ABSTRACT
Availability and selection of macroinvertebrate prey is important to explain temporal and spatial variation in growth among stream salmonids. However, few studies contain information to identify such relationships. Our objectives were to quantify drift and benthic macroinvertebrate prey availability and selection by brown trout on a seasonal basis in five streams across three years in southeastern Minnesota. Few taxa were dominant in diets and the environment with considerable variability in drifting and benthic prey within streams and seasons. Brown trout consistently selected only one or two taxa, and displayed neutral or negative selection for other taxa. In general, large-bodied, energy-rich benthic prey were selected over other more abundant aquatic macroinvertebrate taxa and drifting prey. Foraging patterns suggested a preference of benthic feeding. Electivity of benthos and drift varied spatially and temporally with a negative relationship between the total proportion of prey available and prey electivity. In general, seasonal growth and prey electivity were not related across all streams, but were positively related within two of five streams. Understanding seasonal and spatial relationships among growth, prey availability, and prey selection may aid future management of streams, as climate change is expected to alter physical conditions and biological communities of streams.
Introduction
Growth of stream-dwelling salmonids varies greatly among seasons, reflecting the highly seasonal nature of temperate stream environments (Letcher et al. Citation2002). Reduced temperature, light, and macroinvertebrate productivity have been linked with low or negative growth in many streams (Metcalfe and Thorpe Citation1992); however, in southeastern Minnesota, there is considerable spatial and seasonal variation relative to maximum growth rates of brown trout (Dieterman et al. Citation2004; Dieterman et al. Citation2012; French et al. Citation2014). Groundwater-dominated streams in this region support highly productive populations of brown trout, but demonstrate variation in production and diversity of aquatic macroinvertebrates (Troelstrup and Perry Citation1989; Waters Citation2000).
Invertebrate availability and accessibility on a seasonal and temporal scale may influence brown trout prey selectivity, potentially affecting growth in small groundwater-dominated streams where aquatic insects are the major prey items (Elliott Citation1970; Hunt and Krokhin Citation1975; Pedley and Jones Citation1978; Allan Citation1981). Trout are opportunistic generalists and some studies have linked growth with the abundance of drifting invertebrate prey (Wilzbach et al. Citation1986; Erkinaro and Niemelä Citation1995). Many studies emphasize the importance of drifting prey to trout diet, whereas the contribution of benthos has been less studied (Tippets and Moyle Citation1978; Johansen et al. Citation2010. Past research in southeastern Minnesota provides evidence for drift feeding of brown trout during summer (Newman Citation1987) with neutral selection of terrestrial drift (Laudon et al. Citation2005), and a switch to benthic feeding during fall and winter (Newman Citation1987; Grant Citation1999; Anderson Citation2012; French Citation2014).
Studies in temperate climates have reported a lack of similarity in the composition of macroinvertebrate assemblages and foraging patterns of brown trout, and few studies have assessed these patterns across streams, seasons, and years (Stoneburner and Smock Citation1979; Allan Citation1987; Shearer et al. Citation2002; Leung et al. Citation2009). In general, studies of fish growth, prey resources, and selection in temperate climates are largely restricted to spring, summer, and fall. Few investigations have addressed winter dynamics of macroinvertebrates and trout during cold periods in more than one stream (Lord Citation1933; Maciolek and Needham Citation1952; Cunjak et al. Citation1987; Cunjak and Power Citation1987; Heggenes et al. Citation1993; Fochetti et al. Citation2003; Utz and Hartman Citation2007; White and Harvey Citation2007; Johansen et al. Citation2010). Furthermore, many studies are limited to milder climates (White and Harvey Citation2007), or diets without addressing prey preference or availability (Cunjak et al. Citation1987; Cunjak and Power Citation1987). Several investigations have only examined prey in the drift (e.g. Heggenes et al. Citation1993; Simpkins and Hubert Citation2000) or benthos (e.g. Fochetti et al. Citation2003). Finally, studies of growth of brown trout and prey availability in groundwater-dominated streams of southeastern Minnesota are limited. Recent work examined winter diet and growth (French Citation2014; French et al. Citation2014), and winter prey selectivity (Anderson et al. Citation2016) in southeastern Minnesota; however, no study has simultaneously addressed brown trout growth and prey selectivity of both benthic and drifting macroinvertebrates, and spanned multiple seasons, years, and streams. Thus, variation in the diet and growth of brown trout in southeastern Minnesota may reflect differences in prey assemblages and selectivity on a seasonal and spatial scale.
Our goals were to quantify drift and benthic macroinvertebrate prey availability and selection by brown trout on a seasonal basis in five streams across three years in southeastern Minnesota. The objectives were to: (1) characterize seasonal and spatial trends in the assemblages of benthic and drifting prey; (2) determine whether brown trout select prey relative to availability in the environment, and identify seasonal and spatial trends in how prey are selected; and (3) investigate seasonal and spatial relationships between brown trout growth and prey selectivity. Understanding seasonal and spatial relationships among growth, foraging, prey availability, and prey selection may aid in the future management of groundwater-dominated streams, as climate change is expected to alter physical conditions and biological communities of streams in this region. Identifying the prey taxa most important to the growth of brown trout will provide managers with critical information for taking actions that mitigate the impacts of climate change on groundwater-dominated streams in southeastern Minnesota.
Methods
Study area
This study was conducted in five groundwater-dominated streams located in the Driftless Ecoregion of southeastern Minnesota (Omernik and Gallant Citation1988) (). This region was relatively unaffected by the most recent glaciation, and is characterized by sandstone valleys, limestone bluffs, and 181 groundwater-dominated streams that comprise 1268 stream kilometers (MNDNR Citation2003). These coldwater streams are supplied with fertile water by a high number of active springs, and yield diverse and abundant assemblages of aquatic invertebrates (Waters Citation1977) and productive populations of brown trout (Thorn and Ebbers Citation1997). Brown trout, first introduced to the region in 1888 (Thorn et al. Citation1997), are now the most abundant salmonid species in southeastern Minnesota (Thorn Citation1990).
Sample sites
To investigate how spatial and temporal differences in prey availability and selection may relate to differences in fish growth, site selection was based on stream accessibility, fish abundance, and a gradient of growth among streams reported by Dieterman et al. (Citation2004). Physical characteristics (e.g. thermal regime, discharge area) of the selected streams were typical of groundwater-dominated streams of southeastern Minnesota. Within each stream, a reach of ∼ 200 m was selected for fish and invertebrate sampling. Most streams contained varying degrees of habitat improvement for trout management (Thorn et al. Citation1997). Study streams were ‘summer-cold/winter-warm’ and remained free of ice during winter.
Fish collection, diet, and growth measurements
Brown trout were sampled in each stream on four to six sample dates per year in 2010–2013. Logistical limitations and brown trout spawning in fall resulted in some disparity among the number of sample dates within each season across streams. Fish were collected using a Smith Root® (Washington, USA) LR 20B backpack electrofisher. Following a single pass of electrofishing along the entire study reach, captured fish were placed within in-stream holding pens, anesthetized with an immobilizing dose of clove bud oil, weighed (± 1 g), and measured (± 1 mm TL). Up to 150 brown trout (>100 mm TL) per stream per year were tagged in the anterior portion of the body cavity with 9 mm passive integrated transponder (PIT) tags (Biomark Inc.; Idaho, USA) to measure growth (G; g/day). Random subsamples of up to 30 fish on each sampling date were selected to examine diet composition using gastric lavage. When possible, the subsample contained 10 fish within each of the following size ranges to evaluate diets across a variety of available age classes: 100 and 199 mm, 200 and 299 mm, and >300 mm. Captured fish were placed into a holding pen within the stream to recover from anesthesia and then released into the study site. Stomach contents were preserved in 95% ethanol in the field. In the laboratory, aquatic macroinvertebrates in stomach samples were sorted, identified to the lowest practical taxonomic group, and counted. Only intact specimens or fragments greater than one-half an intact individual were counted.
Instantaneous daily growth (G; g/day) was calculated for all tagged fish recaptured on at least two, subsequent sampling events using the following equation:where W represents weight (g), and t represents the number of days between sampling events.
Invertebrate collection
Drifting macroinvertebrates were collected within 24 hours preceding or following fish collections. Four drift nets (45 cm × 25 cm, with a 1 m long bag and 363 um Nitex mesh) were placed in a contiguous line perpendicular to the shoreline in a randomly selected riffle within the study reach. Water velocity and depth was measured in three locations across the mouth of each net using a Marsh-McBirney Flo-Mate™ Model 2000 Flowmeter. Sampling was conducted within approximately one hour of sunrise and one hour before sunset, the period when salmonids actively feed on macroinvertebrate drift during summer, spring, and fall. During winter, drift nets were set approximately one hour before sunset and remained in the stream for a minimum of 12 hours. Contents of the nets were passed through a 125 μm sieve, preserved in 70% ethanol, and transferred to the lab for processing.
In the laboratory, macroinvertebrates were picked, sorted, and identified to the lowest practical taxonomic group that was reasonable for accuracy (typically Family or Genus) using a dissection microscope. Samples were sorted for a maximum of four hours. Nearly all drift samples (339 of 345, or 98%) were completely sorted within four hours; however, if sorting was not completed, the percentage of the sample processed was estimated, recorded, and multiplied by the total count of each taxon already sorted.
Benthic macroinvertebrate samples were collected immediately following fish collection with a Waters-Knapp modification of a Hess sampler (0.086 m2). Five samples were taken from riffles randomly selected within each reach by disturbing the substrate for three minutes. As with drift samples, benthic collections were passed through a 125 μm sieve prior to preservation in 70% ethanol. Macroinvertebrates were picked from each sample, sorted, and identified by taxa using a dissection microscope. Samples were sorted for a maximum of four hours. Nearly all benthic samples (215 of 224, or 96%) were completely sorted within four hours. However, if sorting was not completed in four hours, the percentage of the sample processed was estimated, recorded, and multiplied against counts of individuals already sorted for each taxon.
Analysis
A multiple response permutation procedure (MRPP) (Zimmerman et al. Citation1985; McCune and Grace Citation2002) was performed in program R (version 3.1.2) to compare brown trout diet and taxonomic composition of drift and benthos between streams and seasons. MRPP makes no distributional assumptions (Smith Citation1998) and a distance matrix is calculated using any number of possible distance measures. Each analysis was performed using proportions of each invertebrate taxon, determined by dividing the number of individuals from a given taxon by the total number of individuals collected on each sample date. Only taxa >1% of total invertebrates were used in the analysis. Proportions were arcsine transformed prior to analysis, and separate tests were run for drift and benthos. Probability of type I error was calculated using a randomization algorithm that allows for comparison between observed δ (weighted mean within-group distance) and the randomized δ distribution. This probability value expresses the likelihood of generating a random δ smaller than the observed value. An effect size of A was also calculated asand represents observed within-group homogeneity relative to what could be expected by chance (McCune and Grace Citation2002). For this study, A provides a measure of the overall agreement among the relative quantity and diversity of invertebrates within the group designated (e.g. stream, season, and year). Within-group homogeneity is greater than the random observation when A > 0 and less when A < 0. The A-value is useful in attaching ecological significance to observed differences among groups because it is independent of sample size (McCune and Grace Citation2002).
A Kruskal–Wallis test (Sokal and Rohlf Citation1981) was used to determine whether there was a significant difference among means of the relative proportion of taxonomic groups among seasons and streams. This test does not indicate the difference between means, only whether the difference is statistically significant. For this analysis, all prey data were expressed as a proportion or percentage contribution of a particular taxon to the overall collection. Prey categories comprising <5% of total prey were combined into a single ‘Other’ category.
Patterns in drift and benthic prey selectivity across streams and seasons were analyzed with the Manly–Chesson index (α) (Manly Citation1974; Chesson Citation1978, Citation1983) using the numerical proportion of each prey category in brown trout diets:where ri is the proportion of food item i in the diet, pi is the proportion of food item i in the environment, and m is the number of food items in the environment. Invertebrate categories that comprised <5% of total diet and available prey were combined into a single ‘Other’ category.
Values of α range from 0 (complete avoidance) to 1 (complete preference). When α = 1/m, prey are consumed in proportion to abundance in environment, whereas α > 1/m indicates preference, and α < 1/m indicates avoidance. Manley–Chesson's index allows for temporal and spatial comparisons among selectivity values even if the relative abundances of prey types in the environment change (Chesson Citation1983). Mean selectivity and the value for random feeding can be evaluated by testing the null hypothesis that α is equal to 1/m, using a t-test comparing mean αi with 1/m for each prey category to identify significant trends in prey selection within seasons and streams (Chesson Citation1983).
Overall electivity, or preference, of each prey item (i) was determined by centering the estimated values of α on zero using the following:
Electivity () scales from −1 to 1; where −1 indicates total avoidance of a prey; 0 indicates a prey item taken in proportion to its abundance; and 1 indicates total preference for a prey. Prey electivity was calculated as the mean of the absolute value of
. A Kruskal–Wallis test was performed to compare brown trout prey electivity among streams and seasons.
The relationship between mean fish growth (g/day) and mean prey selectivity (α) on a seasonal basis was evaluated with a simple linear regression, using data from all streams combined and using data from each individual stream at a stream-scale.
Results
Diet composition
The composition of brown trout diets was similar across seasons (MRPP, A < 0.01, p = 0.31) for all streams combined (). In contrast, diet composition differed across streams (MRPP, A = 0.11, p < 0.01) for all seasons combined ().
Table 1. Seasonal variation in prey assemblages and diet composition of brown trout across five streams in southeastern Minnesota, 2011–2013, based on a MRPP test.
Table 2. Variation in prey assemblages and brown trout diet composition among five streams in southeastern Minnesota, 2011–2013, based on a MRPP test.
Drift composition
Taxonomic composition of drifting invertebrates across seasons was significantly different (MRPP, A = 0.04, p = 0.01) for all streams and sample dates combined (). Mean proportional availability of taxa also varied significantly within all seasons. Chironomidae (mostly larval and pupal forms) was proportionally the most abundant drifting prey overall ( = 0.59, H = 11, d.f. = 3, p < 0.01); and within each season, ranging from 0.39 in winter (H = 160, d.f. = 3, p < 0.01) to 0.72 in fall (H = 81.4, d.f. = 3, p < 0.01).
Overall, the total proportion of drifting macroinvertebrates varied significantly across seasons (H = 27.4, d.f. = 4, p < 0.01); the highest proportion of drift was collected in spring ( = 0.47), and the lowest proportion was collected in winter (
= 0.07). In addition, the mean proportional distribution of the following drifting prey varied across seasons: Brachycentrus (H = 16.5, d.f. = 3, p < 0.01), Chironomidae (H = 28.5, d.f. = 3, p < 0.01), and Simuliidae (H = 13.2, d.f. = 3, p < 0.01). In spring, the highest relative proportions were collected for Brachycentrus (
= 0.54), Chironomidae (
= 0.44), and Simuliidae (
= 0.66), whereas the lowest proportions of these taxa were collected in winter (
= 0.06, 0.04, and 0.05, respectively).
The distribution of drifting prey types also varied significantly across streams when data from all sample dates and seasons were combined (MRPP, A = 0.06, p < 0.01) (). Chironomidae was the most abundant drifting prey collected in all streams, with the highest mean proportion in Daley Creek ( = 0.66, H = 121, d.f. = 3, p < 0.01), followed by Beaver Creek (
= 0.60, H = 104, d.f. = 3, p < 0.01).
Although there was no variation in the mean proportion of total drift collected across streams, the mean proportional distribution of the following drifting prey types did vary: Brachycentrus (H = 62.7, d.f. = 4, p < 0.01), Chironomidae (H = 13.2, d.f. = 4, p < 0.01), and Baetis (H = 9.4, d.f. = 4, p = 0.05). Interestingly, the highest mean proportions of all drifting prey taxa were collected in Garvin Brook, and ranged from 0.28 of all Baetis to 0.63 of all Brachycentrus.
Benthic composition
Taxonomic composition of benthic assemblages was significantly different across seasons (MRPP, A = 0.02, p = 0.05) (), and for the relative proportion of benthic prey within each season. Chironomidae comprised 26% of benthic prey (H = 121, d.f. = 3, p<0.01), and was the most abundant taxon collected in summer ( = 0.33, H = 92.9, d.f. = 3, p < 0.01) and fall (
= 0.25, H = 59.1, d.f. = 3, p < 0.01). Brachycentrus was proportionally the most abundant prey in spring (
= 0.28, H = 84.1, d.f. = 3, p < 0.01) and winter (
= 0.26, H = 23.5, d.f. = 3, p < 0.01), and the second most available prey overall (
= 0.23).
Relative proportional distributions of many benthic prey taxa varied across seasons for all streams and sample dates combined, similar to the drift. Distributions of Baetis, Elmidae, and Gammarus were relatively similar across seasons, whereas distributions of Brachycentrus (H = 13.2, d.f. = 3, p < 0.01), Chironomidae (H = 14.1, d.f. = 3, p < 0.01), and Simuliidae, predominately Simulium, (H = 13.1, d.f. = 3, p < 0.01) were significantly different. Proportions of Brachycentrus ( = 0.36) and Simuliidae (
0.48) were highest during spring and lowest during fall (
= 0.17,
= 0.05, respectively), whereas the proportion of benthic Chironomidae (
= 0.42) was highest during summer and lowest during winter (
= 0.12). Relative contributions of total benthic prey varied significantly by season, and were highest in summer (
= 0.33) and lowest in winter (
= 0.18) (H = 7.5, d.f. = 3, p = 0.05).
The taxonomic composition of benthic assemblages was significantly different among streams (MRPP, A = 0.13, p < 0.01) (), as were the relative proportions of prey types collected within each stream for all sample dates and seasons combined. Chironomidae was the most abundant benthic prey collected in Trout Run Creek ( = 48.0, H = 100.7, d.f. = 6, p < 0.01), Daley Creek (
= 34.3, H = 121, d.f. = 6, p < 0.01), and Gribben Creek (
= 21.4, H = 70.7, d.f. = 6, p < 0.01). Brachycentrus was most abundant in Garvin Brook (
= 22.5, H = 40.8, d.f. = 6, p < 0.01) and Beaver Creek (
= 37.4, H = 116, d.f. = 6, p < 0.01).
The relative proportional distributions of all benthic taxa, with the exception of Chironomidae (H = 7.2, d.f. = 4, p = 0.11), varied significantly across streams. Garvin Brook contained the highest proportions of Brachycentrus ( = 0.52, H = 77.5, d.f. = 4, p<0.01) and Baetis (
= 0.29, H = 12.9, d.f. = 4, p = 0.01), whereas approximately 75% of all Elmid beetles were collected in Beaver Creek (H = 130.9, d.f. = 4, p < 0.01). Daley Creek contained the highest proportions of Simuliidae (
= 0.43, H = 20.3, d.f. = 4, p < 0.01) and Gammarus (
= 0.53, H = 48.7, d.f. = 4, p < 0.01). The highest relative proportion of all benthic prey was collected in Garvin Brook (
= 0.32) followed by Beaver Creek (
= 0.21), whereas Gribben Creek contained the least (
= 0.11, H = 14.1, d.f. = 4, p < 0.01).
Prey selection
Overall
Mean electivity () of brown trout in all streams varied significantly among prey taxa, when benthos and drift were combined (H = 135.8, d.f. = 7, p < 0.01). Brown trout selected Physella (
= 0.32) and Gammarus (
= 0.06), and avoided all other taxa.
Selection of Physella was significantly greater than neutral (1/m = 0.13) ( = 0.37, d.f. = 40, p < 0.01) in the benthos, whereas brown trout avoided Brachycentrus (
= 0.08, d.f. = 45, p < 0.05), Elmidae (
= 0.02, d.f. = 31, p < 0.01), and Baetis (
= 0.07, d.f. = 42, p < 0.01) (). Electivity of drifting Physella (
= 0.40, d.f. = 31), Brachycentrus (
= 0.20, d.f. = 43, p < 0.01), and Gammarus (
= 0.29, d.f. = 5, p < 0.01) was significantly greater than neutral selection (1/m = 0.14); however, brown trout avoided Chironomidae (
= 0.09, d.f. = 49, p < 0.05) and Simuliidae (
= 0.02, d.f. = 39, p < 0.01) ().
Table 3. Mean prey selectivity (Manly-Chesson index, αi; SD) overall for brown trout collected from five streams in southeastern Minnesota, 2011–2013. Values significantly different (*p < 0.05), from 1/m are indicated by ‘+’ for positive selection and ‘ for negative selection.
Prey electivity by season
Mean electivity () of brown trout in all streams also varied significantly among prey taxa on a seasonal basis, when benthos and drift were combined: winter (H = 34.5, d.f. = 9, p < 0.01), spring (H = 65.1, d.f. = 7, p < 0.01), summer (H = 43.6, d.f. = 7, p < 0.01), and fall (H = 19.6, d.f. = 6, p < 0.01). Limnephilidae was the most highly selected prey during winter (
= 0.55) and the second most highly selected prey in spring (
= 0.17). Physella was the most selected prey type during spring (
= 0.32) and fall (
= 0.22), and second most highly selected prey during winter (
= 0.46). Gammarus was most highly selected for during summer (
= 0.15).
In contrast, mean electivity of brown trout was lowest for Ephemerellidae during winter =
0.99), followed by Simuliidae (
=
), which were also avoided during spring (
=
0.72), and summer (
=
0.80). During summer, mean electivity was lowest for Elmidae (
=
0.99).
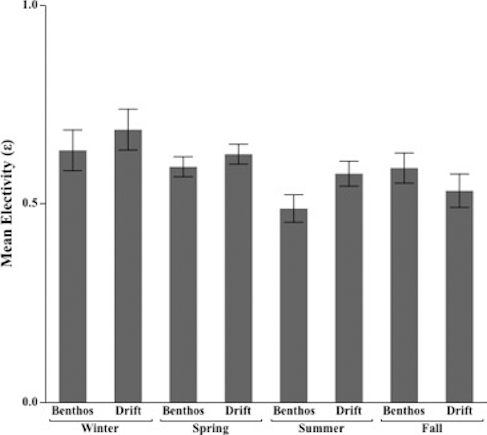
Mean electivity () varied for benthos (H = 8.6, d.f. = 3, p = 0.03) and drift (H = 7.9, d.f. = 3, p = 0.04) among seasons when all sample dates were combined (). In general, brown trout demonstrated highest selectivity toward drifting (
= 0.69) and benthic (
= 0.63) prey during winter. Fish were least selective for drift during fall (
= 0.53) and least selective of benthos during summer (
= 0.49) ().
Figure 2. Mean electivity ( ±1 s.e.) of brown trout by season for benthos (H = 8.6, d.f. = 3, p = 0.04) and drift (H = 7.9, d.f. = 3, p = 0.04) in five streams across southeastern Minnesota, 2011–2013.
Winter selection and electivity: benthos vs. drift. When data from all streams were combined, brown trout avoided Simuliidae ( = 0.02, d.f. = 3, p < 0.01) and Brachycentrus (
= 0.03, d.f. = 5, p < 0.01) in winter in the benthos and mean selection of Simuliidae (
= 0.01, d.f. = 5, p < 0.01), Baetis (
= 0.02, d.f. = 5, p < 0.01), Ephemerellidae (
= 0.0, d.f. = 2, p < 0.01), and Perlidae (
= 0.0, d.f. = 1, p < 0.01) in the drift was significantly lower than neutral (1/m = 0.11) (). Brown trout demonstrated selection of drifting Physella (
= 0.53, d.f. = 3, p = 0.03) during winter, but other values for prey selection in the benthos and drift were not significantly different than random ().
Table 4. Mean prey type selectivity (Manly-Chesson index, αi; SD) by season for brown trout collected from five streams in southeastern Minnesota, 2011–2013. Values significantly different (*p < 0.05), from 1/m are indicated by ‘+’ for positive selection and ‘ for negative selection; n is the number of independent sample dates.
Between streams, brown trout demonstrated differences in electivity () for drifting prey types in winter. Physella was the most selected drifting prey type in Beaver Creek (
= 0.84), Daley Creek (
= 0.89), and Gribben Creek (
= 0.96), whereas brown trout selected Chironomidae in Garvin Brook (
= 0.58) and Brachycentrus in Trout Run Creek (
= 0.98). Gammarus were selected in Daley Creek (
= 0.53) and Gribben Creek (
= 0.42). Drifting Simuliids were avoided during winter in all streams, with mean electivity ranging from
0.97 in Daley Creek to
0.55 in Garvin Brook. In addition, brown trout avoided Baetis and Chironomidae in the drift in all streams except Garvin Brook, where mean electivity was 0.21 and 0.66, respectively.
Electivity of benthic prey also varied across streams during winter. Brown trout selected Gammarus in Daley Creek ( = 0.60), Chironomidae in Garvin Brook (
= 0.48), Limnephilidae in Gribben Creek (
= 0.63), and Physella in Trout Run Creek (
= 0.96) and Beaver Creek (
= 0.99). Brown trout selected Physella in the benthos in all streams, except Daley Creek (
= −0.56). In addition, brown trout avoided Brachycentrus and Simuliidae in the benthos in all streams where these they occurred.
Overall, brown trout electivity of drifting prey varied from = 0.80) in Beaver Creek
to
= 0.36) in Garvin Brook in winter, but electivity was not significantly different across streams (H = 8.9, d.f. = 4, p = 0.06). In contrast, electivity of benthos varied significantly across streams (H = 12.1, d.f. = 4, p = 0.02); brown trout demonstrated the highest electivity in Beaver Creek
= 0.82) and lowest electivity in Garvin Brook
= 0.36).
Spring selection and electivity: benthos vs. drift. When data from all streams were combined, selection of Simuliidae was significantly less than neutral (1/m = 0.13) ( = 0.01, d.f. = 20, p < 0.01) among drifting taxa, whereas brown trout selected Physella (
= 0.37, d.f. = 12, p < 0.01) and Gammarus (
= 0.28, d.f. = 14, p = 0.02) in spring (). Brown trout also avoided Simuliidae (
= 0.07, d.f. = 18, p = 0.03) and Baetis (
= 0.05, d.f. = 17, p < 0.01) in the benthos in spring, but selection of benthic Physella was significantly greater than neutral (1/m = 0.13) (
= 0.34, d.f. = 15, p < 0.01) ().
Mean electivity () for drifting and benthic prey varied in spring between streams. Drifting Physella was selected by brown trout in all streams except Garvin Brook, and ranged from 0.12 in Beaver Creek to 0.84 in Trout Run Creek. Likewise, Physella was the most widely selected benthic prey, with positive electivity in all streams except Beaver Creek, and ranged from 0.12 in Gribben Creek to 0.67 in Daley Creek. Limnephilidae was the most preferred drifting prey in Beaver Creek (
= 0.94) and most preferred benthic prey in Daley Creek (
= 0.75), and Gammarus was the most preferred drifting prey taxon in Beaver Creek (
= 0.38) and Gribben Creek (
= 0.23) in spring. Simuliids were avoided in the benthos and drift in all streams, with the exception of Beaver Creek (
= 0.03). Likewise, brown trout avoided Brachycentrus in the benthos in all streams, with mean electivity from
0.33 in Garvin Brook to
0.83 in Gribben Creek, and as drift in all streams, except Trout Run Creek (
= 0.06). Brown trout avoided Baetis in the benthos and in drift in all streams, except Garvin Brook (
= 0.18). Chironomidae was the only prey avoided in the benthos and drift in all streams in spring.
Overall, mean electivity ( of benthic prey varied significantly across streams in spring (H = 19.2, d.f. = 4, p < 0.01), whereas electivity for drifting prey did not (H = 4.1, d.f. = 4, p = 0.30). Brown trout in Daley Creek demonstrated highest electivity for drift
= 0.69) and benthos
= 0.73), but fish were least selective for drift in Garvin Brook
= 0.55) and least selective for benthos in Beaver Creek
= 0.35).
Summer selection and electivity: benthos vs. drift. When data from all streams were combined, brown trout did not select Chironomidae ( = 0.09, d.f. = 12, p = 0.02), Simuliidae (
= 0.01, d.f. = 12, p < 0.01), Baetis (
= 0.06, d.f. = 11, p < 0.01), and Formicidae (
= 0.09, d.f. = 10, p = 0.04) from the drift, whereas drifting Brachycentrus (
= 0.29, d.f. = 11, p < 0.01) and Gammarus (
= 0.37, d.f. = 12, p < 0.01) were favored during summer (). Brown trout demonstrated neutral selection (1/m = 0.17) during summer toward all benthic taxa ().
Mean electivity () of drifting and benthic prey varied in summer across streams. Gammarus was the most widely ingested drifting prey, with positive electivity in all streams, except Trout Run Creek. Brachycentrus were selected in Beaver Creek (
= 0.49) and Trout Run Creek (
= 0.67) in summer Chironomidae were avoided in all streams in the drift, except Garvin Brook, drifting Baetis were avoided in all streams, except Daley Creek (
= 0.15) ().
Brachycentrus was selected in Daley Creek ( = 0.89) from the benthos, whereas brown trout selected Gammarus in Garvin Brook (
= 0.46) and Gribben Creek (
= 0.17), Chironomidae in Garvin Brook (
= 0.15), and Elmid beetles in Trout Run Creek (
= 0.24). Simuliidae was the most widely avoided drifting prey in all streams, and ranged from
0.85 in Garvin Brook to
0.98 in Daley Creek. Brown trout in Beaver Creek avoided Elmidae (
=
0.80) during summer in the benthos, whereas brown trout in Daley Creek and Gribben Creek avoided Chironomidae (
=
0.88,
0.50, respectively), and Gammarus (
= 0.57) in Trout Run Creek ().
Mean electivity (of drift in summer differed significantly among streams, and ranged from 0.47 in Beaver Creek to 0.63 in Daley Creek (H = 9.1, d.f. = 4, p = 0.05). Mean electivity of benthos did not vary significantly among streams during summer (H = 3.5, d.f. = 4, p = 0.52).
Fall selection and electivity of benthos vs. drift. No prey were significantly selected during fall from the drift; however selectivity for Simuliidae ( = 0.03, d.f. = 8, p < 0.01) and other taxa (
= 0.08, d.f. = 7, p < 0.01) were significantly less than neutral (1/m = 0.14) (). Selectivity for Physella (
= 0.36, d.f. = 8, p = 0.03) was significantly higher than neutral, whereas selectivity for Elmidae (
= 0.0, d.f. = 4, p < 0.01) was significantly lower than neutral (1/m = 0.14) from the benthos in fall ().
Among streams, mean electivity () of benthic and drifting prey varied during fall. Physella was the most preferred drifting prey in Beaver Creek (
= 0.58), Garvin Brook (
= 0.33), Gribben Creek (
= 0.08), and Trout Run Creek (
= 0.75), but avoided in Daley Creek (
=
1.0). Gammarus was selected in Daley Creek (
= 0.56) and Garvin Brook (
= 0.22) in fall. Drifting Simuliidae was avoided in Beaver Creek (
=
0.86), Daley Creek (
=
0.78), Gribben Creek (
=
0.69), and Trout Run Creek (
=
0.98). Brachycentrus was selected in Beaver Creek (
= 0.56), and Trout Run Creek (
= 0.50), but avoided in Garvin Brook (
=
0.70) and Gribben Creek (
=
0.48).
Physella was the most selected benthic prey in Beaver Creek ( = 0.88) and Gribben Creek (
= 0.76), whereas Chironomidae was selected for in Daley Creek (
= 0.23), Garvin Brook (
= 0.50), and Trout Run Creek (
= 0.66) in fall. Baetis was selected in Garvin Brook (
= 0.78), but avoided in Beaver Creek(
=
0.56), Daley Creek (
=
0.63), Gribben Creek (
=
0.53), and Trout Run Creek (
=
0.82). Elmid beetles were avoided in all streams where they occurred during all, including Beaver Creek, Garvin Brook, and Gribben Creek (
=
1.0).
When all taxa were combined, mean electivity (of drift differed significantly among streams during fall, and ranged from 0.23 in Garvin Brook to 0.70 in Trout Run Creek (H = 12.1, d.f. = 4, p = 0.02). Mean electivity (
of benthos did not vary significantly among streams (H = 3.8, d.f. = 4, p = 0.43).
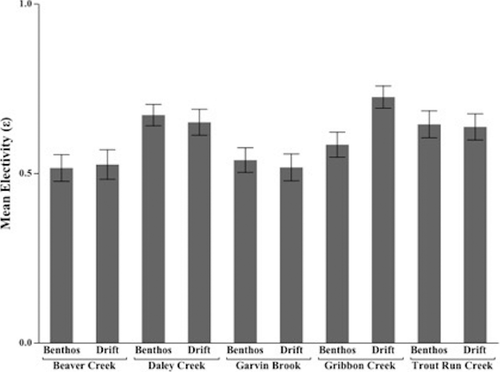
Prey electivity by stream
Mean electivity () of benthic and drifting prey also varied significantly across streams for all seasons combined (). Electivity of drifting prey ranged from 0.51 in Garvin Brook to 0.72 in Gribben Creek (H = 12.6, d.f. = 4, p = 0.01). Mean electivity ranged from 0.51 in Beaver Creek to 0.67 in Daley Creek (H = 23.5, d.f. = 4, p < 0.01) for benthos ().
Figure 3. Mean electivity ( ±1 s.e.) of brown trout for benthos (H = 12.6, d.f. = 4, p = 0.01) and drift (H = 23.5, d.f. = 4, p < 0.01), in five streams across southeastern Minnesota, 2011–2013.
Beaver Creek. In Beaver Creek, selection of Brachycentrus ( = 0.26, d.f. = 6, p < 0.01) and Physella (
= 0.35, d.f. = 6, p = 0.03) was significantly higher than neutral (1/m = 0.11), but selection of Chironomidae (
= 0.03, d.f. = 7, p < 0.01), Simuliidae (
= 0.01, d.f. = 7, p < 0.01), and Baetis (
= 0.05, d.f. = 7, p < 0.01) was significantly lower (). Selection of Physella (
= 0.37, d.f. = 7, p = 0.03) in the benthos was significantly higher than neutral selection (1/m = 0.10), but Hydropsychidae (
= 0.03, d.f. = 6, p < 0.01), Chironomidae (
= 0.08, d.f. = 6, p = 0.05), and Elmidae (
= 0.01, d.f. = 7, p < 0.01) were avoided in Beaver Creek ().
Table 5. Mean prey type selectivity (Manly-Chesson index, αi; SD) for brown trout from five streams in southeastern Minnesota, 2010–2013. Values significantly different (p < 0.05) from 1/m are indicated by ‘+’ for positive selection and ‘ for negative selection; n is the number of independent sample dates.
Overall, mean electivity () of drifting prey was similar across seasons in Beaver Creek (H = 2.5, d.f. = 3, p = 0.45), but electivity of benthos differed significantly, and ranged from 0.35 during spring to 0.83 during winter (H = 13.9, d.f. = 3, p < 0.01).
Daley Creek. Brown trout selected drifting Gammarus in Daley Creek ( = 0.46, d.f. = 9, p < 0.01), but avoided Chironomidae (
= 0.07, d.f. = 5, p = 0.03), Simuliidae (
= 0.02, d.f. = 5, p < 0.01), Baetis (
= 0.05, d.f. = 7, p = 0.05), and Formicidae (
= 0.04, d.f. = 5, p < 0.01) when compared to neutral feeding (1/m = 0.14) (). No benthic prey were positively selected; however, brown trout in Daley Creek selected Baetis
= 0.01, d.f. = 9, p < 0.01) in the benthos significantly less than neutral selection (1/m = 0.17) ().
Mean electivity () of brown trout of the drift (H = 3.6, d.f. = 3, p = 0.31) and benthos (H = 3.4, d.f. = 3, p = 0.34), was similar across seasons in Daley Creek.
Garvin Brook. When compared to neutral feeding (1/m = 0.17), brown trout did not select drifting Simuliidae in Garvin Brook ( = 0.03, d.f. = 9, p< 0.01) (). Brown trout favored Chironomidae (
= 0.24, d.f. = 9, p = 0.05) in the benthos, but selection of Glossosoma (
= 0.04, d.f. = 8, p < 0.01) and Elmidae (
= 0.02, d.f. = 9, p < 0.01) was significantly lower than neutral (1/m = 0.13) ().
Overall, mean electivity () of drifting prey varied significantly across seasons at Garvin Brook (H = 8.7, d.f. = 3, p = 0.03), and ranged from 0.22 during fall to 0.56 during spring, but was similar for benthos across seasons (H = 5.3, d.f. = 3, p = 0.15).
Gribben Creek. Selection toward Physella in the drift ( = 0.41, d.f. = 7, p = 0.03) by brown trout in Gribben Creek was significantly higher than neutral feeding (1/m = 0.10), but Brachycentrus (
= 0.05, d.f. = 9, p < 0.01), Simuliidae (
= 0.01, d.f. = 10, p < 0.01), and Ephemerellidae (
= 0.0, d.f. = 5, p < 0.01) were avoided (). Of benthic prey, brown trout in Gribben Creek selected Physella (
= 0.34, d.f. = 9, p < 0.01) and Limnephilidae (
= 0.44, d.f. = 3, p = 0.04) significantly higher than neutral selection (1/m = 0.10), but Brachycentrus (
= 0.04, d.f. = 9, p < 0.01), Simuliidae (
= 0.02, d.f. = 8, p < 0.01), Baetis (
= 0.05, d.f. = 8, p < 0.01), and Ephemerellidae (
= 0.0, d.f. = 5, p < 0.01) were avoided ().
Mean electivity () of drifting prey was similar across seasons at Gribben Creek (H = 2.6, d.f. = 3, p = 0.45), but differed significantly for benthic prey (H = 11.0, d.f. = 3, p = 0.01), and ranged from 0.38 during summer to 0.79 during winter.
Trout Run Creek. Brown trout in Trout Run Creek selected drifting Brachycentrus ( = 0.34, d.f. = 8, p = 0.03) and Physella (
= 0.60, d.f. = 5, p<0.01) significantly higher than neutral selection (1/m = 0.14), but Chironomidae (
= 0.08, d.f. = 8, p = 0.02), Simuliidae (
= 0.01, d.f. = 9, p<0.01), and Baetis (
= 0.03, d.f. = 8, p < 0.01) were avoided (). Brown trout positively selected Physella (
= 0.52, d.f. = 7, p < 0.01) in the benthos, but Hydroptilidae (
= 0.03, d.f. = 4, p < 0.01) and Brachycentrus (
= 0.03, d.f. = 8, p < 0.01) were avoided when compared to neutral selection (1/m = 0.14) ().
Mean electivity () was similar across all seasons at Trout Run Creek for drifting prey (H = 4.5, d.f. = 3, p = 0.22) and benthos (H = 1.6, d.f. = 3, p = 0.66).
Selectivity: prey abundance and growth
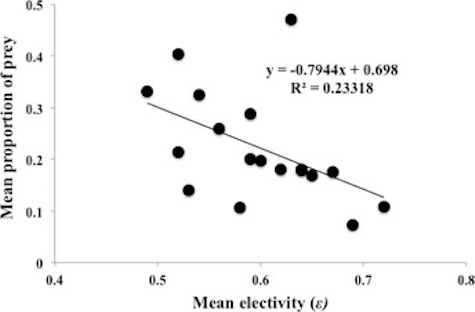
Overall, there was a negative correlation between mean prey electivity () and mean proportional abundance of prey (r2 = 23.3, F = 4.9, p = 0.04) across all sample sites and seasons; as the mean proportion of prey increased, the mean electivity of prey decreased ().
Figure 4. Relationship between mean proportion of total prey available and mean prey electivity across all sample dates, 2011–2013, in five streams in southeastern Minnesota (r2 = 0.23, F = 4.9, p = 0.04).
Seasonal prey selectivity and mean growth was only positively correlated in Daley Creek (p = 0.04) and Garvin Brook (p = 0.03) ().
Table 6. Linear regressions between mean daily growth of brown trout and mean prey selectivity on a seasonal basis among streams and among seasons within each sample site.
Discussion
Broad patterns
We found broad variation in the composition and availability of aquatic macroinvertebrates assemblages in the benthos and drift across streams in southeastern Minnesota, similar to other studies (Troelstrup and Perry Citation1989; Waters Citation2000). Benthic and drifting macroinvertebrate assemblages were dominated by relatively few taxa, which was not unexpected based on other studies (Steingrímsson and Gíslason Citation2002; Kara and Alp Citation2005). However, despite the low richness of dominant taxa overall, the proportional contributions of each varied among seasons and streams. The proportion of available drift was highest in spring and lowest in winter, which mirrors the typical pattern for drift abundance in temperate streams, where a spring maximum may be a function of higher discharge and increased density of benthic macroinvertebrates (Hynes Citation1970). Garvin Brook also contained the highest relative proportions of all benthos and drift collected; however, distributions of specific prey taxa varied widely among streams. Seasonal distributions of benthic taxa also varied, with most taxa occurring in highest proportional abundances during spring or summer. Overall, the greatest proportion of benthos was collected in summer, and similar to drift, the least in winter.
The present study also found broad variation in the feeding patterns of brown trout on a seasonal basis across several streams and demonstrated that prey selection was not proportional to the environmental density of macroinvertebrates, similar to other studies (Sagar and Glova Citation1995; MacNeil et al. Citation2000; Crespin De Billy and Usseglio‐Polatera Citation2002). Brown trout are visual feeders, thus prey preference and capture probability are likely influenced by the accessibility, size, color, mobility, and degree of exposure of various prey types (Rader Citation1997; Crespin De Billy and Usseglio‐Polatera Citation2002). Across all streams, brown trout favored Physella in the benthos and avoided Elmidae, Baetis, and Brachycentrus, whereas selectivity of all other benthic prey was either neutral or not significant. Electivity of prey may be related to size-selective predation or prey behavior. For example, other studies documented avoidance of Baetis by brown trout, despite being active and abundant in many streams (Mathooko Citation1996; Fochetti et al. Citation2003; Sánchez-Hernández and Cobo Citation2013). Baetis has been documented to alter behavior in response to predation risk thereby reducing vulnerability to benthic feeding fish, including brown trout (Kohler and McPeek Citation1989).
Selectivity () of drift indicated that brown trout favored Physella, Gammarus, and Brachycentrus, but avoided Chironomidae and Simuliidae. The positive selection by brown trout for Gammarus and Physella, and avoidance of Dipterans, is consistent with studies in other regions (Cada, Loar, Sale Citation1987; Pender and Kwak Citation2002; Fochetti et al. Citation2003; Johnson et al. Citation2007; Sanchez-Hernandez et al. Citation2011), although Pender and Kwak (Citation2002) found positive selection for Chironomidae among age-0 trout. Physella did not constitute a high proportion of either prey availability or prey consumed, but brown trout consistently favored Physella in the benthos and drift. Physella is large-bodied and more abundant than other aquatic mollusks in southeastern Minnesota, and are energetically rich (5275 j/g; Cummins and Wuycheck Citation1971). Brown trout often display size selectivity, preferentially feeding on large-bodied prey items (Newman and Waters Citation1984). Brown trout generally selected against encased Brachycentrus larvae in the benthos, but demonstrated positive electivity toward encased Limnephilidae larvae in both drift and benthos of streams where this taxon occurred. Preference toward Limnephilids may reflect size selectivity in the present study, as the mean size of Limnephilidae individuals (12.5 mm) was substantially larger than Brachycentrus (6.8 mm). Other studies in southeastern Minnesota reported that large-bodied Limnephilids also comprised a majority of brown trout prey consumed by dry weight in winter (French et al. Citation2014; Anderson et al. Citation2016) whereas small-bodied prey, including Glossosoma and Chironomidae, became increasingly abundant in stomachs in late winter (French et al. Citation2014).
Seasonal and spatial patterns
Gammarus was a dominant aquatic invertebrate taxon among drift in southeastern Minnesota, and Waters (Citation1972) and Newman and Waters (Citation1984) found positive selection of Gammarus by brown trout throughout the year. Overall, Gammarus was a favored drifting macroinvertebrate taxon in our study; however, availability varied among streams and seasons. Low water temperature in winter does not correlate with a reduction in Gammarus, and brown trout demonstrated selection for this taxon in winter in Minnesota (Newman and Waters Citation1984; French et al. Citation2014; Anderson et al. Citation2016) and in other regions (Bridcut and Giller Citation1995). In the present study, brown trout ingested Gammarus from the benthos and drift during winter in streams where this taxon occurred, but positive selection toward Gammarus was only significant during spring and summer.
Chironomidae were common in brown trout stomachs during all seasons and in most streams. Chironomidae comprised the largest proportion of drifting prey in all streams and overall, and was among the two most dominant benthic prey taxa. However, brown trout did not select Chironomidae in the benthos and drift, except in Garvin Brook. Relative to other aquatic macroinvertebrate taxa, Chironomidae are small-bodied, and although widely abundant, may be avoided by brown trout in favor of larger, more conspicuous prey. In Garvin Brook, the highest electivity for Chironomidae occurred in winter, when Diamesa mendotae can account for up to 75% of all Chironomidae available, and approximately 15% of all benthos (Jane Mazack, unpublished data). D. mendotae is among the largest Chironomid larvae available to brown trout in winter (mean length ≈ 812 mm) (Anderson et al. Citation2016), and brown trout positively selected for D. mendotae in two of three streams studied, including Beaver Creek, in winter (Anderson et al. Citation2016). The caloric value provided by D. mendotae and other winter-emerging Chironomids may benefit populations by providing energy in winter when abundances of other aquatic invertebrate taxa are low. Stable isotope analysis has provided evidence that Chironomids are among the most important contributors toward brown trout growth in winter in southeastern Minnesota (French et al. Citation2014), and even a marginal contribution of Chironomidae to brown trout diets in winter may significantly influence annual growth rates (Anderson et al. Citation2016). Climate change may reduce abundances of D. mendotae in southeastern Minnesota in winter (Anderson Citation2012), thus brown trout may face consequences that include lower rates of growth and survival in winter, especially because other benthic macroinvertebrate taxa are generally lower in winter.
Foraging strategies: drift vs. benthic selection
Trout are opportunistic generalists throughout the year (Lord Citation1933; Maciolek and Needham Citation1952; Reimers Citation1957; Cunjak and Power Citation1987; Ozvarol et al. Citation2011), and other studies emphasize the importance of drifting prey to the trout diet, including a widely cited study that emphasized brown trout taking less than 15% of their prey from the benthos (Bachman Citation1984). However, numerous studies of prey selection demonstrate that brown trout foraging strategies (drift-feeding vs. epibenthic feeding) vary seasonally and spatially. Waters (Citation1972) emphasized that there is no distinct drift fauna, but rather, benthic invertebrates enter the drift due to several abiotic and biotic factors. Overall, drift represents a mixture of drift densities, which depends on the species present in the benthos, and their propensity to drift. Therefore, assessing diet selectivity from field data when the same prey can be selected from the water column or substratum is not often feasible without direct underwater observations. However, we were able to infer patterns in foraging modes by separately assessing the selectivity of benthos and drift. On a seasonal basis, brown trout demonstrated higher selectivity of drift, and were less selective for benthos in all seasons, except fall. This pattern was especially prominent in summer, when benthic prey types were not selected and availability of benthic macroinvertebrates and drift was highest (Cochran-Biederman Citation2015). Positive electivity for Brachycentrus in the drift was an exception to a generally observed pattern, but coincided with documented peaks in annual pupal density (June and July), larval size (June and early July), and larval drift (August and September) (Krueger and Cook Citation1984).
Our study suggests that brown trout select drifting taxa in summer, and that epibenthic feeding is important in all streams, and especially in winter, as observed by other studies in our region (Anderson Citation2012; French Citation2014), and elsewhere (Cunjak and Power Citation1987). Additionally, the consistent preference and consumption of gastropods also supports a benthic mode of feeding, which has been observed in salmonids of subarctic rivers (Brittain and Eikeland Citation1988; Amundsen et al. Citation1999; Steingrímsson and Gíslason Citation2002; Johnson et al. Citation2007).
An explanation for the higher degree of selectivity of drift relative to benthos may be that brown trout are predominately feeding epibenthically, whereby our electivity analyses using drift may not actually represent prey from the water column. Epibenthic feeding varies widely among trout populations, and seasonal or spatial differences in drift density or availability of actively drifting versus less-mobile prey may influence which feeding mode is dominant. Other studies have shown that salmonids feed on benthos when drift was depleted (Nislow et al. Citation1998; Nakano et al. Citation1999) and that brown trout were more likely to feed on benthos when less-mobile prey types were abundant (McIntosh and Townsend Citation1995). Fausch et al. (Citation1997) found an adaptive shift from drift to epibenthic feeding in dolly varden (Salvelinus malma) as drift was experimentally reduced.
Numerous studies have identified a positive selection by brown trout for terrestrial insects (see review by Hunt and Krokhin Citation1975), but not in the present study. Terrestrial macroinvertebrates may not contribute a large proportion of total drift or diet of brown trout, compared to other regions, where terrestrial inputs contributed up to 53% of stream drift and 82% of brown trout diet (Dahl Citation1998). Laudon et al. (Citation2005) found brown trout in Valley Creek, Minnesota exhibited neutral selection for terrestrial prey in summer, which only comprised 3% of all available drift and diet. Similarly, we found on a few notable occasions, stomachs contained high proportions of terrestrial prey in response to a temporary spike in availability (e.g. ants and aphids following a significant rainfall event in summer, and Chironomidae during a large emergence in late spring) that may not have been reflected in our broad comparisons of electivity due to a low frequency of these events relative to the number of sampling events.
Our sampling typically included smaller brown trout (100–300 mm in TL; age-0 – 2+), which may feed epibenthically to evade predation, especially in winter when drift rates are lowest and there are fewer terrestrial and emerging aquatic insects (Grant Citation1999). This pattern was also evident in other winter studies of brown trout diet in southeastern Minnesota (Anderson et al. Citation2016; French Citation2014; French et al. Citation2014), and for salmonids in other regions where nocturnal benthic feeding in winter was attributed to lower capture efficiency of drift because of reduced light from ice cover, elevated turbidity, lower drift rates, and avoidance of predators (Tippets and Moyle Citation1978; Cunjak and Power Citation1986; Jørgensen and Jobling Citation1992; Heggenes et al. Citation1993; Fraser and Metcalfe Citation1997; Johansen et al. Citation2010).
We found brown trout selected large-bodied taxa in winter and tended to avoid small-bodied taxa. Larger prey may be more obvious and susceptible to capture by visual predators, such as brown trout. Selectivity in winter may be because of prey accessibility, reflecting a reduced ability to see and capture smaller prey items, and not necessarily that the brown trout are actively avoiding these prey. However, Chironomids comprised over half of all stomach contents in much of the year, including winter, but were the most abundant benthic and drifting prey type in all streams. Thus, the abundance of Chironomidae in the environment may result in a high occurrence of incidental or accidental consumption.
Associations of selectivity with growth and prey abundance
Stable isotope analysis of brown trout diets in southeastern Minnesota provided evidence that the dominant prey taxa observed in stomach contents also contributed toward growth (French et al. Citation2014). Overall, prey resources in all streams and seasons appeared to be abundant and available. There was substantial variation in diet, prey, and patterns of selectivity on a seasonal and spatial basis, but the majority of prey available in the environment and consumed were represented by relatively few taxa. Feeding was likely shaped by a generalist or opportunist strategy that reflects site-specific prey abundance and accessibility or size.
Mean instantaneous growth of brown trout and mean prey selectivity were not correlated on a seasonal basis across streams, but growth increased significantly with selectivity of drift in Daley Creek. In Daley Creek, brown trout consistently selected and consumed Physella, the most energetically-rich of all prey available, and Gammarus, which has been positively correlated with brown trout biomass in southeastern Minnesota (Kwak Citation1993). Daley Creek contained the fastest growing brown trout and the highest abundance of Gammarus and proportion of drifting Chironomids in our study (Cochran-Biederman Citation2015). Overall selectivity was highest in Daley Creek in winter and spring when ∼70% of annual fish growth occurred.
A potential limitation of the present study is that brown trout stomach samples were taken at variable times of the day between early morning and early evening, therefore, observed diets may not have accurately represented feeding over a 24-hour period. For example, fish could have selected certain prey taxa in the sampling period, but the stomach contents may have been dominated by other macroinvertebrates consumed earlier in the day, perhaps relative to diel patterns in macroinvertebrate activity. Few of the prey taxa in this study were of terrestrial origin, however, if sampling occurred before terrestrial drift peaked (Elliott Citation1970), these taxa may have been under-represented. In addition, the present study did not account for variation in prey size or potential differences in selectivity among brown trout of varying sizes and/or ages. Although some studies report an ontogenetic shift toward larger prey among older salmonids (e.g. Steingrímsson and Gíslason Citation2002; Montori et al. Citation2006; Sánchez-Hernández Cobo Citation2013; Sánchez-Hernández et al. Citation2013), others have not found consistent correlations between prey size and fish length or age of brown trout (Sánchez-Hernández and Cobo Citation2015), and no relationship between gape size and prey ingestion (Newman Citation1987; Rincón and Lobón-Cerviá Citation1999). Sánchez-Hernández and Cobo (Citation2015) postulated that the lack of relationship might have been due to sampling predominately brown trout <300 mm in total length. Similarly, we collected relatively few brown trout >300 mm in total length, suggesting that size-selectivity may not have been a factor shaping patterns in prey electivity in our study.
Conclusion
We found macroinvertebrate assemblages and prey preference varied significantly across time and space, but only a few taxa represented a majority of prey selected. In general, brown trout selected large-bodied, energy-rich benthic prey over other more abundant aquatic macroinvertebrate taxa and drifting prey. Although analyses of stomach contents revealed that brown trout diets typically reflected prey that were most abundant in the environment, the selection of energy-dense benthic prey taxa, and the possible consequences for growth, warrants future study with bioenergetics modeling.
We provide information about the foraging patterns of brown trout, which could be useful in helping fisheries managers identify and manage key macroinvertebrate taxa on a year-round basis. In addition, this detailed knowledge about the preferences of brown trout and composition of aquatic macroinvertebrate communities can be combined with life history data and bioenergetics modeling to predict the future consequences of climate change on trophic structures in groundwater-dominated streams in southeastern Minnesota.
Acknowledgments
We thank the following individuals for helpful comments on a previous version of this manuscript: Dr. Douglas Dieterman, Dr. Raymond Newman, and Dr. Len Ferrington Jr. We thank Trevor Biederman, Karin Jokela, Dustan Hoffman, and many undergraduate students for assistance in the field and lab. We thank Kevin Stark for assistance with creating the map included in this manuscript. All animals used in this study were handled according to animal use and care guidelines established by the University of Minnesota IACUC committee.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Jennifer Lynn Cochran-Biederman
Jennifer L. Cochran-Biederman is an assistant professor in the Biology Department at Winona State University (Winona, Minnesota).
Bruce Vondracek
Bruce Vondracek is professor emeritus in the Department of Fisheries, Wildlife and Conservation Biology at the University of Minnesota.
References
- Allan JD. 1981. Determinants of diet of brook trout (Salvelinus fontinalis) in a mountain stream. Can J Fish Aquat Sci. 38:184–192.
- Allan JD. 1987. Macroinvertebrate drift in a Rocky Mountain stream. Hydrobiologia. 144:261–268.
- Amundsen PA, Bergersen R, Huru H, Heggberget, TG. 1999. Diel feeding rhythms and daily food consumption of juvenile Atlantic salmon in the River Alta, northern Norway. J Fish Biol. 54:58–71.
- Anderson AM. 2012. Winter-emerging chironomidae (Insecta: Diptera) in Minnesota trout streams (dissertation). St. Paul (MN): University of Minnesota.
- Anderson AM, Mittag E, Middleton B, Vondracek B, Ferrington LC Jr. 2016. Winter diets of Brown Trout populations in southeastern Minnesota and the significance of winter-emerging invertebrates. Trans Am Fish Soc. 145:206–220.
- Bachman RA. 1984. Foraging behavior of free-ranging wild and hatchery brown trout in a stream. Trans Am Fish Soc. 113:1–32.
- Bridcut EE, Giller PS. 1995. Diet variability and foraging strategies in brown trout (Salmo trutta): an analysis from subpopulations to individuals. Can J Fish Aquat Sci. 52:2543–2552.
- Brittain JE, Eikeland TJ. 1988. Invertebrate drift—a review. Hydrobiologia. 166:77–93.
- Cada GF, Loar JM, Sale MJ. 1987. Evidence of food limitation of rainbow and brown trout in southern Appalachian soft-water streams. Trans Am Fish Soc. 116:692–702.
- Chesson J. 1978. Measuring preference in selective predation. Ecology. 59:211–215.
- Chesson J. 1983. The estimation and analysis of preference and its relationship to foraging models. Ecology. 64:1297–1304.
- Crespin De Billy V, Usseglio‐Polatera P. 2002. Traits of brown trout prey in relation to habitat characteristics and benthic invertebrate communities. J Fish Biol. 60:687–714.
- Cochran-Biederman JL. 2015. Seasonal patterns in growth, diet, and prey availability of Brown Trout Salmo trutta in groundwater-dominated streams in the Driftless Ecoregion (dissertation). St. Paul (MN): University of Minnesota.
- Cummins KW, Wuycheck JC. 1971. Caloric equivalents for investigations in ecological energetics. Vol. 18. Mitteeilungen: Internationale Vereinigung ftir theoretische und angewandte Limnologie p. 1–158.
- Cunjak RA, Power G. 1986. Seasonal changes in the physiology of brook trout, Salvelinus fontinalis (Mitchill), in a sub-Arctic river system. J Fish Biol. 29:279–288.
- Cunjak RA, Power G. 1987. The feeding and energetics of stream‐resident trout in winter. J Fish Biol. 31:493–511.
- Cunjak RA, Curry A, Power G. 1987. Seasonal energy budget of brook trout in streams: implications of a possible deficit in early winter. Trans Am Fish Soc. 116:817–828.
- Dahl J. 1998. Effects of a benthivorous and a drift-feeding fish on a benthic stream assemblage. Oecologia. 116:426–432.
- Dieterman DJ, Thorn WC, Anderson CS. 2004. Application of a bioenergetics model for brown trout to evaluate growth in southeast Minnesota streams. St. Paul (MN): Fisheries and Wildlife Division, Minnesota Department of Natural Resources.
- Dieterman DJ, Hoxmeier RJH, Staples DF. 2012. Factors influencing growth of individual brown trout in three streams of the upper Midwestern United States. Ecol Freshw Fish. 21:483–493.
- Elliott JM. 1970. Diel changes in invertebrate drift and the food of trout Salmo trutta L. J Fish Biol. 2:161–165.
- Erkinaro J, Niemelä E. 1995. Growth differences between the Atlantic salmon parr, Salmo salar, of nursery brooks and natal rivers in the River Teno watercourse in northern Finland. Environ Biol Fish. 42:277–287.
- Fausch KD, Nakano S, Kitano S. 1997. Experimentally induced foraging mode shift by sympatric charrs in a Japanese mountain stream. Behav Ecol. 8:414–420.
- Fochetti R, Amici I, Argano R. 2003. Seasonal changes and selectivity in the diet of brown trout in the River Nera (Central Italy). J Freshw Ecol. 18:437–444.
- Fraser NHC, Metcalfe NB. 1997. The costs of becoming nocturnal: feeding efficiency in relation to light intensity in juvenile Atlantic salmon. Funct Ecol. 11:385–391.
- French WE. 2014. Protected from the elements: winter ecology of Brown Trout in groundwater buffered streams (dissertation). St. Paul. (MN): University of Minnesota.
- French WE, Vondracek B, Ferrington Jr LC, Finlay JC, Dieterman DJ. 2014. Winter feeding, growth and condition of brown trout Salmo trutta in a groundwater-dominated stream. J Freshw Ecol. 29:187–200.
- Grant GC. 1999. Growth, spawning behavior and feeding strategies of trout in a small Midwestern stream (dissertation). St. Paul (MN): University of Minnesota.
- Heggenes J, Krog OMW, Lindås OR, Dokk JG. 1993. Homeostatic behavioural responses in a changing environment: brown trout (Salmo trutta) become nocturnal during winter. J Anim Ecol. 62:295–308.
- Hunt RL, Krokhin E. 1975. Coupling of land and water systems. New York (NY): Springer-Verlag; Chapter 10, Food relations and behavior of salmonid fishes; p. 137–151.
- Hynes HBN. 1970. The ecology of running waters. Toronto: University of Toronto Press.
- Johansen M, Thorstad EB, Rikardsen A, Koksvik J, Ugedal O, Jensen AJ, Saksgård L, Næsje TF. 2010. Prey availability and juvenile Atlantic salmon feeding during winter in a regulated subarctic river subject to loss of ice cover. Hydrobiologia. 644:217–229.
- Johnson RL, Coghlan SM, Harmon T. 2007. Spatial and temporal variation in prey selection of brown trout in a cold Arkansas tailwater. Ecol Freshw Fish. 16:373–384.
- Jørgensen EH, Jobling M. 1992. Feeding behaviour and effect of feeding regime on growth of Atlantic salmon, Salmo salar. Aquaculture. 101:135–146.
- Kara C, Alp A. 2005. Feeding habits and diet composition of brown trout (Salmo trutta) in the upper streams of River Ceyhan and River Euphrates in Turkey. Turk J Vet Anim Sci. 29:417–428.
- Kohler SL, McPeek MA. 1989. Predation risk and the foraging behavior of competing stream insects. Ecology. 70:1811–1825.
- Krueger CC, Cook EF. 1984. Life cycles and drift of Trichoptera from a woodland stream in Minnesota. Can J Zool. 62:1479–1484.
- Kwak TJ. 1993. Influence of physical and biotic factors on trout production dynamics in southeastern Minnesota streams (dissertation). St. Paul (MN): University of Minnesota.
- Laudon MC, Vondracek B, Zimmerman JK. 2005. Prey selection by trout in a spring-fed stream: terrestrial versus aquatic invertebrates. J Fresh Ecol. 20:723–733.
- Letcher BH, Gries G, Juanes F. 2002. Survival of stream-dwelling Atlantic salmon: effects of life history variation, season, and age. Trans Am Fish Soc. 131:838–854.
- Leung ES, Rosenfeld JS, Bernhardt JR. 2009. Habitat effects on invertebrate drift in a small trout stream: implications for prey availability to drift-feeding fish. Hydrobiologia. 623:113–125.
- Lord RF. 1933. Type of food taken throughout the year by brook trout in a single Vermont stream with special reference to winter feeding. Trans Am Fish Soc. 63:182–197.
- Maciolek JA, Needham PR. 1952. Ecological effects of winter conditions on trout and trout foods in Convict Creek, California, 1951. Trans Am Fish Soc. 81:202–217.
- MacNeil C, Elwood RW, Dick JT. 2000. Factors influencing the importance of Gammarus spp. (Crustacea: Amphipoda) in riverine salmonid diets. Arch Hydrobiol. 149:87–107.
- Manly BFJ. 1974. A model for certain types of selection experiments. Biometrics. 30:281–294.
- Mathooko JM. 1996. Rainbow trout (Oncorhynchus mykiss Walbum) as a potential natural drift sampler in a tropical lotic ecosystem. Limnologica. 26:245–254.
- McCune B, Grace JB. 2002. Analysis of ecological communities. Gleneden Beach (OR): MjM Software Design.
- McIntosh AR, Townsend CR. 1995. Contrasting predation risks presented by introduced brown trout and native common river galaxias in New Zealand streams. Can J Fish Aquat Sci. 52:1821–1833.
- Metcalfe NB, Thorpe JE. 1992. Anorexia and defended energy levels in over-wintering juvenile salmon. J Anim Ecol. 61:175–181.
- Minnesota Department of Natural Resources (US). 2003. Fisheries long-range plan for trout stream resource management in southeast Minnesota, 2004–2009. St. Paul (MN): Section of Fisheries.
- Montori A, Tierno De Figueroa JM, Santos X. 2006. The diet of the brown trout Salmo trutta (L.) during the reproductive period: size‐related and sexual effects. Int Rev Hydrobiol. 91:438–450.
- Nakano S, Kawaguchi Y, Taniguchi Y, Miyasaka H, Shibata Y, Urabe H, Kuhara N. 1999. Selective foraging on terrestrial invertebrates by rainbow trout in a forested headwater stream in northern Japan. Ecol Res. 14:351–360.
- Newman RM. 1987. Comparison of encounter model predictions with observed size-selectivity by stream trout. J N Am Benthol Soc. 6:56–64.
- Newman RM, Waters TF. 1984. Size-selective predation on Gammarus pseudolimnaeus by trout and sculpins. Ecology. 65:1535–1545.
- Nislow KH, Folt C, Seandel M. 1998. Food and foraging behavior in relation to microhabitat use and survival of age-0 Atlantic salmon. Can J Fish Aquat Sci. 55:116–127.
- Omernik JM, Gallant AL. 1988. Ecoregions of the upper Midwest states. Washington (DC): Environmental Protection Agency; ( EPA publication; 600/3-88/037).
- Ozvarol ZAB, Yildirim A, Ozvarol Y. 2011. Feeding ecology of various length-classes of brown trout (Salmo trutta) in different streams of Coruh River, Turkey. Kafkas Universitesi Veteriner Fakultesi Dergisi. 17:377–382.
- Pedley RB, Jones JW. 1978. The comparative feeding behaviour of brown trout, Salmo trutta L. and Atlantic salmon, Salmo salar L. in Llyn Dwythwch, Wales. J Fish Biol. 12:239–256.
- Pender DR, Kwak TJ. 2002. Factors influencing brown trout reproductive success in Ozark tailwater rivers. Trans Am Fish Soc. 131:698–717.
- Rader RB. 1997. A functional classification of the drift: traits that influence invertebrate availability to salmonids. Can J Fish Aquat Sci. 54:1211–1234.
- Reimers N. 1957. Some aspects of the relation between stream foods and trout survival. Calif Fish Game. 43:43–69.
- Rincón PA, Lobón-Cerviá J. 1999. Prey-size selection by brown trout (Salmo trutta L.) in a stream in northern Spain. Can J Zool. 77:755–765.
- Sagar PM, Glova GJ. 1995. Prey availability and diet of juvenile brown trout (Salmo trutta) in relation to riparian willows (Salix‐spp.) in three New Zealand streams. New Zeal J Mar Fresh. 29:527–537.
- Sánchez-Hernández J, Cobo F. 2013. Foraging behaviour of brown trout in wild populations: can population density cause behaviourally-mediated foraging specializations? Anim Biol. 63:425–450.
- Sánchez-Hernández J, Cobo F. 2015. Adaptive flexibility in the feeding behaviour of brown trout: optimal prey size. Zool Stud. 54:1–9.
- Sánchez-Hernández J, Vieira-Lanero R, Servia MJ, Cobo F. 2011. First feeding diet of young brown trout fry in a temperate area: disentangling constraints and food selection. Hydrobiologia. 663:109–119.
- Sánchez-Hernández J, Servia MJ, Vieira-Lanero R, Cobo F. 2013. New advances and contributions to fish biology. Croatia: InTech; Chapter 9, Ontogenetic dietary shifts in a predatory freshwater fish species: the brown trout as an example of a dynamic fish species; p. 271–298.
- Shearer KA, Hayes JW, Stark JD. 2002. Temporal and spatial quantification of aquatic invertebrate drift in the Maruia River, South Island, New Zealand. New Zeal J Mar Fresh. 36:529–536.
- Simpkins DG, Hubert WA. 2000. Drifting invertebrates, stomach contents, and body conditions of juvenile rainbow trout from fall through winter in a Wyoming tailwater. Trans Am Fish Soc. 129:1187–1195.
- Smith EP. 1998. Randomization methods and the analysis of multivariate ecological data. Environmetrics. 9:37–51.
- Sokal RR, Rohlf EJ. 1981. Biometry: the principles and practice of statistics in biological research. New York (NY): W. H. Freeman.
- Steingrímsson SÓ, Gíslason GM. 2002. Body size, diet and growth of landlocked brown trout, Salmo trutta, in the subarctic River Laxá, North-East Iceland. Environ Biol Fishes. 63:417–426.
- Stoneburner DL, Smock LA. 1979. Seasonal fluctuations of macroinvertebrate drift in a South Carolina Piedmont stream. Hydrobiologia. 63:49–56.
- Thorn WC. 1990. Investigational Report 401: Evaluation of special regulations for trout in southeast Minnesota streams. St. Paul (MN): Minnesota Department of Natural Resources, Section of Fisheries.
- Thorn WC, Ebbers M. 1997. Brook trout restoration in southern Minnesota. In: RE Gresswell, P Dwyer, RH Hamre, editors. Wild trout VI – putting the native back in wild trout. Bozeman (MT): Trout Unlimited, Inc.; p. 188–192.
- Thorn WC, Anderson CS, Lorenzen WE, Hendrickson DL, Wagner JW. 1997. A review of trout management in southeast Minnesota streams. N Am J Fish Manag. 17:860–872.
- Tippets WE, Moyle PB. 1978. Epibenthic feeding by rainbow trout (Salmo gairdneri) in the McCloud River, California. J Anim Ecol. 47:549–559.
- Troelstrup NH, Perry JA. 1989. Water quality in southeastern Minnesota streams: observations along a gradient of land use and geology. J Minn Acad Sci. 55:6–13.
- Utz RM, Hartman KJ. 2007. Identification of critical prey items to Appalachian brook trout (Salvelinus fontinalis) with emphasis on terrestrial organisms. Hydrobiologia. 575:259–270.
- Waters TF. 1972. The drift of stream insects. Annu Rev Entomol. 17:253–272.
- Waters TF. 1977. Streams and rivers of Minnesota. Minneapolis (MN): University of Minnesota Press.
- Waters TF. 2000. Wildstream: a natural history of the free-flowing river. Minneapolis (MN): Riparian Press.
- White JL, Harvey BC. 2007. Winter feeding success of stream trout under different streamflow and turbidity conditions. Trans Am Fish Soc. 136:1187–1192.
- Wilzbach MA, Cummins KW, Hall JD. 1986. Influence of habitat manipulations on interactions between cutthroat trout and invertebrate drift. Ecology. 67:898–911.
- Zimmerman GM, Goetz H, Mielke PW Jr. 1985. Use of an improved statistical method for group comparisons to study effects of prairie fire. Ecology. 66:606–611.