ABSTRACT
Many important components of animal behavior, growth, and reproduction are tightly linked to environmental conditions, particularly for ectothermic freshwater organisms. For amphibian species, factors such as temperature and rainfall can be physiologically limiting and alter activity levels. The effects of environmental conditions on terrestrial amphibian movement have been well characterized, but less is known about the importance of these factors in aquatic habitats. Here we investigate the impact of temperature and rainfall on the activity of a pond-dwelling amphibian using capture patterns of aquatic adult red-spotted newts (Notophthalmus viridescens). Data on newt captures, air temperature, and rainfall were collected for 6 years (2009, 2011, 2013–2016) during the winter breeding season at a wetland in the Piedmont region of North Carolina, USA. In 2016, we collected more detailed data on the size and sex of captured newts, as well as recording water temperature. Overall, temperature played a significant role in determining newt activity, while rainfall had little effect. As expected, and consistent with findings on amphibian activity in terrestrial systems, newt captures increased with increasing air temperature. During the 2016 breeding season we found sex-based changes in activity in response to temperature, with a higher proportion of males captured during warmer temperatures and a reduction in the male capture bias during colder temperatures. We found no evidence of size-based shifts in activity in response to temperature. This study increases our knowledge of amphibian responses to temperature while in aquatic habitats. Although the duration of time spent in aquatic habitats can be highly variable between species, these breeding locations are critical for the persistence of populations and activity levels, and reproductive success in these wetlands can be highly impacted by future changes in environmental conditions.
Introduction
Activity and behavior in freshwater organisms are determined by a variety of environmental factors, with temperature being one of the most important abiotic variables influencing activity in aquatic habitats (Dunham et al. Citation1989; Glanville and Seebacher Citation2006; Buckley et al. Citation2012). Temperature is particularly important for ectothermic organisms, which include most major groups of freshwater taxa, because their body temperature mirrors environmental temperature (i.e. Huey and Berrigan Citation2001; Ojanguren et al. Citation2001). As a result, activity in these species, including invertebrates, fish, and amphibians, can be physiologically limited under relatively hot or cold conditions based on the thermal tolerance range of the species (Gvoždík Citation2005; Buckley et al. Citation2012). Measuring ectotherm activity provides a biologically relevant indicator of how behavior and associated physiological processes of animals may change under variable environmental temperatures (Gillooly et al. Citation2002; Byström et al. Citation2006).
Amphibians are critical components of biomass in many ecosystems (Gibbons et al. Citation2006), and significant population decreases or extirpation of amphibian species can reduce biodiversity and alter ecosystem structure (Ranvestel et al. Citation2004; Whiles et al. Citation2006). Changes in abiotic factors, such as temperature, have the potential to alter disease dynamics (Rohr and Raffel Citation2010), breeding behavior (Blaustein et al. Citation2001), and movement patterns (Todd and Winne Citation2006), all of which can lead to population decline. For amphibians, as in other ectotherms, environmental temperatures determine activity periods and the time available for important functions, such as energy gain and reproduction (Huey and Stevenson Citation1979; Dunham et al. Citation1989).
Pond-breeding amphibians are known for dramatic seasonal migrations where individuals move from terrestrial to aquatic habitats for reproduction. The timing of migrations, successful breeding, and the suitability of aquatic habitat for larval development are highly dependent on environmental conditions (Semlitsch Citation1985; Semlitsch Citation2008; Connette et al. Citation2011). An important focus of amphibian conservation efforts has been on the quantity and quality of terrestrial non-breeding habitat as a critical factor limiting the persistence of pond-breeding populations due to its importance for growth of metamorphosed juveniles and sustaining breeding adults (Semlitsch and Bodie Citation2003). Thus, a majority of studies on amphibian activity focus on the effects of abiotic factors on terrestrial life stages. Both temperature and rainfall can impact terrestrial surface activity for amphibians, where the risks of cutaneous water loss are balanced against food availability and foraging success (Spotila Citation1972; Roe and Grayson Citation2008). These environmental factors also play an important role in reproductive cues and the success of migrations to breeding locations (Gill Citation1978; Semlitsch Citation1985; Todd and Winne Citation2006).
Although aquatic environments change less dramatically on a diel time period than terrestrial environments (Stolp Citation1988), environmental variation can impose physiological limits on aquatic amphibian activity and impact behavior, such as foraging or mating opportunities within breeding ponds (Newman Citation1992; Bartolini et al. Citation2015). Rainfall may still be an important influence on amphibian activity in ponds and wetlands, despite the aquatic habitat, due to water depth, the influx of resources than can arrive in rain events, and the associated changes in water chemistry and turbidity (Reading and Clarke Citation1995). The effects of temperature and rainfall may differentially influence individuals based on sex, stage, or size and result in varied responses between individuals in these categories to changes in environmental conditions (Marvin Citation2003).
Aquatic breeding habitats for amphibians are often temporary, placing increased importance on aquatic activity periods and the ability to take advantage of this ephemeral resource. On the other hand, some pond-breeding amphibian species can spend prolonged periods of time in aquatic habitats rather than immediately returning to the terrestrial habitat after breeding, and some species can remain in stable aquatic habitats for their entire life cycle (Russell et al. Citation2005). Despite the importance of the aquatic habitat to pond-breeding amphibians, whether for short breeding periods or longer residency, fewer studies have examined the role of environmental factors on the activity of aquatic adults.
Eastern red-spotted newts (Notophthalmus viridescens) are a common amphibian species associated with ponds and wetlands across the eastern United States and Canada (Petranka Citation1998). Most populations have four distinct life stages: aquatic egg, aquatic larvae, terrestrial eft (juvenile), and adult. In some populations, particularly in the southern part of the range, larvae can remain in stable aquatic habitats as pedomorphic gilled adults without a terrestrial juvenile stage (Harris Citation1987). Typically, larvae metamorphose into a terrestrial eft stage. After 2–8 years as efts, individuals reach sexual maturity and return to breeding ponds where they can either remain as fully aquatic residents or migrate between a pond and the terrestrial habitat in subsequent seasons (Sever Citation2006). This behavior, termed partial migration, is influenced by a variety of factors including geographic location, time of breeding season, and climatic conditions (Sever Citation2006; Grayson and Wilbur Citation2009). Male and female newts have been shown to differ in their migratory strategies, mate-seeking behavior, and their use of pond habitat (Grayson et al. Citation2012). Females migrate at higher rates and can remain in terrestrial habitats to skip breeding years, while males are more likely to remain in the breeding pond as residents or return to breed every year (Grayson et al. Citation2011). These differences in partial migration can ultimately result in male-biased sex ratios of newts in breeding habitats.
In this study, we used captures from unbaited surface minnow traps to examine the effects of environmental variation on water column activity patterns of aquatic adult newts during the winter breeding season in North Carolina, USA. We measured the effect of daily temperature and rainfall on newt activity, as well as on the sex and size composition of captured newts. Capture data from six years of sampling (2009–2015, except for 2010 and 2012), as well as more detailed data in 2016 on sex and size were used to compare these patterns in newt activity across time. Overall, we predicted that (1) newt activity would be positively correlated with temperature and the presence of rainfall, (2) warmer temperatures would increase male activity and captures, and (3) larger newts would be more active in colder temperatures than smaller newts.
Methods
Study site and long-term trapping study
We conducted our study in Mecklenburg County, North Carolina, USA at Cowan's Ford Wildlife Refuge (35.3775°N, 80.9658°W) in a 0.93 ha wetland with shrubs and grasses surrounded by mixed pine and hardwood forest (Kern et al. Citation2013). The wetland was filled with water for the duration of our study. In North Carolina, red-spotted newts primarily mate in the winter months (Harris et al. Citation1988). Although formal year-round surveys have not been conducted in this population, our observations from pitfall traps and opportunistic sampling during breeding seasons suggest that this population is partially migratory.
A long-term trapping array was established in this wetland in 2009. Forty unbaited plastic minnow traps (approximately 425 mm long, with a 5 mm inner opening diameter) were set by attaching each trap to a 1.2 m PVC pole along designated paths in the wetland. Trap locations were distributed as evenly as possible, at least 1 m apart, in areas of similar water depth and vegetation density, avoiding areas of thick grasses. Traps were maintained at surface level in the water with an internal float, ensuring that they were deep enough for only surface level individuals to become trapped. This wetland was sampled using minnow traps in the same configuration each year from 2009 to 2016 (excluding 2010 and 2012) during 5–6 consecutive weeks in January through March. During the aquatic sampling period, we recorded the number of newts found in each trap daily. Rainfall (mm) and air temperature (°C) data were recorded every 30 minutes and were taken from a nearby weather station on the Davidson College Ecological Preserve located 13.2 km away from Cowan's Ford. Minimum, maximum, and mean air temperatures were determined from the time series data for each day. Minimum temperature was chosen as the temperature variable for statistical analysis because it is relevant to the lower thermal limit for newt activity in the aquatic environment during the winter months.
Additional data collection in 2016
During 2016, the traps were checked daily from 16 January to 26 February. In addition to counting the number of captured individuals, we also recorded sex in this sampling year. Males were assigned based on the presence of a bright yellow spot behind the cloaca, as well as other identifying characteristics often seen during the breeding season (black cornification on the feet and underside of the hind legs, a swollen cloaca, and large tail fin; Dawley Citation1984). The snout-vent length (SVL; mm) and mass (g) for each captured newt was measured on a subset of two trapping occasions per week during the sampling period. In addition to air temperature data from the weather station, a datalogger recorded water temperature (°C) every hour near the water's surface with minimum, maximum, and mean temperatures determined for each day (Onset HOBO Pendant, Model UA-001). Consistent with the long-term sampling analysis, only minimum temperatures were used for the statistical analyses of the 2016 data.
Statistical analyses
In order to assess how newt activity responded to environmental conditions, we used a generalized linear mixed effects model with a Poisson distribution to determine how the daily number of newts captured over the course of six seasons was affected by minimum air temperature and the presence of rainfall. Temperatures analyzed were from midnight-midnight on the sampling day, with the minimum temperature occurring in the morning before the traps were checked. The model included a random effect of year to account for any variation among the seasons not explained by temperature or rainfall. We then tested the correlation between minimum air temperature and minimum water temperature using 2016 data, the only period where both variables were available.
Using only data collected in 2016, we used linear regression to determine the effect of minimum daily water temperature on the sex ratio (proportion male) of the newts captured on a given day. The proportion males captured were arcsine square root transformed. Rainfall was not included in this analysis because there were so few days with rain. We also conducted a linear regression to determine the effect of minimum daily water temperature on the mean SVL of all newts captured on a given day. As both mass and SVL are generally correlated, only SVL was used as a measure of body size in our study because mass measurements can be influenced by newt hydration and feeding status (Caetano and Leclair Citation1996). Rainfall was not included in the 2016 analyses because only one day in this subset of the data had rain. All analyses were conducted in R version 3.4.1 (R Core Team Citation2017).
Results
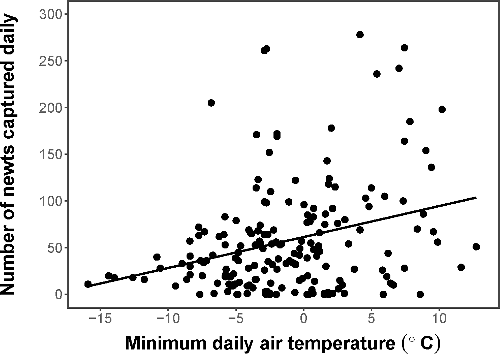
Over the five years of sampling at Cowan's Ford, the members of the Davidson College Herpetology Lab recorded 8,865 red-spotted newt captures. During the winter breeding season surveys we conducted, minimum air temperature ranged from −15.91 to 12.72°C (mean = −1.22°C) and minimum water temperature in 2016 ranged from −0.77 to 12.21°C (mean = 4.92°C). Minimum daily air temperature and minimum daily water temperature in 2016 were positively correlated (Pearson's correlation coefficient = 0.816). Across all years, increasing minimum air temperature significantly increased the number of newts captured on a given day (z = 20.017, p < 0.001; ). The presence or absence of rainfall had no significant effect on daily newt capture numbers (z = 1.309, p = 0.192).
Figure 1. Total number of newts captured daily across all years of sampling (2009, 2011, 2013–2016). There was a significant increase in newt captures with increasing minimum air temperature (°C).
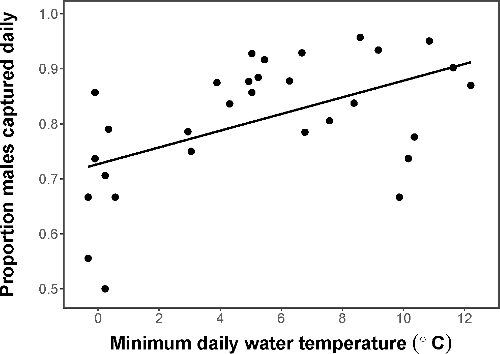
During our 2016 sampling, we recorded 2,079 total red-spotted newt captures consisting of 1,812 male and 267 female captures. The capture sex ratio ranged from a 1:1 male to female ratio up to a 23:1 male to female ratio, with the average capture sex ratio being 7:1 males to females (+/− 5 males SE). While the capture sex ratio was either equal or male-biased for all trapping occasions, we did find differences in activity between the sexes based on temperature. The arcsine transformed proportion of males captured on a given day significantly increased (more males than females) with increasing minimum water temperature (adjusted R2 = 0.23, F1,30 = 10.31, p = 0.003; ). This relationship was statistically similar when the values for proportion female were not transformed (adjusted R2 = 0.22, F1,30 = 9.65, p = 0.004). In other words, the sex ratio was less male-biased in colder temperatures.
Figure 2. Proportion of males captured daily in 2016. Daily captures were always male-biased or equal (0.5). The proportion of males captured in a given day increased with increasing minimum water temperature (°C).
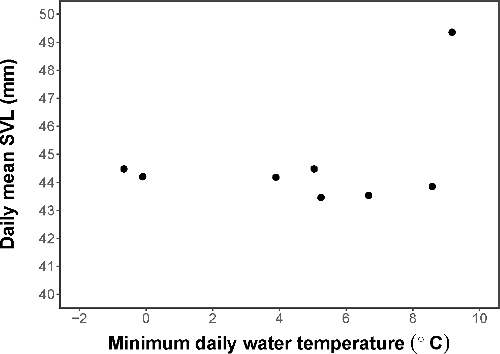
We measured body size of 761 captured newts in 2016, where SVL ranged from 36.8 to 59.06 mm (mean = 45.15 mm ± 0.14 SE). The mean SVL of newts captured on a given day was not affected by the minimum water temperature on that day (adjusted R2 = 0.003, F1,6 = 1.02, p = 0.3519; ).
Discussion
Our results indicate that temperature can play a large role in determining the activity of red-spotted newts in aquatic habitats. A wide range of studies on terrestrial life-stages of amphibians have shown that air temperature and rainfall are important predictors of activity levels (e.g. Heatwole Citation1962; Roe and Grayson Citation2008; Connette et. al Citation2011; Oswald et al. Citation2015). The results of this study also indicate the importance of temperature in breeding wetlands. Across all years of our analysis, our data show that warmer air temperatures result in increased newt activity during the breeding season, but this relationship was not seen for rainfall. Our sampling in 2016 demonstrated the correlation between air and water temperature, and we found sex, but not size-based, differences in the response to temperature.
Based on amphibian studies in terrestrial habitats and the general response of ectothermic animals to temperature, the response to temperature in aquatic newts was expected. Previous studies on terrestrial life stages of amphibians have shown the importance of temperature for migrations, habitat choice, surface activity, and feeding (e.g. Baldauf Citation1952; Heatwole Citation1962; Semlitsch Citation1985; Verrell and Halliday Citation1985). In ectotherms, physiological rates are highly dependent on temperature, and a wide variety of processes have temperature-dependent responses. These processes include foraging rates, prey handling, digestion, mass conversion efficiency, mate finding, and reproductive rates (i.e. Huey and Berrigan Citation2001; Byström et al. Citation2006; Englund et al. Citation2011). The increase in newt activity in response to temperature likely represents the combination of several of these processes becoming more favorable under warmer temperatures during the winter breeding season of this population.
Although other studies have indicated that rainfall is a significant factor in determining activity in terrestrial breeding migrations of amphibians (Heatwole Citation1962; Todd and Winne Citation2006), we did not observe a relationship between aquatic adult newt activity and the presence of rain in this study. This could be attributed to the relatively few days of rain during the study period or the treatment of rainfall as a categorical rather than a continuous variable. Our initial hypothesis regarding the effects of rainfall on aquatic activity was based on changes in resources or associations with changes in other abiotic factors, but these changes may require larger rain events. Additionally, it would be expected that aquatic life-stages, which are not limited by the evaporative water loss, would be less dependent and sensitive to rainfall than life stages in the terrestrial environment.
In 2016, we captured substantially more male than female newts, resulting in daily capture sex ratios that typically were highly male-biased. This could be attributed to our sampling method, which used unbaited traps set at the surface of the water and only captured newts active in the upper water column. Our study was also limited to the winter breeding season, when male mate searching is expected to be at its peak. Male newts exhibit intense mate seeking reproductive behavior and aggressively court females, resulting in higher visibility, activity patterns, and capture rates than females (Verrell Citation1983; Gabor et al. Citation2000). Female newts have been shown to spend more time hidden in vegetation and the substrate, which would reduce their capture probability in our traps (Grayson et al. Citation2012). Thus, the male-bias in our capture data is likely not reflective of the true sex ratio of the population. An alternative explanation for our capture sex ratio is that the population does have a skewed sex ratio with a greater proportion of males in the population (Attum et al. Citation2002) or that males remain in the pond longer than females and are present in higher numbers during the breeding season and a greater proportion of the female population remained in the terrestrial habitat (‘skipping breeding’; Bloch and Grayson et al. Citation2010). Mark-recapture methods that identify individuals would be necessary to accurately estimate population size or to test for differences in detection between the sexes. The biased sex ratio of red-spotted newt captures is an important consideration for management efforts that aim to assess the status of populations.
When examining capture differences in response to temperature between males and females, our data supported our hypothesis that water temperature can have sex-specific effects on newt activity. In addition to the generally male-biased sex ratio, both males and females were captured more frequently under warmer water temperatures. Alongside these general capture patterns, we found that the capture sex ratio changed in response to temperature, with a greater proportion of males captured in warmer temperatures and the proportion of females increasing under cooler temperatures. The decrease in male-bias at lower temperatures may be reflective of aggressive male newt behavior during the breeding season (Grayson et al. Citation2012), where female newts may be capitalizing on reduced male activity under cooler temperatures. An alternative explanation could also be that the sexes differ in their physiological performance in relation to temperature (Whitehead et al. Citation1989).
While we predicted that smaller individuals would be less active in colder temperatures, we did not find differences in the response to water temperature based on the mean SVL of captured newts, suggesting that temperature does not differentially reduce the activity of smaller adult newts. Given their overall small size and the high conductance of aquatic environments, it is likely that newt body temperature closely matches their surrounding environment and any size-based differences in activity in response to temperature would be due to differing behavioral responses. Although we found no overall relationship between body size and temperature, we did capture the largest newts on average during one of the warmest days of the study. A future study that monitors activity into the warmer, non-breeding season would provide more insight into this question.
Our study documents the effects of temperature and rainfall on red-spotted newt behavior in breeding ponds and highlights the importance of considering capture biases associated with unbaited surface minnow traps. Further studies utilizing more intensive mark-recapture analyses, and additional sampling of additional microhabitats habitats within the wetland during the breeding and non-breeding season could further elucidate our male-biased capture rates. We also recommend that future studies examining red-spotted newts include the effects of environmental variables on newt activity, and consider utilizing multiple sampling techniques in addition to passive trapping. Understanding the intersection between temperature and activity is particularly important for ectothermic organisms, where rapid changes in the environment can shift activity periods and ultimately influence the time available for essential functions (Huey et al. Citation2010). More broadly, the variation in red-spotted newt activity and behavior due to environmental conditions seen in our study can serve as a guide for understanding behavior patterns and their relationship with temperature in other amphibian species throughout the eastern United States for important life stages in aquatic habitat.
Acknowledgments
We thank Lenny Lampel and Stephen Hutchinson (Mecklenburg Division of Nature Preserves and Natural Resources) for access to the wetland at Cowan's Ford Wildlife Refuge and assisting with permit acquisition (NC Collecting Permit: 16-SC00068). We thank Shannon Pittman for her guidance with this project. We also thank the members of the Davidson Herpetology Lab, particularly Jaeyoung Lee and Kristen Gillespy, with their assistance in the field. Protocols for this study were approved by the Davidson College IACUC (Protocol no. 3-00-09).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Emma R. Johnson
Emma R. Johnson received a BS in Environmental Studies from Davidson College in 2017 and works for the School for Field Studies at their Centre for Himalayan Environment and Development Studies in Paro, Bhutan.
Brielle L. Bowerman
Brielle L. Bowerman received a BS from Davidson College in 2017. She currently attends the Dental College of Georgia at Augusta University.
Meagan A. Thomas
Meagan A. Thomas received a BS from Towson University in 2012 and her MS from Eastern Illinois University in 2014. She is currently a Research Manager at Davidson College in North Carolina.
Lily M. Thompson
Lily M. Thompson received her BS from Centre College in 2010 and her MS from Virginia Commonwealth University in 2014. She is currently a Lab Research Coordinator at the University of Richmond in Virginia.
Kristine L. Grayson
Kristine L. Grayson received her BS from Davidson College in 2003 and her PhD from University of Virginia in 2010. She is currently an assistant professor of Biology at the University of Richmond.
References
- Attum O, Eason P, Cobbs G. 2002. Effects of collection on weight, length, and sex ratio of red-spotted newts, Notophthalmus viridscens. J Herpetol. 36:703–707.
- Baldauf RJ. 1952. Climatic factors influencing the breeding migration of the spotted salamander, Ambystoma maculatum (Shaw). Copeia. 1952:178–181.
- Bartolini T, Butail S, Porfiri M. 2015. Temperature influences sociality and activity of freshwater fish. Environ Biol Fishes. 98:825–832.
- Blaustein AR, Belden LK, Olson DH, Green DM, Root TL, Kiesecker JM. 2001. Amphibian breeding and climate change. Conserv Biol. 15:1804–1809.
- Bloch AM, Grayson KL. 2010. Reproductive costs of migration for males in a partially migrating, pond-breeding amphibian. Can J Zool. 88:1113–1120.
- Buckley LB, Hurlbert AH, Jetz W. 2012. Broad-scale ecological implications of ectothermy and endothermy in changing environments. Global Ecol Biogeogr. 21:873–885.
- Byström P, Andersson J, Kiessling A, Eriksson L. 2006. Size and temperature dependent foraging capacities and metabolism: consequences for winter starvation mortality in fish. Oikos. 115:43–52.
- Caetano MH, Leclair R. 1996. Growth and population structure of red-spotted newts (Notophthalmus viridescens) in permanent lakes of the Laurentian Shield, Quebec. Copeia. 1996:866–874.
- Connette GM, Price SJ, Dorcas ME. 2011. Influence of abiotic factors on activity in a larval stream salamander assemblage. Southeastern Nat. 10:109–120.
- Dawley EM. 1984. Identification of sex through odours by male red-spotted newts, Notophthalmus viridescens. Herpetologica. 40:101–105.
- Dunham AE, Grant BW, Overall KL. 1989. Interfaces between biophysical and physiological ecology and the population ecology of terrestrial vertebrate ectotherms. Physiol Zool. 62:335–355.
- Englund G, Öhlund G, Hein CL, Diehl S. 2011. Temperature dependence of the functional response. Ecol Lett. 14:914–921.
- Gabor CR, Krenz JD, Jaeger RG. 2000. Female choice, male interference, and sperm precedence in the red-spotted newt. Behav Ecol. 11:115.
- Gibbons JW, Winne CT, Scott DE, Willson JD, Glaudas X, Andrews KM, Todd BD, Fedewa LA, Wilkinson L, Tsaliagos RN, et al. 2006. Remarkable amphibian biomass and abundance in an isolated wetland: implications for wetland conservation. Conserv Biol. 20:1457–1465.
- Gill DE. 1978. The metapopulation ecology of the red-spotted newt, Notophthalmus viridescens (rafinesque). Ecol Monogr. 48(2):145–166.
- Gillooly JF, Charnov EL, West GB, Savage VM, Brown JH. 2002. Effects of size and temperature on developmental time. Nature. 417:70–73.
- Glanville EJ, Seebacher F. 2006. Compensation for environmental change by complementary shifts of thermal sensitivity and thermoregulatory behavior in an ectotherm. J Exp Biol. 209:4869–4877.
- Grayson KL, Bailey LL, Wilbur HM. 2011. Life history benefits of residency in a partially migrating pond-breeding amphibian. Ecology. 92:1236–1246.
- Grayson KL, De Lisle SP, Jackson JE, Black SJ, Crespi EJ. 2012. Behavioral and physiological female responses to male sex ratio bias in a pond-breeding amphibian. Front Zool. 9:24.
- Grayson KL, Wilbur HM. 2009. Sex‐and context‐dependent migration in a pond‐breeding amphibian. Ecology. 90:306–312.
- Gvoždík L. 2005. Does reproduction influence temperature preferences in newts? Can J Zool. 83:1038–1044.
- Harris RN. 1987. Density-dependent paedomorphosis in the salamander Notophthalmus viridescens dorsalis. Ecology. 68:705–712.
- Harris R, Alford R, Wilbur H. 1988. Density and phenology of Notophthalmus Viridescens Dorsalis in a natural pond. Herpetologica. 44:234–242.
- Heatwole H. 1962. Environmental factors influencing local distribution and activity of the salamander, Plethodon cinereus. Ecology. 43:460–472.
- Huey R, Berrigan D. 2001. Temperature, demography, and Ectotherm fitness. Am Nat. 158:204–210.
- Huey RB, Losos JB, Moritz C. 2010. Are lizards toast? Science. 328:832–833.
- Huey RB, Stevenson RD. 1979. Integrating thermal physiology and ecology of ectotherms: a discussion of approaches. Am Zool. 19:357–366.
- Kern MM, Nassar AA, Guzy JC, Dorcas ME. 2013. Oviposition site selection by spotted salamanders (Ambystoma maculatum) in an isolated wetland. J Herpetol. 47:445–449.
- Marvin GA. 2003. Aquatic and terrestrial locomotor performance in a semiaquatic plethodontid salamander (Pseudotriton ruber): influence of acute temperature, thermal acclimation, and body size. Copeia. 2003:704–713.
- Newman RA. 1992. Adaptive plasticity in amphibian metamorphosis. Bioscience. 42:671–678.
- Ojanguren AF, Reyes-Gavilán FG, Braña F. 2001. Thermal sensitivity of growth, food intake and activity of juvenile brown trout. J Therm Biol. 26:165–170.
- Oswald HR, Waldron JL, Welch SM, Bennett SH, Mousseau TA. 2015. Environmental effects on southern two-lined salamander (Eurycea cirrigera) nest-site selection. Copeia. 103:7–13.
- Petranka J. 1998. Salamanders of the United States and Canada. Washington (DC): Smithsonian Institution Press.
- R: A language and environment for statistical computing. 2017. Vienna (AT): R Foundation for Statistical Computing; [accessed 2016]. https://www.R-project.org/.
- Ranvestel AW, Lips KR, Pringle CM, Whiles MR, Bixby RJ. 2004. Neotropical tadpoles influence stream benthos: evidence for the ecological consequences of decline in amphibian populations. Freshwater Biol. 49:274–285.
- Reading CJ, Clarke RT. 1995. The effects of density, rainfall and environmental temperature on body condition and fecundity in the common toad, Bufo bufo. Oecologia. 102:453–459.
- Roe AW, Grayson KL. 2008. Terrestrial movements and habitat use of juvenile and emigrating adult eastern red-spotted newts, Notophthalmus viridescens. J Herpetol. 42:22–30.
- Rohr JR, Raffel TR. 2010. Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proc Natl Acad Sci. 07:8269–8274.
- Russell AP, Bauer AM, Johnson MK. 2005. Migration in amphibians and reptiles: an overview of patterns and orientation mechanisms in relation to life history strategies. New York (NY): Springer.
- Semlitsch RD. 1985. Analysis of climatic factors influencing migrations of the salamander Ambystoma talpoideum. Copeia. 1985:477–489.
- Semlitsch RD. 2008. Differentiating migration and dispersal processes for pond-breeding amphibians. J Wildl Manage. 72:260–267.
- Semlitsch RD, Bodie RJ. 2003. Biological criteria for buffer zones around wetland and riparian habitats for amphibians and reptiles. Conserv Biol. 17:1219–1228.
- Spotila JR. 1972. Role of temperature and water in the ecology of lungless salamanders. Ecol Monogr. 42:95–125.
- Sever DM. 2006. The “false breeding season” of the red-spotted newt, Notophthalmus viridescens. Bull Chicago Herpetol Soc. 41:149–153.
- Stolp H. 1988. Microbial ecology: Organisms, habitats, activities. New York (NY): Cambridge University Press.
- Todd BD, Winne CT. 2006. Ontogenetic and interspecific variation in timing of movement and responses to climatic factors during migrations by pond-breeding amphibians. Can J Zool. 845:715–722.
- Verrell PA. 1983. The influence of the ambient sex ratio and intermale competition on the sexual behavior of the red-spotted newt, Notophthalmus viridescens (Amphibia: Urodela: Salamandridae). Behav Ecol Sociobiol. 13:307–313.
- Verrell P, Halliday T. 1985. Reproductive dynamics of a population of smooth newts, Triturus vulgaris, in Southern England. Herpetologica. 41:386–395.
- Whiles MR, Lips KR, Pringle CM, Kilham SS, Bixby RJ, Brenes R, Connelly S, Colon-Gaud JC, Hunte-Brown M, Huryn AD, et al. 2006. The effects of amphibian population declines on the structure and function of Neotropical stream ecosystems. Front Ecol Environ. 4:27–34.
- Whitehead PJ, Puckridge JT, Leigh CM, Seymour RS. 1989. Effect of temperature on jump performance of the frog Limnodynastes tasmaniensis. Physiol Biochem Zool. 62:937–949.