 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A long-term fish community monitoring program was established by the Oklahoma Department of Wildlife Conservation Streams Program in 2016. One of the primary goals of this program is to evaluate contemporary fish species distributions in Oklahoma and draw inferences regarding changes in those distributions over time. In 2016, fish community surveys took place from late June to early August at a total of 48 sites within the upper Red River basin. Compared to the most comprehensive historical sampling effort within the basin, contemporary surveys detected an additional eight species while three species historically present were not detected in 2016. Multivariate generalized linear model results indicated significant differences in community structure between historical and contemporary surveys. Univariate testing paired with Sum-of-LR analyses revealed differences in community structure were largely driven by increases in generalist fish species (e.g. Green Sunfish and Common Carp) and decreases in small-bodied specialist cyprinids (e.g. Chub Shiner). Although changes in species occurrences may be partially driven by differences in sampling methodology and effort, changes across multiple stream reaches likely reveal real trends.
Introduction
The negative effects of anthropogenic-induced stressors on the earth's flora and fauna, particularly extirpation and extinction, are well documented (Ehrlich and Wilson Citation1991; Vitousek et al. Citation1997; Dirzo et al. Citation2014; Wilting et al. Citation2017). Increases in imperilment of biota in freshwater ecosystems, specifically freshwater fishes, have been documented in North America in the past several decades. Approximately 40% of freshwater fish species in North America are listed as threatened, imperiled or endangered (Jelks et al. Citation2008). This is particularly concerning for freshwater ecosystems undergoing increased levels of anthropogenic alterations such as instream water-withdrawals, creation of hydroelectric impoundments, groundwater pumping for agricultural purposes, and urban ‘beautification’ (Hermoso Citation2017; Pennock Citation2017; Perkin et al. Citation2017). Fish communities in the Midwest and Interior Plains portion of the United States have drastically changed as a result of modifications to lotic ecosystems (Dodds et al. Citation2004; Gido et al. Citation2010; Hoagstrom et al. Citation2011). These modifications have led to expanded distributions of non-native and generalist species coupled with contracted distributions of specialist species and homogenization of fish communities (Smith et al. Citation2014; Matthews and Marsh-Matthews Citation2015; Perkin et al. Citation2017).
Long-term biodiversity monitoring efforts can detect small declines in species ranges prior to larger collapses or local extirpation of populations, as well as facilitate understanding of anthropogenic effects on the community as a whole (Nielsen et al. Citation2009; Magurran et al. Citation2010; Ward-Campbell et al. Citation2017). This is especially critical for fish communities in lotic waters of the Interior Plains as they consist of many species with short life spans and reproductive strategies (i.e. pelagic-broadcast spawning; PBS) that make them exceptionally vulnerable to range reductions and local extirpation resulting from anthropogenic activities (Platania and Altenbach Citation1998; Pennock, Gido, et al. Citation2017). PBS species are particularly sensitive to anthropogenic alterations as they depend on unfragmented river reaches and adequate streamflow to keep ova suspended until they hatch (Luttrell et al. Citation1999; Perkin and Gido Citation2011).
In 2016, the Oklahoma Department of Wildlife Conservation (ODWC) Streams Program established a long-term fish community monitoring program with a primary goal to identify fish assemblage changes through time across lotic waters of Oklahoma. The study design of the monitoring program allows for a detection-adjusted occupancy modeling approach, which enables the calculation of population parameters unbiased by detection error using presence–absence data and accurate tracking of changes in species occurrence across time ( MacKenzie et al. Citation2006). The current monitoring program plans to resample drainages throughout the state on a decadal rotation. This rotation schedule limits immediate robust statistical comparisons of communities due to the long time-frame needed to compare detection-adjusted occupancies of species and changes in community structure. However, initial baseline surveys present an opportunity for retrospective analysis and comparison to historical data-sets that can provide critical foundational knowledge pertaining to changes in the state of the fish community (Smith et al. Citation2014). In 2016, the ODWC Streams Program conducted extensive sampling in the upper Red River basin. Fortunately, thorough sampling of the ichthyofaunal communities of the upper Red River basin by Taylor et al. (Citation1991) provided an opportunity for a comparative study. Unfortunately, this data-set does not precede the construction of Denison Dam and Lake Texoma in 1945, which greatly modified this system. It does offer insight into community shifts over the past 30 years. The objective of this study was to test for differences in fish community structure between contemporary surveys and the Taylor et al. (Citation1991) study.
Methods
Study area
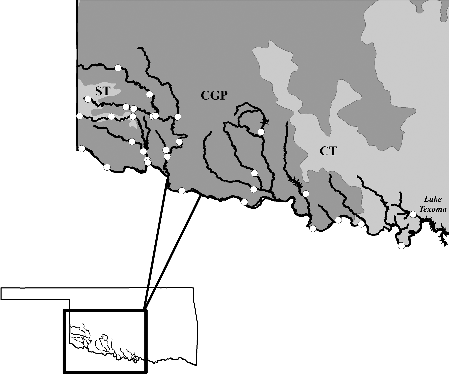
The upper Red River basin of Oklahoma spans approximately 38,300 km2 from the Texas–Oklahoma border in southwest Oklahoma to the upper reaches of Lake Texoma in southcentral Oklahoma (). The major lotic systems in the Red River basin include the Red River proper, the North Fork of the Red River, the Elm Fork of the Red River, and the Salt Fork of the Red River. These larger streams are characterized by shallow, braided, sand-bed channels with large S-shaped meanders (Matthews Citation1988). Smaller streams of the region typically fall into either of the two types: systems with incised banks, high turbidity, and silt-mud substrates, or high-gradient systems with low turbidity and cobble to boulder sized substrates Ecoregions within the upper Red River basin include the Southwestern Tablelands (ST), Central Great Plains (CGP), and the Cross Timbers (CT). The ST are characterized by sub-humid grassland and semiarid rangeland, little to no cropland, and scattered red-hued canyons, mesas, and badlands. In contrast, much of the CGP is cropland with some grassland scattered with shrubs and low trees. A unique feature of the CGP region is its many subsurface salt deposits that can cause high salinity in some streams. The CT region is characterized by native grasslands scattered with sparse Blackjack Oak Quercus marilandica and Post Oak Quercus stellate trees. Large portions of the CT region are used for pastureland and oil exploration (EPA Citation2013).
Figure 1. Sites sampled by both historical (1989) and contemporary (2016) fish community surveys located in the upper Red River basin of Oklahoma. Level III ecoregions within the drainage include Southwest Tablelands (ST), Central Great Plains (CGP), and Cross Timbers (CT).
Before construction of Lake Texoma, the upper Red River basin was potentially home to 11 current Oklahoma fish Species of Greatest Conservation Need (SGCN): Shovelnose Sturgeon Scaphirhynchus platorhynchus, Blue Sucker Cycleptus elongatus, Shorthead Redhorse Moxostoma macrolepidotum, Black Buffalo Ictiobus niger, Plains Minnow Hybognathus placitus, Prairie Chub Macrhybopsis australis, Red River Shiner Notropis bairdi, Chub Shiner Notropis potteri, Red River Pupfish Cyprinodon rubrofluviatilis, Paddlefish Polyodon spathula, and Alligator Gar Atractosteus spatula (Miller and Robinson Citation2004; Crews et al. Citation2005). The basin is also home to many recreationally important species: Largemouth Bass Micropterus salmoides, Spotted Bass Micropterus punctulatus, Smallmouth Bass Micropterus dolomieu, Black Crappie Pomoxis nigromaculatus, White Crappie Pomoxis annularis, Walleye Sander vitreus, Striped Bass Morone saxatilis, Blue Catfish Ictalurus furcatus, Channel Catfish Ictalurus punctatus, Flathead Catfish Pylodictus olivaris, White Bass Morone chrysops, and other members of the sunfish (i.e. Centrarchidae) family (e.g. Bluegill Lepomis macrochirus and Green Sunfish Lepomis cyanellus). It is worth noting that several sport fish are only present because of stockings outside of their native ranges (e.g. Smallmouth Bass and Walleye).
Fish surveys
Historical data were collected at 87 sites across the upper Red River basin by Taylor et al. (Citation1991) from March to late June. Each site was sampled once using a seine (4.5 × 1.5 m, 9.5-mm mesh) for a total of one hour.
Contemporary collections occurred at 48 sites in the upper Red River basin. Sites were selected in a stratified random fashion so that a representative number of samples were collected from each ecoregion (based on total area) and stream type (based on elevation, stream size, and dominant substrate). Sites were surveyed from late June to early August in 2016. Each site was surveyed a total of three times, generally one week apart, to generate replicate surveys for future, additional statistical analyses. Fish assemblages were primarily sampled using a seine (3 × 1.5 m, 6.0-mm mesh). A minimum of 20 seine hauls, covering a minimum longitudinal distance of 150 m were conducted at each site when conditions allowed. The majority of fishes were identified in the field and released. Voucher and unidentified specimens were preserved in 10% formalin and identified using Buchanan and Robison (Citation1988), Plieger (Citation1997), and Miller and Robison (Citation2004).
Analyses
Thirty-one of the 48 contemporary sites were also sampled during the 1989 surveys. Therefore, these 31 paired sites were retained for multivariate analyses. Due to differences in sampling effort (three replicate visits to one site in 2016 versus one sample per site in 1989) only species presence–absence data were used for multivariate analyses. Significant differences between historical and contemporary fish communities were tested using a model-based approach employing simultaneous generalized linear models (GLMs) of multivariate data (ManyGLM; Wang et al. Citation2017; Warton et al. Citation2015). Due to the use of binary (presence/absence) data, a GLM with a binomial error distribution and a complementary log-log link function was used with the formula:where Yji is the presence/absence of fish species j found at site i, and the fixed effect of time (historical or contemporary) for species j at site i. Residual plots from the ManyGLM procedure showed little to no pattern, indicating that the binomial error distribution was appropriate. This procedure fits a GLM to each species and the log-likelihood ratios (LR) for each species are summed to create a Sum-of-LR that can be used as a test statistic via randomization (Warton et al. Citation2015; McCain et al. Citation2016). This analysis was repeated using one randomly selected sampling event per site from contemporary surveys to examine the potential effect of sampling effort on the model.
Using the anova.ManyGLM procedure, univariate test statistics and accompanying p values were generated using 999-iteration bootstrapping corrected for multiple testing for each species. For species that significantly differed between sampling periods in the anova.ManyGLM procedure, the percent contribution to Sum-of-LR was calculated as the proportion of LR for an individual model to the Sum-of-LR (McCain et al. Citation2016). For all analyses, results were considered significant at α = 0.05 using a LR statistic. Because of sensitivities with rare species, species occurring in less than 5% of all paired samples were removed prior to analyses.
To further quantify changes in distribution of particular species, naïve site occupancy (not corrected for detection) was calculated for each species in both historical and contemporary collections at sites that were sampled in both time periods. Naïve site occupancy () was calculated as the proportion of sites occupied by a given species (MacKenzie et al. Citation2006). Change in occupancy for paired sites (∆
) was calculated by subtracting the historical from the contemporary percentage of sites occupied. Specific attention was given to changes in
for SGCN.
Differences in species richness between paired sites were tested using paired t-tests. All analyses were performed in R (R version 3.3.4; R Core Team Citation2017) using the vegan (Oksanen et al. Citation2017) and MVabund packages (Wang et al. Citation2017).
Results
Fifty-three species representing 12 families were collected from 48 sites encompassing 17 streams in the upper Red River basin in contemporary surveys. Three species present in historical surveys were not collected in contemporary surveys, while eight species not collected during historical surveys were collected in contemporary surveys (). Only 4 of the 11 potential SGCN were sampled. No significant difference between species richness values for historical (10.8 ± 2.7; mean ± SD) and contemporary (10.3 ± 3.7) surveys was observed (paired t30 = 0.76, p = 0.46).
Table 1. Number of sites with species that were absent from either historical or contemporary sampling efforts.
Multivariate GLMs indicated significant differences between historic and contemporary fish community structure (LR1,61, p < 0.01). Univariate tests indicated that occurrences of eight species, Common Carp Cyprinus carpio, Gizzard Shad Dorosoma cepedianum, Western Mosquitofish Gambusia affinis, Channel Catfish, Green Sunfish, Bluegill, Inland Silverside Menidia Beryllina, and Chub Shiner were significant drivers of observed differences (). The GLM used to account for differences in sampling effort also indicated significant differences between historic and contemporary fish community structure (LR1,61, p < 0.01). Univariate tests indicated that occurrences of three species, Emerald Shiner Notropis atherinoides (12.3%), Green Sunfish (19.3%), and Chub Shiner (30.0%) were significant drivers of observed differences.
Table 2. Analysis of deviance table generated from the ManyGLM procedure for fish species occurring in both historic and contemporary samples.
Notable differences in were observed for many species. The largest increases in
were observed for Green Sunfish (+74%), Gizzard Shad (+54%), and Channel Catfish (+49%). Species with the largest decreases were Chub Shiner (−48%), Emerald Shiner (−29%), and Plains Minnow (−16%). All PBS species had decreases in
except for Silver Chub Macrhybopsis storeriana ().
Table 3. Change in historical (1989) and contemporary (2016) naïve occupancy () rates at paired sites. Highlighted species were found to be significantly different between historical and contemporary surveys in univariate analyses ().
Discussion
Declines in many species occurrences were observed despite increased sampling effort. Museum collections of fish community surveys within the basin reviewed by Wilde et al. (Citation1996) also reported basin-wide declines in relative abundance of two SGCN: Plains Minnow and Red River Shiner. The current study also observed declines in the naïve occupancy for both species and targeted sampling efforts may be warranted.
Furthermore, it is alarming that of the 20 sites where Chub Shiner were collected in 1989, none were captured in 2016. Seasonal shifts in Chub Shiner abundances do not provide a likely explanation for the observed non-detections in 2016 as Wilde et al. (Citation1996) listed 81 historical collections within the basin where Chub Shiner were collected within the same months as the contemporary survey. Additionally, a report by Ruppel et al. (Citation2017) detailing fish community and targeted Prairie Chub sampling within the Red River mainsteam and tributaries on the Texas side of the upper Red River basin also failed to collect any Chub Shiner, despite sampling 36 sites and 20 reaches from September 2015 to September 2016. The last known collection of Chub Shiner that the authors are aware of consists of one individual taken from the Salt Fork in 2004 (2016 email from W.J. Matthews, unreferenced). Changes in species distributions may be interpreted with caution because of differences in sampling methodology and effort between historical and contemporary surveys. However, as described by Smith et al. (Citation2014), increased effort in contemporary surveys should yield conservative estimates of species decline, which makes the apparent disappearance of Chub Shiner and the overall decrease in of PBS in recent years more concerning.
In contrast, when interpreting increases in species range and site occupancy, the reader should keep in mind that increased effort and temporal fluctuations in species’ abundances may contribute to these patterns. It is likely that increased effort would account for increased occupancy of rare or uncommon species within the basin such as Silver Chub and River Shiner Notropis blennius (Miller and Robison Citation2004). Silver Chub and River Shiner were sampled twice and once, respectively, but they were not collected in historical surveys. Additionally, some species for which increases were found (e.g. Common Carp and Longnose Gar Lepisosteus osseus) have been shown to be rare in seine samples (catch per unit effort [cpue] <0.1 per haul) in large prairie rivers of Oklahoma (Utrup and Fisher Citation2006). Therefore, it is logical that increased effort would increase the likelihood of detecting these species. Conversely, it would be expected that for those species that Utrup and Fisher (Citation2006) documented cpue greater than 0.1 per seine haul (River Carpsucker Carpoides carpio 1.07, Smallmouth Buffalo Ictiobus bubalus 0.34, Plains Minnow 19.23, Prairie Chub 0.13, Emerald Shiner 13.22, Red River Shiner 1.88, Red River Pupfish 0.28, and Plains Killifish Fundulus zebrinus 0.35) to be collected by both historical and contemporary surveys given a conservative threshold of 10 seine hauls per sampling event. Contemporary surveys exceeded this threshold (median = 20 hauls per site) while historical sites sampled for an hour likely met or exceeded 10 hauls per site (2017 email from C. Taylor; unreferenced). Regardless, decreases in for most of these ‘common’ species were documented between historical and contemporary surveys. In an effort to better gauge the effects of unequal sampling effort, an additional multivariate analysis in MVabund using one randomly selected sampling event per site from contemporary surveys was used. The results of this analysis reduced the number of significant species from eight to three. This analysis represents the most conservative estimate of changes in species distributions. Actual shifts likely reside somewhere between this model and the initial model included in the results section.
While fish communities of the upper Red River basin have been shown to be somewhat resilient to extreme fluctuations, several of the species for which declines in were documented include native PBS species particularly sensitive to fragmentation of riverine habitat when coupled with drought (Ross et al. Citation1985; Perkin and Gido Citation2011; Perkin et al. Citation2015). This ecological ‘ratcheting’ described by Perkin et al. (Citation2015) refers to a scenario where species become locally extirpated due to severe drought because source populations are unable to recolonize fragmented habitats. Past local extirpations of fish species within the basin have been attributed to fragmentation resulting from construction of impoundments on both the North Fork of the Red River and the Wichita River, Texas (Winston et al. Citation1991; Wilde et al. Citation1996). In both cases, known PBS species (e.g. Prairie Chub, Chub Shiner, Plains Minnow, Red River Shiner, and Silver Chub) were either locally extirpated, or exhibited consistent declines in abundance in fragmented reaches. In the current study, declines in
for cyprinids were largely attributed to sites on the Salt Fork of the Red River. Plains Minnow was the only PBS that was collected in the middle to upstream reaches of the Salt Fork. None of the remaining PBS species, nor Emerald Shiner, were collected at any of the four sites on the Salt Fork except for one occurrence (site 17) located just upstream (approximately 6.2 rkm) from the confluence with the Red River (). Although Emerald Shiner are considered lithophilic species, they are known to respond negatively to impoundments (Wilde et al. Citation1996; Pennock, Bender, et al. Citation2017). Although the Salt Fork is considered unfragmented, investigation of aerial photography by the authors revealed what appear to be a series of man-made structures modifying stream channel morphology between Elmer and Olustee, OK. These structures were typically accompanied by make-shift roads running perpendicular to the river directly downstream. Given the link between PBS species and fragmentation, it is possible that these structures could explain non-detections at sites in the upper Salt Fork as they may be inhibiting recolonization following the intense regional droughts of 2011 and 2012.
Despite differences in effort, it should be noted that similar investigations concerning temporal shifts in stream fish communities have found increases in generalist centrarchid species coupled with community homogenization at both the hydraulic unit and level III ecoregion scale (Smith et al. Citation2014; Matthews and Marsh-Matthews Citation2015). Changes in in the present study indicate both increases in occurrence of native generalists (e.g. Green Sunfish) and non-native generalists (e.g. Common Carp). These changes across multiple reaches likely reveal real trends. This is especially true in instances where increased efforts yielded less detection of species that have been shown to be sensitive to anthropogenic modifications in other parts of their range. It should also be noted that these retrospective analyses were completed on an already altered stream fish community as evidenced by the fact that contemporary and historical (1989) surveys failed to detect seven and six, respectively, pre-Lake Texoma Oklahoma fish SGCN in this basin. A comparison of upper Red River fish communities pre-Lake Texoma would highlight the loss of large migratory species (e.g. Paddlefish and Blue Sucker), as well as large-bodied lotic specialists (e.g. Shovelnose Sturgeon) that our current study failed to detect (Riggs and Moore Citation1949; Wilde et al. Citation1996).
Continued monitoring of stream fish communities within the basin is warranted to monitor trends in sensitive species. The 2016 surveys will serve as valuable baseline data for future analyses. These future analyses may elucidate statistically significant shifts in fish community structure and identify drivers of those shifts (Edge et al. Citation2016). Sampling methodology within the current framework allows for future statistical analyses that enable identification of factors leading to non-detection of species through both ecological and detection processes. This makes calculation of detection-corrected site occupancies possible (Mackenzie et al. Citation2002). This method has particular utility for large-scale biodiversity monitoring programs and will be the foundational analysis used for monitoring stream fish communities by the ODWC Streams Program in the future (Pellet and Schmidt Citation2005; Anderson et al. Citation2012).
Acknowledgments
Financial support for this publication was provided by the ODWC and Sport Fish Restoration Grant F13AF00191. We thank R. Farney, C. Tackett, M. Howery, D. Rodger, J. Perry, C. Porter, B. Matthews, D. Warton, J. McCain, D. McDonald, C. Taylor, W. Heather, J. Burroughs, and R. Ryswyk for assistance. This work would not have been possible without the cooperation of many generous landowners.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Trevor A. Starks
Trevor A. Starks is a fisheries technician in the streams program for the ODWC. He received a BS degree in Fisheries, Wildlife, and Conservation Biology from Kansas State University in 2013 and a MS degree in Fisheries and Aquatic Ecology from Oklahoma State University in 2015.
Anthony W. Rodger
Anthony W. Rodger is a fisheries biologist in the streams program for the ODWC. He received a BS degree in Wildlife and Fisheries Sciences from South Dakota State University in 2013 and a MS degree in Fisheries Science from Texas A&M University in 2015.
Donnie King
Donnie King is a fisheries technician in the stream program for the ODWC. He received a BS degree in Biology from Northeastern State University in 2012.
Matthew Skoog
Matthew Skoog is a fisheries biologist in the streams program for the ODWC. He received a BS degree in Aquatic Biology from Bemidji State University and a MS degree in Aquaculture/Fisheries from the University of Arkansas at Pine Bluff.
References
- Anderson GB, Freeman MC, Hagler MM, Freeman BJ. 2012. Occupancy modelling and estimation of the Holiday Darter species complex with the Etowah River system. Trans Am Fish Soc. 141:34–45.
- Buchanan TM, Robison H. 1988. Fishes of Arkansas. Fayetteville (AR): The University of Arkansas Press.
- Crews A, Hawkinson B, Duffy G, Namminga H, Howery M, Hatcher R, Suttles R, Amend S, Shropshire T. 2005. Oklahoma comprehensive wildlife conservation strategy. Oklahoma City (OK): Oklahoma Department of Wildlife Conservation. ( State Wildlife Grant T-2-P-1).
- Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B. 2014. Defaunation in the anthropocene. Science. 345:401–406.
- Dodds WK, Gido KB, Whiles MR, Fritz KM, Matthews WJ. 2004. Life on the edge: the ecology of Great Plains prairie streams. BioScience. 54:205–216.
- Edge CB, Fortin M, Jackson DA, Lawrie D, Stanfield L, Shrestha N. 2016. Habitat alteration and habitat fragmentation differentially affect beta diversity of stream fish communities. Landscape Ecol. 32:647–662.
- Ehrlich PR, Wilson EO. 1991. Biodiversity studies: science and policy. Science. 253:758–762.
- [EPA] Environmental Protection Agency. 2003. Primary distinguishing characteristics of level III ecoregions of the continental United States. [ accessed 2017 Jan 5]. https://www.epa.gov/eco-research/level-iii-and-iv-ecoregions-continental-united-states.
- Gido KB, Dodds WK, Eberle ME. 2010. Retrospective analysis of fish community change during a half-century of landuse and streamflow changes. J N Am Benthol Soc. 29:970–987.
- Hermoso V. 2017. Freshwater ecosystems could become the biggest losers of the Paris Agreement. Global Change Biol. 23:3433–3436.
- Hoagstrom CW, Brooks JW, Davenport SR. 2011. A large-scale conservation perspective considering endemic fishes of the North American plains. Biol Conser. 144:21–34.
- Jelks HL, Walsh SJ, Burkhead NM, Contreras-Balderas S, Diaz-Pardo E, Hendrickson DA, Lyons J, Mandrak NE, McCormick F, Nelson JS, et al. 2008. Conservation status of imperiled North American freshwater and diadromous fishes. Fisheries. 33:372–407.
- Luttrell GR, Echelle AA, Fisher WL, Eisenhour DJ. 1999. Declining status of two species of the Macrhybopsis aestivalis complex (Teleostei: Cyprinidae) in the Arkansas River Basin and related effects of reservoirs as barriers to dispersal. Copeia. 4:981–989.
- MacKenzie DI, Nichols JD, Lachman GB, Droege S, Royle JA, Langtimm CA. 2002. Estimating site occupancy rates when detection probabilities are less than one. Ecology. 83:2248–2255.
- MacKenzie DI, Nichols JD, Royle JA, Pollock KH, Bailey LL, Hines JE. 2006. Occupancy estimations and modelling: inferring patterns and dynamics of species occurrence. Burlington (MA): Academic Press.
- Magurran AE, Baillie SR, Buckland ST, Dick JM, Elston DA, Scott EM, Smith RI, Somerfield PJ, Watt AD. 2010. Long-term datasets in biodiversity research and monitoring: assessing change in ecological communities through time. Trends Ecol Evol. 25:574–582.
- Matthews WJ. 1988. North American prairie streams as systems for ecological study. J N Am Benthol Soc. 7:387–409.
- Matthews WJ, Marsh-Matthews E. 2015. Comparison of historical and recent fish distribution patterns in Oklahoma and western Arkansas. Copeia. 103:170–180.
- McCain JSP, Cull DJ, Schneider DC, Lotze HK. 2016. Long-term shift in coastal fish communities before and after the collapse of Atlantic Cod (Gadus morhua). ICES J Mar Sci. 75:1415–1426.
- Miller RJ, Robison HW. 2004. Fishes of Oklahoma. Norman (OK): University of Oklahoma Press.
- Nielsen SE, Haughland DL, Bayne E, Schieck J. 2009. Capacity of large-scale, long-term biodiversity monitoring programmes to detect trends in species prevalence. Biodivers Conserv. 18:2961–2978.
- Oksanen JF, Blanchet G, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, et al. 2017. Vegan: community ecology package version 2.4-2. [ accessed 2016 Dec 1]. http://CRAN.R-project.org/package=vegan.
- Pellet J, Schmidt BR. 2005. Monitoring distributions using call surveys: estimating site occupancy, detection probabilities and inferring absence. Biol Conserv. 123:27–35.
- Pennock CA. 2017. Beautification of Great Plains rivers: a perspective on the use and appreciation of aquatic resources. Fisheries. 42:83–87.
- Pennock CA, Bender D, Hofmeier J, Mounts JA, Waters R, Weaver VD, Gido KB. 2017. Can fishways mitigate fragmentation effects on Great Plains fish communities? Can J Fish Aquat Sci. 75:121–130.
- Pennock CA, Gido KB, Perkin JS, Weaver VD, Davenport SR, Caldwell JM. 2017. Collapsing range of an endemic Great Plains minnow, Peppered Chub Macrhybopsis tetranema. Am Mid Nat. 177:57–68.
- Perkin JS, Gido KB. 2011. Stream fragmentation thresholds for a reproductive guild of Great Plains fishes. Fisheries. 36:371–383.
- Perkin JS, Gido KB, Cooper AR, Turner TF, Osborne MJ, Johnson ER, Mayes KB. 2015. Fragmentation and dewatering transform Great Plains stream fish communities. Ecol Monogr. 85:73–92.
- Perkin JS, Gido KB, Falke J, Fausch KD, Crockett H, Johnson ER, Sanderson J. 2017. Groundwater declines are linked to changes in Great Plains stream fish assemblages. Proc Natl Acad Sci. 114:7373–7378.
- Platania SP, Altenbach CA. 1998. Reproductive strategies and eggs types of seven Rio Grande basin cyprinids. Copeia. 1998:559–569.
- Plieger WL. 1997. The fishes of Missouri. Jefferson City (MO): Missouri Department of Conservation.
- R Core Team. 2017. R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. http://www.R-project.org/.
- Riggs CD, Moore GA. 1949. Some new records of Paddlefish and Sturgeon for Oklahoma. Proc Oklahoma Acad Sci. 30:16–18.
- Ross ST, Matthews WJ, Echelle AA. 1985. Persistence of stream fish assemblages: effects of environmental change. Am Nat. 126:24–40.
- Ruppel DS, Sotola VA, Gurbuz OA, Martin NH, Bonner TH. 2017. Endangered species research projects for the Prairie Chub. San Marcos (TX): Texas State University.
- Smith CD, Fischer JR, Quist MC. 2014. Historical changes in Nebraska's lotic fish assemblages: implications of anthropogenic alterations. Am Mid Nat. 172:160–184.
- Taylor CM, Winston MR, Mattews WJ. 1991. Distribution and abundance of sport and forage fishes of the upper Red River drainage (above Lake Texoma) in Oklahoma. Norman (OK): University of Oklahoma. Sport Fish Restoration Grant F-48-R.
- Utrup NJ, Fisher WL. 2006. Development of a rapid bioassessment protocol for sampling fish in large prairie rivers. N Am J Fish Manage. 26:714–726.
- Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. 1997. Human domination of earth's ecosystems. Science. 277:494–499.
- Wang Y, Naumann U, Wright S, Edelbuettel D, Warton D. 2017. MVAbund: statistical methods for analysing multivariate abundance data version 3.12. [ accessed 2016 Dec 10]. https://CRAN.R-project.org/package=mvabund.
- Ward-Campbell B, Cottenie K, Mandrak NE, McLaughlin R. 2017. Fish assemblages in agricultural drains resilient to habitat change caused by drain maintenance. Can J Fish Aquat Sci. 999:1–11.
- Warton KI, Foster SD, De'ath G, Stoklosa J, Dunstan PK. 2015. Model-based thinking for community ecology. Plant Ecol. 216:669–682.
- Wilde GR, Weller RR, Smith CD, Jimenez Jr. R. 1996. Review and synthesis of existing fish collection records for the upper Red River basin upstream from Lake Texoma. Lubbock (TX): Texas Tech University.
- Wilting HC, Schipper AM, Bakkenes M, Meijer JR, Huibregts MAJ. 2017. Quantifying biodiversity losses due to human consumption: a global-scale footprint analysis. Environ Sci Technol. 51:3298–3306.
- Winston MR, Taylor CM, Pigg J. 1991. Upstream extirpation of four minnow species due to damming of a prairie stream. Trans Am Fish Soc. 120:98–105.
