Abstract
In this study, the concept of functional traits was used to classify zooplankton into functional groups and their seasonal dynamics were determined in relation to physico-chemical factors in a highly turbid and nutrient-rich wetland. This study revealed a seasonal variation of both physico-chemical factors and biomass of zooplankton functional groups. Water temperature (TEP), total phosphorus (TP), ferrous ion (Fe2+) and total carbon (TC) were significantly higher in summer while water transparency (SD) was notably higher in spring. Zooplankton functional group biomass was higher in summer (5.11 mg/L) followed by winter, autumn and spring (0.91, 0.72 and 0.28 mg/L, respectively). Large copepods and cladoceran carnivore (LCC) was the dominant functional group in spring accounting for about 86.7% of the total biomass. In summer, MCF (Middle copepods and cladocerans filter feeders) was the dominant group probably because of the optimal temperature and availability of nutrients. Pearson correlation and RDA analysis results suggested that Fe2+, TP and TEP were the major factors influencing zooplankton functional groups. Notably, turbidity was not the main factor despite the fact that Small Xingkai Wetland Lake is a very turbid lake. The variation of zooplankton functional groups among seasons highlights the role of physico-chemical factors in structuring zooplankton composition.
Introduction
Wetlands are dynamic and highly productive ecosystems providing habitat for primary organisms such as zooplankton and phytoplankton which plays a critical role in aquatic systems. Zooplankton are highly diverse organisms that play a critical role as grazers in wetland foodwebs, acting as a link of energy transfer from primary producers to the tertiary consumers such as birds and fish. Ruhl and Smith (Citation2004) noted that zooplankton not only support the higher trophic levels in wetlands but also sustain the benthic and microbial communities. More importantly, zooplankton play essential roles in shaping climate change phenomena (Richardson Citation2008). The ability of wetlands to act as sink of greenhouse gas species to some extent depend on the biological interactions. By grazing on phytoplankton, zooplankton enhances carbon cycle that could be fixed or locked up in the wetland sediments. Recently, scientists have been trying to understand the biological, chemical and physical interactions in wetlands ecosystems (Ma and Yu Citation2013; Huszar et al. Citation2015). Several hypotheses have been proposed by these scientists and among them is that there exists spatial-temporal variation in physico-chemical parameters as well as biological community structure (flora and fauna) in wetland ecosystems (Declerck et al. Citation2007; Dube et al. Citation2017). And according to Sun et al. (Citation2010), Shi et al. (Citation2015) and Kagalou et al. (Citation2010), zooplankton functional groups are very sensitive to changes in physico-chemical variables in aquatic systems.
The Small Xingkai Wetland Lake is part of larger Xingkai Lake National Nature Reserve which is the biggest freshwater lake in Northeast Asia. The reserve was designated as a RAMSAR site in 2001 and in 2007 it was recognized by UNESCO as a Man and Biosphere reserve. Small Xingkai Wetland Lake provides protection to rare Lake Forest wetlands and it is endowed with rich biodiversity of both flora and fauna. In addition, the lake is an important hub for agricultural products, fisheries and water resources for the local people (Haiqian Citation2011). As a result of economic and human population growth, Small Xingkai Wetland Lake is experiencing immense pressure, such as reclamation of its wetland into agricultural farms, loss of biodiversity and pollution (Xiangcan and Pingyang Citation2006). The water quality of the lake has deteriorated dramatically with turbidity and eutrophication steadily raising (Kang et al. Citation2009).
The impacts of water quality change in aquatic systems can be mirrored in disruption of organisms such as zooplankton (Špoljar et al. Citation2011). Due to their functional feeding behavior, zooplankton species are regarded as good indicators of water quality. However, studies of zooplankton species have been given less attention in Small Xingkai Wetland Lake despite the fact that they form an important component of aquatic food webs. The objectives of this study are: (1) to identify and classify zooplankton of Small Xingkai Wetland Lake into functional groups and (2) to highlight the seasonal dynamics of zooplankton functional groups and their relationship to physico-chemical factors. We hypothesized that seasonal changes in zooplankton functional groups could be explained by physico-chemical factors. The findings of this present study will help fill the lacunae in understanding ecological community structure of the highly turbid, nutrient-rich Small Xingkai Wetland Lake which represents a RAMSAR protected area with few studies conducted so far (Kang et al. Citation2009; Wang et al. Citation2013). Also this is the first study on zooplankton functional groups in the Lake.
Materials and methods
Study area
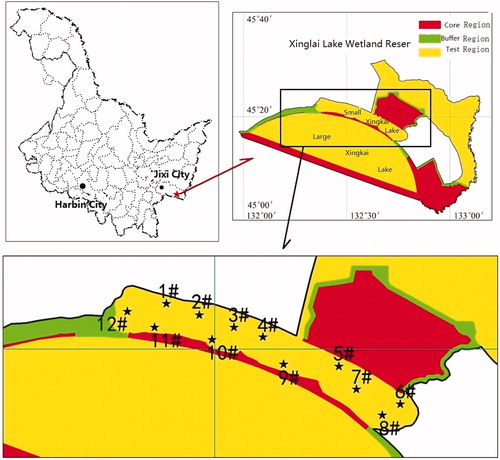
The study was conducted in Small Xingkai Wetland Lake located at 45°N 132 °W in Heilongjiang Province, Northeast China (). The Small Xingkai Wetland Lake is separated from the Large Xingkai Lake by a natural sand mound which is 35 km long, 5–6 m above the mean lake level with trees growing on it. The total area of Small Xingkai Wetland Lake is 140 km2 with an average water depth of 3 m and maximum water storage 5.05 × 108 m3 (Haiqian Citation2011). Flood-diversion sluices were constructed between Small Xingkai Wetland Lake and Large Xingkai Lake to allow the flow of Muling River into the larger lake. The main sources of water to the Small Xingkai Wetland Lake are river discharge, diversion from canals and direct precipitation. The study area is a typical monsoonal climate region with a mean annual precipitation and temperature of 750 mm and 3.1 °C, respectively (Wang et al. Citation2006; Haiqian Citation2011). The lake is the main source of livelihoods for the local communities and its catchment is used for agricultural activities. Small Xingkai Wetland Lake is also important as habitat for flora and fauna including rare and endangered plants species, birds such as white-neped crane (Grus vipio) and Red-crowned cranes (Grus japonensis) and freshwater species of fish (Xiangcan Citation1999; Xiangcan and Pingyang Citation2006; Haiqian Citation2011). Twelve sites (1#–12#) were selected for this study and sampled during spring, summer, autumn and winter. Sites 1#, 2#, 3#, 4# and 6# are located on the northern side of the lake near rivers. In the northern part of the lake there is Jixi city, human settlements and farming activities. Sites 7# and 12# are located at the center of the lake while 8#, 9#, 10# and 11# are on the southern side of the lake. Tourism is the main activity on the southern part of the Small Xingkai Wetland Lake.
Sampling collection and laboratory analysis
Water temperature (TEP), conductivity (COND), ammonium (NH4+) and chloride ions (Cl−) were measured in situ using a portable multi-probe (YSI 6600, YSI Inc., USA) while water transparency (SD) and turbidity (TURBID) were determined using Secchi disk and turbidimeter, respectively. Triplicate water samples for nutrients analysis were collected on a monthly basis, placed in ice box and transported to laboratory for analysis. Total phosphorus (TP), total nitrogen (TN), chemical oxygen demand (CODcr), biological oxygen demand (BOD5), ferrous ion (Fe2+) and ferric ion (Fe3+) were determined using the standard methods for China (MEP Citation2002). For the zooplankton samples, 20 L of lake water from water surface and 0.5 m above the bottom were collected using a Schindler sampler and filtered through plankton net (64 µm mesh size). The concentration samples were then fixed using formaldehyde solution (4% final concentration). Protozoa and rotifer samples were obtained by taking 1-L subsamples to form the 20-L pooled sample. The samples were preserved with Lugol's iodine and formaldehyde and allowed to sediment in 1-L jar for more than 48 hours. The supernatant water was carefully removed and the residue was then collected and made to a known volume of 30 mL according to Huang (Citation1981). Zooplankton specimen identification and counting was carried using a light microscope according to specialized species keys (Yeatman Citation1959; Chen et al. Citation1974; Chiang and Du Citation1979; Kotov et al. Citation2013). Dry weight (mg) obtained from length–weight relationship was used to compute biomass by dividing by the volume of water (L) filtered (Zuo et al. Citation2003; Sun et al. Citation2010).
Description and classification of zooplankton functional group
According to the researchers, zooplankton functional traits are the morphological, behavioral or phonological characteristics that shape its ecological role and fitness in their living environment (Sun et al. Citation2010; Benedetti et al. Citation2015; Shi et al. Citation2015). Reproduction, mode of feeding, trophic level and interaction among organisms have been used to categorize zooplankton (Shi et al. Citation2015), however, size has been considered as the basic principle for classification of zooplankton functional group. The sampled zooplankton in the Small Xingkai Wetland Lake were classified into six functional groups: protozoa filter feeders (PF), rotifer filter feeders (RF), small copepods and cladocerans filter feeders (SCF), middle copepods and cladocerans filter feeders (MCF), middle copepods and cladocerans carnivore (MCC) and large copepods and cladocerans carnivore (LCC). The PF and RF are passive filter feeders feeding on bacteria, algae and organic detritus. SCF group included those species with body size <0.7 mm and were mainly dominated by small copepods and cladocerans (Microcyclops javanus and Bosmina coregoni). The MCF group consists of zooplankton individuals with body length in the range of 0.7–1.5 mm and was mainly dominated by Moina chankensis, and Epischura chankensis species. The MCF group plays a key role in aquatic food web of being a source of food for fish and also they filter feed algae hence controlling algal blooms. Similarly, the body length of MCC functional group ranges between 0.7–1.5 mm just like MCF, however, MCCs are carnivores feeding on other zooplankton. By feeding on other zooplankton, MCF competes with fish for food resources in the lake. Species in this group included Moina chankensis, Epischura chankensis and Diaphanosoma chankensis. The LCC group included those zooplankton species with body length >1.5 mm and were dominated by Cyclops strenuous. This group preys on other zooplankton and it forms an important food source for fish (). Previous studies have shown that biomass is an important factor when modeling and evaluating spatial and seasonal variation of zooplankton functional groups (Pitois and Fox Citation2006; Shi et al. Citation2015). For this study, biomass computed in mg/L was used in the analysis.
Table 1. Description and classification of zooplankton functional group of the Small Xingkai freshwater lake.
Data analysis
To determine the seasonal variation of physico-chemical variables, one-way ANOVA with SPSS version 15.0 statistical package for windows was used. A multivariate of ordination method was performed (software CANOCO 4.5) to identify the effect of physico-chemical variables on the biomass of zooplankton functional group. Detrended correspondence analysis (DCA) was used to see whether the species data was linear or unimodal prior to employing redundancy analysis (RDA). From the DCA results, the maximum gradient length of the axis did not exceed three standard deviations hence prompting the use of RDA. All continuous environmental variables were log10 transformed. Zooplankton functional groups biomass was log(1 + x) transformed before analysis in order to normalize the data. During the RDA analysis, Monte Carlo simulation was employed to test the significance of physico-chemical variable in explaining the zooplankton functional groups under unrestricted model of 999 permutations.
Results
Seasonal variation of physico-chemical factors
The mean values of physico-chemical variables obtained in Small Xingkai Wetland Lake are presented in . Mean water temperature (TEP) differed significantly seasonally (F(3, 44) = 1932, p < 0.001) with higher significant values in summer than in winter (p < 0.001), autumn (p < 0.001) and spring (p < 0.001). Water transparency (SD) values also varied seasonally (F(3, 44) = 9.78 p < 0.001) with spring recording the highest values. Mean Conductivity (COND) in the Small Xingkai Wetland Lake were similar in all seasons (F(3, 44) = 0.53 p = 0.67). The turbidity (TURBID) of the lake was very high as shown in , however, its mean values did not vary seasonally but relative high value was recorded in autumn (99.62 NTU) while spring had the lowest value (63.84 NTU). It is quite clear from the results that the nutrients of the lake were enriched. Conversely no seasonal variations were observed for TN (p = 0.68) and TP (p = 0.20). Values of total organic carbon (TOC), total carbon (TC) and inorganic carbon (IC) showed significant seasonal variation (p < 0.001) with summer having high mean values. Post hoc analysis further indicated that TOC values for autumn differ significantly with spring, summer and winter while IC mean values differed between spring and summer, spring and autumn; and autumn and winter. Ferrous ion (Fe2+) and Fe3+ were significantly high in summer than in spring, autumn and winter (p < 0.001). On the other hand, BOD5 and CODCr were significantly higher in spring and relatively lower in summer.
Table 2. Physico-chemical variables recorded in Small Xingkai Wetland Lake based on season, values are expressed as means ± standard error.
Table 3. Zooplankton species listed according to their functional groups and their percentage contribution to their total biomass in Small Xingkai Wetland Lake.
Seasonal variation of zooplankton community
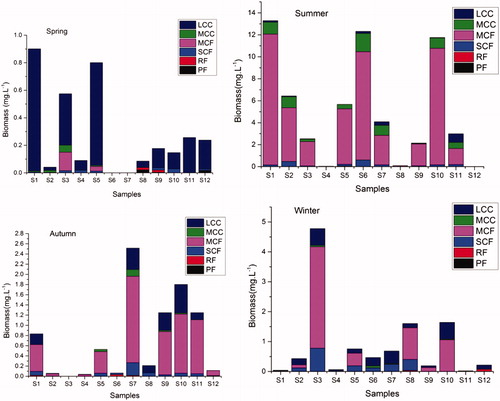
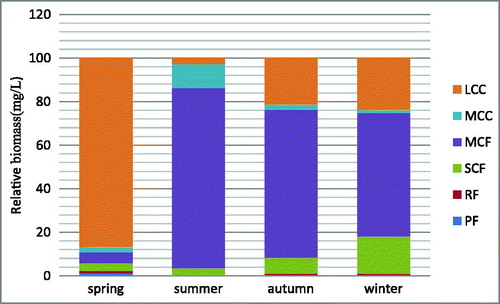
In total, 23 zooplankton species belonging to four taxonomic categories were identified during the study conducted in spring, summer, autumn and winter 2014 in the Small Xingkai Wetland Lake (). Out of this 8 were copepods (34.78%), 6 rotifers (26.09%), 5 protozoa (21.74%) and 4 cladocerans (17.39). Summer recorded the highest number of species 13 followed by spring with 12, autumn and winter had 11 and 9 species, respectively. Zooplankton functional group biomass exhibited seasonal and spatial variability ( and ) and the values were higher in summer at 5.11 mg/L followed by winter, autumn and spring (0.91, 0.72 and 0.28 mg/l, respectively). In spring, approximately 86.67% of the total biomass contribution was of LCC while in summer, autumn and winter MCF functional group had the highest biomass contribution of about 82.71%, 67.93% and 56.83%, respectively (). LCC and SCF were the second and third most contributors of the total biomass in both autumn and winter, respectively. Protozoa filter feeders (PF) and RF were seldom collected during the study period. The relatively highest contribution by PF to the total biomass was in spring of about 1.34% and no PF was collected in summer and winter.
Correlation analysis between functional groups and water environment factors
Correlation coefficient between physico-chemical variables and biomass of zooplankton functional groups were listed in . From the results most of the water environmental factors were correlated with MCF and MCC functional groups. Ferrous ion (Fe2+), TP and TEP significantly influenced MCF and MCC groups positively while SD, Cl− and BOD5 had a negative influence. Turbidity showed a weak influence on MCF and MCC functional group. On the other hand, PF group was very sensitive to turbidity and SD but weakly influenced by TP, CODCr and NH4+. LCC which formed the largest proportion of the total biomass in spring and RF group showed no sensitivity with the environmental factors. Similarly, correlation analysis between zooplankton functional groups revealed that SCF, MCF and MCC were significantly positive correlation to each other while PF, RF and LCC groups showed no correlation with other functional groups ().
Table 4. Correlation coefficients between physico-chemical variables and the biomass of zooplankton functional groups.
Table 5. Correlation coefficients between the biomass of zooplankton functional groups (n = 45).
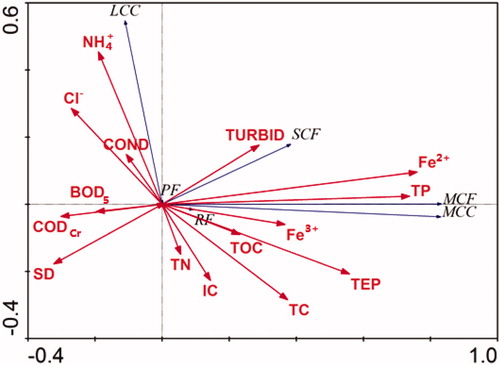
RDA analysis of zooplankton functional group with physico-chemical variables
RDA was carried out to determine the relationship between zooplankton functional groups and environmental factors. The Monte Carlo test was significant for the first axis and all canonical axes (p < 0.001), suggesting that these environmental variables are important factors in explaining the group compositions. The eigenvalue for RDA axis 1 (0.687) and axis 2 (0.002) together explained 68.9% of the species variance out of which 68.7% of the total variability was of axis 1 (). Axis 1 was positively related with Fe2+ (0.760), TP (0.738) and TEM (0.558) while axis 2 was positively related with NH4+-N (0.454) and Cl− (0.286) (). SCF, MCF and MCC groups were positively related with Fe2+, TP, TEM and TC, and negatively related with Cl− and NH4+-N. On the contrary, LCC group was negatively related with Fe2+, TP, TEM and TC and positively related with Cl− and NH4+-N. All the zooplankton functional groups were positively and negatively related with SD and TURBID, respectively.
Table 6. Redundancy analysis results for zooplankton functional groups.
Discussion
Classification of organisms into their functional groups has been proposed to be the best way of understanding the linkage of community structure in aquatic systems (Hébert et al. Citation2016). Previous studies have demonstrated over time that zooplankton functional groups can be used in biogeochemical models in marine ecosystems (Sun et al. Citation2010). And as a result, scientists have classified zooplankton into functional groups based on the specific question to be addressed. Unfortunately, there are no existing studies on zooplankton in order to compare with in the highly turbid and nutrient-rich Small Xingkai Wetland Lake. Through the mode of feeding and size determination, the zooplankton were grouped into six functional groups PF, RF, SCF, MCF, MCC and LCC as indicated in our methods. Although the identification and characterization of the zooplankton functional groups can address the question at hand, there is need to consider biotic interactions, reproduction and biogeochemical roles in the future.
Seasonal variation of zooplankton functional groups biomass
Spatial and temporal variation in zooplankton biomass, species composition and diversity have been well studied in aquatic ecosystems (Sun et al. Citation2010; Benedetti et al. Citation2017; Dube et al. Citation2017) with little attention in highly turbid and nutrient-rich wetlands (Dorak and Temel Citation2015). Compared to oligotrophic systems, zooplankton biomass has been found to be higher in turbid and nutrients-rich ecosystems probably because of the absence of submerged plants, poor visibility of predators or availability of food (Muylaert et al. Citation2003; Špoljar et al. Citation2011; Lu et al. Citation2012). In the present study, zooplankton functional groups biomass showed seasonal variation. In spring, LCC was the dominant zooplankton functional group contributing about 86.67% of the total biomass while in summer MCF was the dominant group composed mainly by Moina chankensis and Epischura chankensis species. LCCs are carnivores feeding on other zooplankters while MCF groups are filter-feeders feeding on bacteria, algae, organic detritus and protozoa. The change in group dominance of LCC in spring to MCF in summer could be due to optimum conditions created by summer temperature and nutrients which support the growth of food resource in the form of algae. Previous studies have indicated that in the presence of algal bloom, zooplankton composition changes regularly with large copepods and cladocerans being replaced by other zooplankton who can filter feed small particles (Blancher Citation1984; Srifa Citation2010). On the other hand, low biomass contribution by the other functional groups in spring can be explained by the relative high water transparency (SD), enabling LCC to visualize its prey (i.e. smaller zooplankton). Protozoa and rotifers, which were main contributors of PF and RF functional groups, showed no obvious variations with seasons and their contribution to the total biomass was very minimal. This observation can be explained by the following arguments; first, RF and PF are filter feeders just like MCF feeding on algae, organic detritus, nano-flagellates and therefore they are depressed through exploitative competition. Second, RFs are preyed upon by LCC and MCC in agreement with previous studies which revealed some cyclopoid copepods are good predators on rotifers hence constraining their population (Brandl Citation2005; Wang et al. Citation2010). The last possible reason could be environmental factors. Studies have shown that change in physico-chemical factors such as nutrients, pH, turbidity and temperature can affect rotifer community structure (Armengol et al. Citation1998; Duggan et al. Citation2002; Wen et al. Citation2017). Changes in rotifer community structure could in turn lead to wavering of foodwebs and stability of the wetland lake.
Zooplankton functional groups driving factors in high turbid and nutrient-rich wetland
Researchers have noted that water temperature, nutrient, pH, bottom-up effect of phytoplankton, top-down effect of predators, turbidity, hydrology and species interaction are essential factors in shaping zooplankton community composition (Srifa Citation2010, Shi et al. Citation2015, Sharma et al. Citation2017). Similarly, our analysis also showed that zooplankton functional groups were influenced by environmental factors that vary with season. Being a highly turbid lake, the authors hypothesized that turbidity could play a crucial role in influencing the biomass of zooplankton functional groups. Although turbidity was found to be higher in the lake, our results did not indicate turbidity as the main driving factor of zooplankton functional group biomass. Our results revealed that ferrous ion (Fe2+) and total phosphorus (TP) were the main factor responsible for zooplankton functional groups biomass dynamics. Nutrients can indirectly impact the biomass of zooplankton functional groups through their influence on phytoplankton productivity. Iron (Fe) which is not the common nutrient specie has been demonstrated as important nutrient affecting the growth of phytoplankton in marine ecosystem (Alderkamp et al. Citation2015). However, its impact on freshwater wetlands is still unclear but some scientists suspect that it might increase the biomass of phytoplankton (Zou et al. Citation2011). Since most of the functional groups were positively correlated with physico-chemical factors indicative of nutrient status in our study, then we deduce that bottom-up controlled by nutrients could be the driving force influencing zooplankton function groups.
Moreover, our analysis has shown that temperature is still a main factor influencing zooplankton in the highly turbid and nutrient-rich wetland. Water temperature is a factor that can positively or negatively directly affect the growth of some zooplankton species (Srifa Citation2010). A study by Kagalou et al. (Citation2010) shows that copepods and cladocerans are affected by water temperature and nutrient enrichments in wetlands. This is reflected in our study during summer where the biomass contribution by Moina chankensis, Diaphanosoma Chankensis and Epischura chankensis species was higher and gradually reduced in autumn and winter. Probably the higher temperature and nutrients recorded in summer attributed to this finding. Note, however, we should be cautious in agreeing with these findings since the physico-chemical variables were measured on monthly basis.
Water transparence (SD) and chloride ion (Cl−) were negatively correlated with MCF and MCC groups while turbidity (TURBID) was positively correlated with most of the groups. Turbidity is an essential factor that influences predator–prey relationship in aquatic system (Van der Gucht et al. Citation2003). Reid et al. (Citation1999) demonstrated that turbidity may reduce prey detection distance and hence predation rate by predators such as fish. Therefore, the positive correlation revealed in this study could imply, but does not prove, that turbidity may lead to low predation pressure on zooplankton (top-down effect) in the highly turbid and nutrient-rich wetland lake. Pearson correlation further revealed a positive relationship between SCF, MCF and MCC groups while PF, RF and LCC showed no relationship with any group. This further shows that zooplankton functional group species in the Small Xingkai Wetland Lake are not influenced by predation or competition but by the availability of food resources (bottom-up effect of phytoplankton). As depicted by RDA results the first two axes explained 68.9% of zooplankton functional groups changes meaning that environmental factors are critical in shaping zooplankton composition in Small Xingkai Wetland Lake.
Conclusion
In this study, the concept of functional traits was used to classify zooplankton into functional groups and their seasonal dynamics in relation with physico-chemical factors were determined in the highly turbid and nutrient-rich wetland. The major findings of our study can be summarized as follows:
| (1) | A total of 23 zooplankton species belonging to four taxonomic categories were identified and classified into six functional groups: protozoa filter feeders (PF), rotifer filter feeders (RF), small copepods and cladocerans filter feeders (SCF), middle copepods and cladocerans filter feeders (MCF), middle copepods and cladocerans carnivore (MCC) and large copepods and cladocerans carnivore (LCC). | ||||
| (2) | Both physico-chemical variables and zooplankton functional groups in the highly turbid nutrient-rich wetland lake vary seasonally. Water temperature (TEP), total phosphorus (TP), ferrous ion (Fe2+) and total carbon (TC) were significant higher in summer than in any other season. | ||||
| (3) | Zooplankton functional group biomass was higher in summer by 5.11 mg/L followed by winter, autumn and spring (0.91, 0.72 and 0.28 mg/L, respectively). Large copepods and cladocerans carnivore (LCC) was the dominant functional group in spring accounting for about 86.67% of the total biomass associated with water transparent (SD). Biomass contribution by the other group was very minimal during spring probably because of the predation by LCC. In summer, middle copepods and cladocerans filter feeders (MCF) was dominant group probably because of the optimal temperature and availability of the nutrients. | ||||
| (4) | Fe2+, TP and TEP are the major factors influencing zooplankton functional groups in the Small Xingkai Wetland Lake. | ||||
Notes on contributors
Chengxue Ma is the teacher of Northeast Forestry University, China. He is majoring in Hydrobiology. Skills and Expertise: water quality, aquatic ecology, freshwater ecology, aquatic science, wetland science, freshwater biology.
Patteson Chula Mwagona is currently a PhD student at Northeast Forestry University, China. He is majoring in Hydrobiology. Skills and Expertise: water quality, aquatic ecology, freshwater ecology, aquatic science, benthic ecology, aquatic macroinvertebrates, freshwater biology.
Hongxian Yu is a professor of Northeast Forestry University, China. She is majoring in Hydrobiology and Wetland Science. Skills and Expertise: water quality, aquatic ecology, freshwater ecology, wetland ecology.
Xiaowen Sun is a boffin of Heilongjiang River Fisheries Research Institute, China. He is majoring in aquatic agriculture. Skills and Expertise: gene map.
Liqun Liang is a boffin of Heilongjiang River Fisheries Research Institute, China. He is majoring in aquatic agriculture. Skills and Expertise: mRNA.
Shahid Mahboob is a professor of King Saud University. He is majoring in water environment. Skills and Expertise: heavy metals, fish cholinesterases, collagen, pesticide.
Acknowledgments
We would like to thank Liu Hua-jin for his tireless support during field work and our families for their moral support. The authors would like to express their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. 1436–11.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alderkamp A-C,. van Dijken GL, Lowry KE, Connelly TL, Lagerström M, Sherrell RM, Haskins C, Rogalsky E, Schofield O, Stammerjohn SE. 2015. Fe availability drives phytoplankton photosynthesis rates during spring bloom in the Amundsen Sea Polynya, Antarctica. Elem Sci Anth. 3: 43.
- Armengol X, Esparcia A, Miracle M. 1998. Rotifer vertical distribution in a strongly stratified lake: a multivariate analysis. Hydrobiologia. 387:161–170.
- Benedetti F, Gasparini S, Ayata S-D. 2015. Identifying copepod functional groups from species functional traits. J Plankton Res. 38(1):159–166.
- Benedetti F, Guilhaumon F, Adloff F, Ayata SD. 2017. Investigating uncertainties in zooplankton composition shifts under climate change scenarios in the Mediterranean Sea. Ecography. doi:10.1111/ecog.02434
- Blancher EC. 1984. Zooplankton-trophic state relationships in some north and central Florida lakes. Hydrobiologia. 109(3):251–263.
- Brandl Z. 2005. Freshwater copepods and rotifers: predators and their prey. In: Herzig A, Gulati RD, Jersabek CD, May L, editors. Rotifera X. Developments in Hydrobiology. 181:475–489.
- Chen Q, Zhang S, Zhu C. 1974. On planktonic copepods of the yellow Sea and the East China Sea. II. Cyclopoida and Harpacticoida. Studia Marina Sinica. 9(2776):24.
- Chiang S-c, Du N. 1979. Fauna sinica; crustacea: freshwater cladocera. Peking, China: Science Press.
- Declerck S, Vanderstukken M, Pals A, Muylaert K, Meester LD. 2007. Plankton biodiversity along a gradient of productivity and its mediation by macrophytes. Ecology. 88(9):2199–2210.
- Dorak Z, Temel M. 2015. The zooplankton community and its relationship with environmental variables in a highly polluted system. Turkey: Golden Horn. J Aquacult Eng Fish Res. 1(2):57–71.
- Dube T, DeNecker L, Van Vuren JH, Wepener V, Smit NJ, Brendonck L. 2017. Spatial and temporal variation of invertebrate community structure in flood-controlled tropical floodplain wetlands. J Freshw Ecol. 32(1):1–15.
- Duggan IC, Green JD, Shiel RJ. 2002. Distribution of rotifer assemblages in North Island, New Zealand, lakes: relationships to environmental and historical factors. Freshw Biol. 47(2):195–206.
- Haiqian L. 2011. Sustainable economic development model based on stakeholders’ collaboration: comparative study between the Heilongjiang Xingkai Lake Biosphere Reserve and Beijing Miyun water source reserve. UNESCO Man and Biosphere Young Scientist Award Final Report.
- Hébert MP, Beisner BE, Maranger R. 2016. A meta‐analysis of zooplankton functional traits influencing ecosystem function. Ecology. 97(4):1069–1080.
- Huang X. 1981. Application of the simplified method of weight determination to various species of planktonic rotifers in Lake Donghu, Wuhan. Acta Hydrobiologia Sin. 7(3):409–416.
- Huszar VL, Nabout JC, Appel MO, Santos JB, Abe DS, Silva LH. 2015. Environmental and not spatial processes (directional and non-directional) shape the phytoplankton composition and functional groups in a large subtropical river basin. J Plankton Res. 37(6):1190–1200.
- Kagalou II, Kosiori A, Leonardos ID. 2010. Assessing the zooplankton community and environmental factors in a Mediterranean wetland. Environ Monit Assess. 170(1):445–455.
- Kang S, Peng X, Zhang L, Liu M, Zhang Y. 2009. The assessment of the present eutrophication status and characteristic analysis of Xingkai Lake. In: Proceedings of the 3rd International Conference on Bioinformatics and Biomedical Engineering. IEEE. p. 1–4.
- Kotov A, Forró L, Korovchinsky N, Petrusek A. 2013. World checklist of freshwater cladocera species. World Wide Web Electronic Publication. http://fada.biodiversity.be/group/show/17.
- Lu M, Liu S-t, Guo Z. 2012. Study on turbidity purification effects of combination of constructed wetland plants on living wastewater. J Shandong Jianzhu Univ. 6:000.
- Ma C, Yu H. 2013. Phytoplankton community structure in reservoirs of different trophic status, Northeast China. Chin J Oceanol Limnol. 31(3):471–481.
- [MEP] Ministry of Environmental Protection, China. 2002. China's national standard: GB3838-2002: Environmental quality standards for surface water. Beijing: Ministry of Environmental Protection.
- Muylaert K, Declerck S, Geenens V, Van Wichelen J, Degans H, Vandekerkhove J, Van der Gucht K, Vloemans N, Rommens W, Rejas D. 2003. Zooplankton, phytoplankton and the microbial food web in two turbid and two clearwater shallow lakes in Belgium. Aquatic Ecol. 37(2):137–150.
- Pitois SG, Fox CJ. 2006. Long-term changes in zooplankton biomass concentration and mean size over the Northwest European shelf inferred from continuous plankton recorder data. ICES J Mar Sci. 63(5):785–798.
- Reid SM, Fox MG, Whillans TH. 1999. Influence of turbidity on piscivory in largemouth bass (Micropterus salmoides). Canadian J Fisheries and Aquatic Sci. 56(8):1362–1369.
- Richardson AJ. 2008. In hot water: zooplankton and climate change. ICES J Mar Sci. 65(3):279–295.
- Ruhl HA, Smith KL. 2004. Shifts in deep-sea community structure linked to climate and food supply. Science. 305(5683):513–515.
- Sharma AS, Gupta S, Singh NR. 2017. Zooplankton community of Keibul Lamjao National Park (KLNP) Manipur, India in relation to the physico-chemical variables of the water. Chin J Oceanol Limnol. 35(3):469–480.
- Shi Y-Q, Sun S, Zhang G-T, Wang S-W, Li C-L. 2015. Distribution pattern of zooplankton functional groups in the Yellow Sea in June: a possible cause for geographical separation of giant jellyfish species. Hydrobiologia. 754(1):43–58.
- Špoljar M, Tomljanović T, Lalić I. 2011. Eutrophication impact on zooplankton community: a shallow lake approach. Holistic Approach Environ. 1(4):131–142.
- Srifa A. 2010. Factors controlling zooplankton dynamics in a subtropical lake during cyanobacterial bloom events [thesis]. Gainesville (FL): University of Florida.
- Sun S, Huo Y, Yang B. 2010. Zooplankton functional groups on the continental shelf of the yellow sea. Deep Sea Res. 57(11):1006–1016.
- Van der Gucht K, Vloemans N, Rommens W, Rejas D. 2003. Zooplankton, phytoplankton and the microbial food web in two turbid and two clearwater shallow lakes in Belgium. Aquatic Ecology. 37(2):137–150.
- Wang G-d, Jiang M, Lu X-g, Wang M. 2013. Effects of sediment load and water depth on the seed banks of three plant communities in the National Natural Wetland Reserve of Lake Xingkai, China. Aquatic Botany. 106:35–41.
- Wang S, Xie P, Geng H. 2010. The relative importance of physicochemical factors and crustacean zooplankton as determinants of rotifer density and species distribution in lakes adjacent to the Yangtze River, China. Limnol Ecol Manage Inland Waters. 40(1):1–7.
- Wang X, Yu S, Liu Z. 2006. Xingkai Lake Reserve in Heilongjiang Province, its main features and effective management. Chin Wildlife. 27(2):29–32.
- Wen X, Zhai P, Feng R, Yang R, Xi Y. 2017. Comparative analysis of the spatio-temporal dynamics of rotifer community structure based on taxonomic indices and functional groups in two subtropical lakes. Scientific Reports. 7(1):578.
- Xiangcan J. 1999. Diagnostic analysis of the lake Xingkai. Beijing, China: Khanka Basin (Chinese Side).
- Xiangcan J, Pingyang J. 2006. Lake Xinkai/Khanka. Experience and lessons learnt brief. World lake database International Lake Environment Committee (ILEC). Kusatsu. p. 447–459.
- Yeatman HC. 1959. Cyclopoida. Freshwater biology. 2nd ed. New York (NY): Wiley; p. 795–815.
- Zou Y-c, Lu X-g, Yu X-f, Jiang M, Guo Y. 2011. Migration and retention of dissolved iron in three mesocosm wetlands. Ecol Eng. 37(11):1630–1637.
- Zuo T, Wang K, Li C. 2003. Length-dry weight relationship of Calanus sinicus in the southern part of the Yellow Sea. J Fisheries China. 27(Special Issue):103–107.