and the variation from year to year was relatively small (Sakai City website [in Japanese]; http://www.city.sakai.lg.jp/kurashi/gomi/kankyo_hozen/kankyokanshi/hokoku/df_filename_742131201706275.html). The area along these river branches comprises mostly agricultural fields, but also includes secondary forest and residential areas. There were eight, one and five weirs (height: 1.0–1.8 m; see ) at the study sites on the Hodoji, Daini Toyoda and Myosho rivers, respectively (). The banks on both sides of the rivers at the study site were protected by concrete.
Mark–release–recapture survey
Field sampling of C. sieboldii was performed every 2 weeks, with a few exceptions, in section 2 of the Hodoji River, between 22 May 2012 and 27 August 2013 (). For the mark–release–recapture survey, specimens of all sizes could be captured with a D-frame hand-net (40-cm wide, 1-mm mesh) because all three rivers are narrow and shallow, with clear water. Any C. sieboldii captured were anaesthetized with ethyl 3-aminobenzoate methanesulfonate salt (Tricaine) (Sigma-Aldrich Japan, Meguro, Tokyo, Japan) and measured for standard length (SL) with an electronic calliper (precision of 0.1 mm; Digimatic Caliper, Mitutoyo Co., Kawasaki, Kanagawa, Japan). We defined SL as the length from the snout tip to the last vertebra. The abdomens of captured fish were lightly pressed to see if they contained sperm or mature eggs. The presence or absence of male nuptial coloration and the pearl organ was also recorded. Newly captured individuals greater than 30 mm SL were marked individually by using combinations of nine colors of fluorescent elastomer (Visible Implant Elastomer, Northwest Marine Technology Inc., Shaw Island, Washington, USA). We excluded individuals less than 30 mm SL because we believed that the injection of fluorescent elastomer would have caused severe damage. If marked fish were recaptured, their individual identities were recorded and they were again measured for SL. The fish were released into the area where they had been caught, after they recovered from the anaesthesia. To evaluate the SL–frequency distribution from each sampling, we calculated the catch per unit effort (CPUE) for each size class as
(Number of captured individuals)/(Number of people sampling)/(Sampling time).
For this survey, there were two or three people sampling and the sampling-time averaged to 30 min (range, 25–50 min).
Population size from 5 June 2012 to 13 August 2013 was estimated by using the Jolly–Seber method (Jolly Citation1965; Seber Citation1965). Growth rates (increase of SL per day) were calculated from the changes in SL in the recaptured individuals.
We also attempted to find marked fish in sections 1 and 3 (upstream and downstream, respectively, of section 2) on 19 July, 29 September, 26 November and 17 December 2012, and on 4 January, 9 April, 23 April, 16 May, 22 June and 27 August 2013. To find out whether the fish moved between the three branches of the river, we performed mark–release–recapture surveys in sections 2, 3 (Hodoji River), 4 (junction of Hodoji and Myosho rivers) and 5 (Myosho River) once every 2 weeks between 30 September and 23 December 2013 by using the same method as described above (). All fish were collected with special permission from the Osaka Prefectural Government.
Results
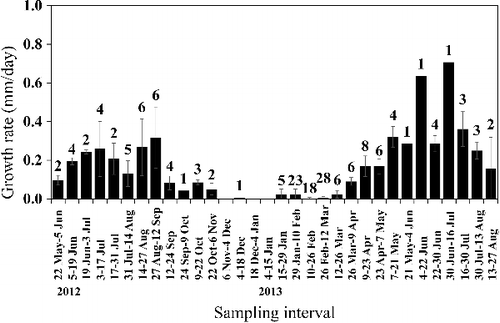
Of the 963 individuals captured in section 2 of the Hodoji River, 275 (29%) were recaptured 1–6 times (446 total recaptures). The longest interval between recaptures was about 15 months. The SL size range with the highest frequency among the total cumulative captures throughout the investigation period (n = 1409) was 30–40 mm, with the frequency decreasing with increasing SL (). The maximum SL was 119 mm, although there were only 19 individuals with SL > 100 mm. Twenty-six (12 males, 14 females) fertile individuals were found between 3 July and 27 August 2012, and 39 (23 males, 16 females) between 7 May and 27 August 2013 (). Fertile males were 68.1–119.0 mm SL and fertile females 71.3–118.2 mm SL. We therefore defined fish at or above 70 mm SL as adults. None of the 26 fertile individuals marked in 2012 were recaptured in 2013. The frequency distribution of the CPUE by size for each month showed a single peak at about 40 mm SL in May 2012 (). After that, the SL with the highest CPUE increased with the season, although the CPUEs decreased between June and October 2012. Similar trends were observed between May and August in 2013. Thus, the lifespan of this species was estimated at 1–2 years. Growth rates were relatively high between June and August in both 2012 and 2013, and lower between November 2012 and March 2013 ().
Figure 2. Distribution of standard lengths of 963 Candidia sieboldii individuals captured during the mark–release–recapture study in section 2 of the Hodoji River, Osaka Prefecture.
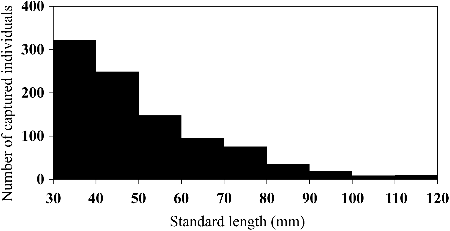
Figure 3. Standard lengths and breeding condition of Candidia sieboldii captured in section 2 of the Hodoji River, Japan. Open and closed circles indicate males and females, respectively, with sperm or eggs and nuptial coloration. Crosses indicate fish that were not fully fertile.
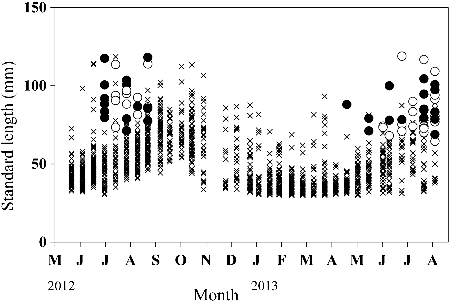
Figure 4. Monthly distribution of the catch per unit effort (CPUE) of Candidia sieboldii by standard length in section 2 of the Hodoji River, Japan.
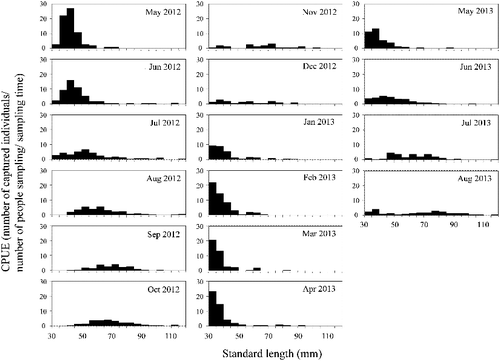
Figure 5. Growth rates (increase in standard length; SL) of Candidia sieboldii between sampling dates in section 2 of the Hodoji River, Japan. Numbers indicate the numbers of recaptures used for calculating growth rates and error bars indicate standard deviations.
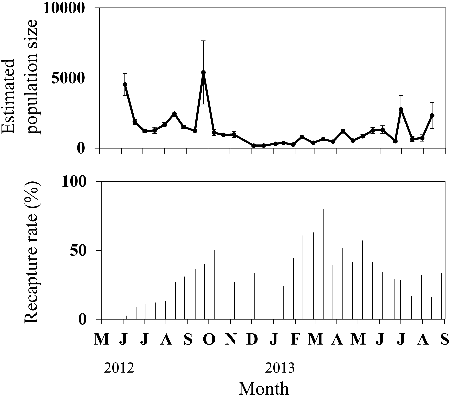
Recapture rates gradually increased with repeat samplings after the third sampling (19 June 2012) and fluctuated between 25% and 80% (). The population size, as estimated by using the Jolly–Seber method, fluctuated between June and October in 2012 and decreased in winter (). In 2013, numbers consistently fluctuated, sometimes exceeding 2000.
Figure 6. Total numbers and 95% confidence intervals for Candidia sieboldii on each sampling date, as estimated by using the Jolly–Seber method (top), and percentage recapture rates (bottom) in section 2 of the Hodoji River, Japan.
We captured 208 fish in section 1 and 297 in section 3, and a single individual that was marked in section 2 was found 500 m downstream from its release point, in section 3. This individual was first captured in section 2 on 27 August 2012 and was recaptured once in section 2 on 12 September 2012. It was then recaptured on 17 December 2012 and 4 January 2013 in section 3.
In our sampling to detect the movement between the three river branches, 409 individuals were marked (154, 127, 35 and 93 individuals in sections 2, 3, 4 and 5, respectively) and there were 25 recaptures (4, 12, 2 and 7 recaptures in sections 2, 3, 4 and 5, respectively). However, no recaptures involved individuals from a different branch.
Discussion
We clarified life-history traits of C. sieboldii including breeding season, size at maturity, growth rate and dispersal. According to Hosoya (Citation2013), the breeding season of this species is from June to July. Our observations of fertile individuals indicated that the breeding season begins in June and continues to August; males with nuptial coloration were seen between May and August. The size at maturity that we found (around 70 mm SL) was almost the same as that of C. temminckii (Miyaji et al. Citation1976), and a little larger than that of Opsariichthys platypus (68 mm total length) (Mizuguchi and Hiyama Citation1969). O. platypus is in a species related to C. sieboldii (Chen et al. Citation2008); we also found this species in the Ishizu River system.
According to Sado and Kimura (Citation2002), eggs of C. sieboldii hatch 47–60 h after fertilization, and the body length of newly hatched fish is 3.50–4.76 mm. Fish with SLs of 30–40 mm first appeared in our samples between November and January and had grown to 70 mm by the following summer. In the case of C. temminckii, eggs hatch 40–53 h after fertilization and the body length of newly hatched fish is 4.90–5.28 mm (Sado and Kimura Citation2002). C. temminckii grows to 15–25, 30–50 and 62–71 mm in 1, 2 and 3 years, respectively (Katano Citation1993). Thus, newly hatched larvae of C. sieboldii are smaller than those of C. temminckii. At our study site, however, growth rates of C. sieboldii after hatching were higher than that of C. temminckii observed by Katano (Citation1998). Katano (Citation1998) also suggested that the growth rates of C. temminckii were highest from April to May, and those of males were higher than those of females from May to August. We found here that the growth rates of C. sieboldii did not differ between males and females, and those during summer were higher than in winter.
Mark–release–recapture methods are often used to determine the dispersal ability of cyprinid fishes (e.g. Goforth and Foltz Citation1998; Bolland et al. Citation2009; Kondo et al. Citation2012). Kondo et al. (Citation2012) reported that, among 550 marked individuals of C. sieboldii released in an artificial still-water canal without weirs, 39 (7%) were captured within 7 days. In their study, the maximum recapture distance from the release point was 2400 m (n = 2) and more than 10 individuals moved more than 1000 m. We found here that only one individual moved 500 m downstream from the release point. There were, however, weirs at our study site that could interfere with fish movement – especially movement from downstream to upstream. Because most of our recapture rates remained higher than 25% throughout the year, we consider this species to be sedentary throughout its life. Molecular analysis revealed significant divergence of this species among branches within the Ishizu River system (H. Matsuoka et al. unpublished data). These observations suggest that the dispersal of this species is restricted by the presence of weirs at the study site.
Although this species has been described as reaching an SL of 150–200 mm (Hosoya Citation2015) or 180 mm (Kimura Citation2009), we found few individuals over 100 mm SL. In addition, no fertile fish marked in 2012 were found in 2013. We therefore hypothesize that most fertile individuals die after the breeding season. According to Katano (Citation1998), the maximum observed size of an individual C. temminckii in the Kiyotaki River, Kyoto Prefecture (located to the north of the Osaka Prefecture) was 157 mm SL; it was 8 years old. Because the study site of Katano (Citation1998) was at a higher elevation (150–200 m) in a deciduous forest, the water temperature, which affects the growth rate, was lower than that in our study. Many related species of freshwater fishes have different altitudinal distributions. Schlosser (Citation1990) studied stream fishes in the United States and suggested that upstream species often have a shorter lifespan, smaller body and earlier sexual maturity than do downstream species. Goto (Citation1998) and Yan et al. (Citation2012) studied populations of Cottus nozawae in Japan and Acrossocheilus fasciatus in China, respectively, and found that the downstream populations had a higher growth rate, larger body and later sexual maturity than did the upstream populations. Generally, C. sieboldii prefers lentic waters in the middle to lower courses of rivers, whereas the related species C. temminckii inhabits clear water in the upper to middle courses (Hosoya Citation2013), although the habitats of the two species often overlap. The life-history traits of C. sieboldii, such as a short lifespan, high growth rate and early sexual maturity, are similar to those reported for upper-stream fishes and might be related to their habitat of small creeks, small river branches and canals.
Our mark–release–recapture results show that the lifespan for C. sieboldii is only 1–2 years for most individuals, and that this species has low dispersal frequency. A short lifespan can increase the risk of breeding failure, and a low frequency of dispersal, restricted by weirs, makes it difficult for a population to become re-established and recover. Because there is concern that populations of this species are declining in several places, conservation measures should be considered to prevent a continued decline in habitat area and population sizes. In other freshwater fishes, decreasing species diversity and population fragmentation have been attributed to weirs and other human artifacts (Wolter Citation2001; Meldgaard et al. Citation2003). According to Kondo et al. (Citation2012), the dispersal distance of C. sieboldii reaches more than 200 m per day in calm water if there is no barrier. Nevertheless, we found here that the frequency of dispersal beyond weirs was quite low. Thus, the lack of movement in our study area can be attributed to direct interference by the weirs. Moreover, in such a small habitat, local extinction could easily happen because, as this study shows, the lifespan of this species is very short. Effectiveness of fish ladders have been reported in various stream fishes (e.g. Souchon and Keith Citation2001; Stantucci et al. Citation2005; Roscoe and Hinch Citation2010; Hatry et al. Citation2016). To conserve this species, therefore, weirs should be removed, or each one should be provided with an efficient fish ladder to facilitate movement throughout the area.
Acknowledgments
We are very grateful to T. Hirowatari, S. Ueda, K. Hanazaki, K. Uehara and S. Aoyama for their invaluable comments and advice. We also thank our laboratory colleagues for their helpful cooperation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Haruka Matsuoka
Haruka Matsuoka earned a master's degree in Life and Environmental Sciences from Osaka Prefecture University, Japan in 2014. His research interests include freshwater fish ecology and conservation.
Norio Hirai
Norio Hirai is an associate professor at Osaka Prefecture University in the Laboratory of Environmental Entomology and Zoology with interests in life history and conservation ecology of small animals such as insects, fishes, amphibians etc.
Minoru Ishii
Minoru Ishii has been a professor of the Graduate School of Life and Environmental Sciences at Osaka Prefecture University, Japan since 1996 after receiving a doctorate from Kyoto University and joining OPU in 1985 and is now serving as a Vice-President of OPU. He has conducted numerous researches in the field of animal ecology, entomology and conservation biology.
References
- Bolland JD, Cowx IG, Lucas MC. 2009. Dispersal and survival of stocked cyprinids in a small English river: comparison with wild fishes using a multi-method approach. J Fish Biol. 74:2313–2328.
- Chen IS, Wu JH, Hsu CH. 2008. The taxonomy and phylogeny of Candidia (Teleostei: Cyprinidae) from Taiwan, with description of a new species and comments on a new genus. Raffles B Zool. 19:203–214.
- Dudgeon D, Arthington AH, Gessner MO, Kawabata ZI, Knowler DJ, Leveque C, Naiman RJ, Prieur-Richard AH, Soto D, Stiassny MLJ, et al. 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev. 81:163–182.
- Goforth RR, Foltz JW. 1998. Movements of the yellowfin shiner, Notropis lutipinnis. Ecol Fresh Fish. 7:49–55.
- Goto A. 1998. Life-history variations in the fluvial sculpin, Cottus nozawae (Cottidae), along the course of a small mountain stream. Environ Biol Fish. 52:203–212.
- Hatry C, Thiem JD, Hatin D, Dumont P, Smokorowski KE, Cooke SJ. 2016. Fishway approach behaviour and passage of three redhorse species (Moxostoma anisurum, M. carinatum, and M. macrolepidotum) in the Richelieu River, Quebec. Environ Biol Fish. 99:249–263.
- Helfman GS. 2007. Fish conservation: a guide to understanding and restoring global aquatic biodiversity and fishery resources. Washington (DC): Island Press.
- Hosoya K. 1993. Cyprinidae. In: Nakabo T, editor. Fishes of Japan with pictorial keys to the species. Tokyo: Tokai University Press; p. 212–230. Japanese.
- Hosoya K. 2013. Cyprinidae. In: Nakabo T, editor. Fishes of Japan with pictorial keys to the species. 3rd ed. Tokyo: Tokai University Press; p. 308–327. Japanese.
- Hosoya K. 2015. Nihon no tansui-gyo [The Japanese freshwater fish]. Tokyo: Yama to keikoku-sya. Japanese.
- Hosoya K, Ashiwa H, Watanabe M, Mizuguti K, Okazaki T. 2003. Zacco sieboldii, a species distinct from Zacco temminckii (Cyprinidae). Ichthyol Res. 50:1–8.
- Jolly GM. 1965. Explicit estimates from capture–recapture data with both death and immigration-stochastic model. Biometrika. 52:225–247.
- Katano O. 1993. Factors affecting mating status of the male dark chub, Zacco temmincki. Physiol Ecol Japan. 29:118–131.
- Katano O. 1998. Growth of dark chub, Zacco temmincki (Cyprinidae), with a discussion of sexual size differences. Environ Biol Fish. 52:305–312.
- Kimura Y. 2009. Nihon no tansui-gyo [The Japanese freshwater fish]. Tokyo: Gakusyu kenkyu-sya. Japanese.
- Kondo M, Ito K, Senge M. 2012. Moving distance of Nipponocypris sieboldii, the host fish species of unionid mussels. Trans Jpn Soc Irrig Drain Reclam Eng (TJSIDRE). 282:51–57. Japanese with English summary.
- Maitland PS. 1995. The conservation of freshwater fish: past and present experience. Biol Conserv. 72:259–270.
- Meldgaard T, Nielsen EE, Loeschcke V. 2003. Fragmentation by weirs in a riverine system: a study of genetic variation in time and space among populations of European grayling (Thymallus thymallus) in a Danish river system. Conserv Genet. 4:735–747.
- Miyaji D, Kawanabe H, Mizuno N. 1976. Gensyoku nihon tansui-gyorui zukan [Picture book of the Japanese freshwater fish]. Osaka: Hoiku-sya. Japanese.
- Mizuguchi K, Hiyama Y. 1969. Reproduction of the Oikawa, Zacco platypus (Temminck and Schlegel), a Cyprinid-I. Sexual characters in the anal fin and maturation. Jpn J Ichthyol. 16:17–23. Japanese with English summary.
- Okazaki T, Watanabe M, Mizuguti K, Hosoya K. 1991. Genetic differentiation between two types of dark chub, Zacco temminckii, in Japan. Jpn J Ichthyol. 38:113–140.
- Osaka Prefecture. 2014. Red list of Osaka prefecture 2014. Osaka: OsakaPrefectural Government. Japanese. [accessed 2017 Feb 11]. http://www.pref.osaka.lg.jp/attach/21490/00148206/p13gyo.pdf
- Roscoe DW, Hinch SG. 2010. Effectiveness monitoring of fish passage facilities: historical trends, geographic patterns and future directions. Fish Fish. 11:12–33.
- Sado T, Kimura S. 2002. Descriptive morphology of the eggs, larvae, and juveniles of two cyprinid fishes belonging to the Zacco temminckii species’ group. Ichthyol Res. 49:245–252.
- Santucci VJ Jr, Gephard SR, Pescitelli SM. 2005. Effects of multiple low-head dams on fish, macroinvertebrates, habitat, and water quality in the Fox River, Illinois. North Am J Fish Mana. 25:975–992.
- Schlosser IJ. 1990. Environmental variation, life history attributes, and community structure in stream fishes: implications for environmental management and assessment. Environ Manage. 14:621–628.
- Seber GAF. 1965. A note on the multiple-recapture census. Biometrika. 52:249–259.
- Secretariat of the Conservation on Biological Diversity. 2010. Global biodiversity outlook 3. Montreal (QC): Secretariat of the Conservation on Biological Diversity.
- Souchon Y, Keith P. 2001. Freshwater fish habitat: science, management, and conservation in France. Aquat Ecosyst Health Manag. 4:401–412.
- Takehana Y, Nagai N, Matsuda M, Tsuchiya K, Sakaizumi M. 2003. Geographic variation and diversity of the cytochrome b gene in Japanese wild populations of Medaka, Oryzias latipes. Zool Sci. 20:1279–1291.
- Watanabe M, Mizuguchi K. 1988. On the two types of the dark chub (Zacco temminckii)-IV. Morphological variation of B-type (fluvial type) [abstract]. 16th Annual Meeting of Ichthyological Society of Japan. 15.
- Wolter C. 2001. Conservation of fish species diversity in navigable waterways. Landscape Urban Plan. 53:135–144.
- Yamaguchi Prefecture. 2002. Red data book – Yamaguchi. Yamaguchi: Yamaguchi Prefectural Government. Japanese.
- Yan YZ, Zhu R, He S, Chu L, Liang YY, Chen YF. 2012. Life-history strategies of Acrossocheilus fasciatus (Barbinae, Cyprinidae) in the Huishui Stream of the Qingyi watershed, China. Ichthyol Res. 59:202–211.