Abstract
This study investigated the physiological and ecological processes of Taxodium distichum in the water-level-fluctuating zone (WLFZ) of the Three Gorges Reservoir (TGR). We determined the contents of non-structural carbohydrates (NSCs), N and P in the aboveground parts and root systems of T. distichum individuals that had experienced 3 yearly flooding cycles. These flooding cycles were driven by the operation of the reservoir in the Zhong County vegetation restoration demonstration area. This flooding inhibited the growth of T. distichum. The plant heights and canopy diameters of the flooded groups were significantly smaller than those of the control group. The flooding facilitated the synthesis of NSCs, and the soluble sugar content in the NSCs increased. The flooding inhibited the uptake of N and P, but the N/P ratios remained stable. The ratios of NSCs to N or P increased. T. distichum adapts well to submergence because of its regulation of NSC synthesis and storage, its balancing of the inputs of photosynthetic products to growth and storage, and its storage of material during exposure to maintain physiological activities during winter submergence and the energy sources required for growth during exposure. This study may provide a useful reference for use in vegetation reconstructions in areas with hydrological characteristics similar to those of the TGR.
Introduction
Flooding is a common environmental stress in nature, but relatively little is known about specific species and how they adjust to flood stress. Precipitation, snowmelt, tides and the construction of artificial water storage facilities may all lead to the occurrence of flooding (Liu et al. Citation2013). The operation of artificial reservoirs may cause seasonal and temporal fluctuations in the water levels of rivers and lakes. The area between the highest and lowest water levels in an impoundment is called the water-level-fluctuating zone (WLFZ) (Lü et al. Citation2015). Plants that grow in this area are often exposed to either long- or short-term flooding stress. This flooding stress may change the environment relative to that the plants became established in, greatly impacting the growth and physiology of these plants (Liu et al. Citation2014).
The Three Gorges Dam is the largest artificial water storage project in the upper reaches of the Yangtze River, and it plays important roles in flood control, power generation and navigation (Zhu et al. Citation2015). According to the operating mode of the Three Gorges Reservoir (TGR), the maximum water level reaches 175 m ASL in winter, and the lowest water level in summer is 145 m ASL; the difference in these levels produces the WLFZ of the reservoir (New and Xie Citation2008). This fluctuation pattern is contrary to the natural hydrological rhythm that existed before the construction of the reservoir; in this rhythm, the highest water levels occurred in summer, and the lowest water levels occurred in winter (Liu et al. Citation2013). A WLFZ with an area of approximately 400 km2 and a maximum elevation difference of 30 m has formed in the TGR, and this WLFZ is characterized by long flooding durations, large flooding depths and out-of-season flooding (Fan et al. Citation2015). Plants sensitive to flooding have been unable to adapt to the complex flooding environment created by the construction of the dam and are gradually dying out. As a result, the biodiversity in the WLFZ has decreased, leading to deterioration of the ecological environment and the degeneration of ecological functions (Wu et al. Citation2004; Jie et al. Citation2012).
In recent years, the impact of the Three Gorges Project on the ecological environment of the reservoir area has received continuous attention (Lü et al. Citation2015). Compared with engineering solutions, biological solutions such as the construction of artificial vegetation are suitable for addressing serious ecological issues such as soil erosion (Wang et al. Citation2009) and improving the ecological quality and ecosystem services in the WLFZ of the TGR (Gomez-Aparicio et al. Citation2004; Fan et al. Citation2015). In particular, the exploration of the physiological and ecological mechanisms of flood tolerance of suitable plants is crucial for conducting vegetation restoration in the WLFZ and is a prerequisite to solving the problems related to the ecological environment of the WLFZ of the reservoir. Previous studies have carried out many evaluations of flood tolerance. For example, these studies have assessed the characteristics of photosynthetic physiology and nutrient elements under submergence (Liu et al. Citation2014; Wang et al. Citation2016), the formation of aerenchyma and adventitious roots under flooding (Thomson et al. Citation1990; Bailey-Serres and Voesenek Citation2008), and the distribution of non-structural carbohydrates (NSCs), which serve as an energy supply (Ye and Zeng Citation2013) and assist in hormonal regulation (Perata and Voesenek Citation2007). Some flood-tolerant species have been assessed in pot experiments under different soil moisture conditions. However, only a few plants survived after they were applied in vegetation recovery in the WLFZ, which indicated the appearance of new problems and challenges related to vegetation recovery in this zone.
Taxodium distichum, which is native to North America, is a deciduous conifer in the family Cupressaceae and has been widely introduced in the rest of the world. Because of its rapid growth and strong adaptability, T. distichum has been selected as a suitable species for vegetation restoration in the WLFZ (Li et al. Citation2005; Li and Zhong Citation2007) and is widely used in vegetation restoration in this zone. Some researchers have studied the responses of the photosynthesis (Pezeshki and Santos Citation1998; Elcan and Pezeshki Citation2002; Vann and Megonigal Citation2002; Stiller Citation2009), growth (Conner et al. Citation1997; Pezeshki et al. Citation1999), and secondary metabolism (Li et al. Citation2010) of this plant to water stress, but most of these studies were one-time, short-term, flooding simulation experiments, and these characteristics differ from the actual hydrological rhythm of the WLFZ in the TGR. Thus, these studies do not accurately reflect the long-term adaptability of this species. Researchers are currently focusing on in situ studies. Recent studies have shown that flooding has a significant effect on the nutrient uptake of T. distichum in the reconstructed vegetation system in the WLFZ of the TGR; specifically, the uptake of N, P, K and other elements closely related to growth is inhibited (Ma et al. Citation2017), and photosynthesis and growth are inhibited by submergence (Wang et al. Citation2016). The results of previous studies on the waterlogging tolerance of plants have shown (Jackson et al. Citation2009; Bailey-Serres and Colmer Citation2014; Voesenek et al. Citation2016) that flood-tolerant species typically employ either an ‘escape’ strategy (a long-term strategy used to tolerate shallow submergence by consuming carbohydrate reserves and increasing stem elongation to raise some stems and leaves above the water surface) or a ‘tolerance’ strategy (a strategy used to survive short-term flooding by reducing the metabolic rate and slowing carbohydrate consumption) to cope with flood stress. The autumn and winter seasons are the dormant period for T. distichum, during which plant growth slows and leaf litter falls. However, these seasons also represent the period in which additional water is stored in the TGR, producing flooding of long duration and having a great submergence depth. Therefore, it is difficult for T. distichum to escape the flood stress in the reservoir region through rapid growth. This plant tends to adopt the ‘tolerance’ strategy (Iwanaga et al. Citation2015) during the long submergence period of the WLFZ, thus placing greater demands on the material reserves of the plants (Ye and Zeng Citation2013).
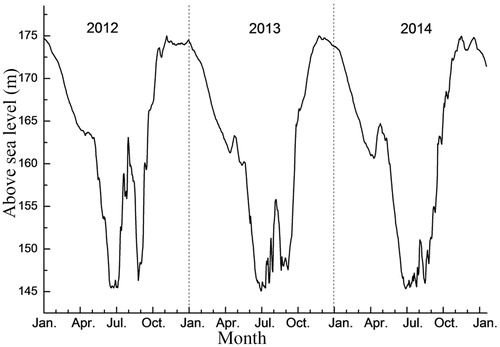
During the flooding cycle, the water level rises gradually from September to October. The water level is maintained at its maximum level of 175 m ASL from November through January of the next year; it then gradually decreases and reaches its lowest level of 145 m ASL in April. The water level is then maintained at this level from June to September. This process is considered to represent one flooding cycle. Under flooding conditions, photosynthesis is inhibited in plants, resulting in a decrease in the source of NSCs. NSCs, including soluble sugar and starch, are important energy sources that drive plant growth and metabolism and metabolic processes (Panda and Sarkar Citation2014). Sugar is the main form of carbohydrates transported in plants, and starch is an important stored form of plant carbon. However, plant self-respiration consumes energy, and plants require the antioxidase system to clear out reactive oxygen species during flooding periods, thus consuming energy (Juan et al. Citation2012). Furthermore, perennials need to germinate new leaves during periods of exposure, which also consumes energy (Chen et al. Citation2013). Therefore, sufficient NSC reserves are key in tolerating flooding. The concentrations of NSCs in plants greatly influence plant growth and ecological responses to environmental changes (Myers and Kitajima Citation2007; Poorter and Kitajima Citation2007). NSCs are the products of plant photosynthesis. All of the physiological and ecological factors that affect photosynthesis also affect the levels of NSCs in plants. Research has shown that the N concentration in leaves is positively correlated with the capacity of leaves to fix and accumulate NSCs (Millard et al. Citation2007). The photosynthetic rate of plant leaves increases significantly with increasing leaf N concentration, and the ability to fix and assimilate CO2 and to produce NSCs is enhanced accordingly (McGroddy et al. Citation2008). Phosphorus is a key element in plant metabolism and energy and protein synthesis; therefore, the photosynthetic capacity of plants and the synthesis of NSCs are affected by the concentrations of both P and N (Reich and Oleksyn Citation2004). The unique hydrological rhythm in the TGR has caused the plants there to become waterlogged during the dormant period. At present, the response of the NSCs, N and P in T. distichum to flooding during the dormant period is unclear and needs to be researched.
The main aims of this study were to determine the growth adaptability of T. distichum after experiencing 3 yearly flooding cycles in the WLFZ; to investigate the contents of NSCs, N and P and their proportions; to understand the physiological and ecological adaptation mechanisms of T. distichum in the WLFZ; and to assess whether this species can adapt to repeated long-term, out-of-season flooding using its material reserves.
Materials and methods
Research region and materials
The research region (107°32′–108°14′ E, 30°03′–30°35′ N; ) is located along the Ruxi River, Shibaozhai, Gonghe Village, Zhong County, Chongqing Municipality, China. The Ruxi River is a first-order tributary of the Yangtze River, and the study area experiences a subtropical, southeastern monsoonal, mountain climate. This region experiences a yearly average temperature of 18.2 °C, an annual cumulative temperature of 5787 °C for days ≥10 °C, an annual frost-free period of 341 days, an annual sunshine duration of 1327.5 h, an annual sunshine ratio of 29%, a yearly total radiation energy of 83.7 × 4.18 kJ/cm2, an annual precipitation of 1200 mm, and a an annual mean relative humidity of 80%. The region experiences high temperatures and heavy rain in summer (which extends approximately from May to late September) and mild temperatures and brief rain in winter (which extends approximately from November to February). The substrate is purple, and the parent material is the typical subtropical purple sandstone of China; this material displays a low degree of rock weathering and a shallow soil layer. Soil erosion is serious where vegetation cover is limited in the Ruxi River Basin.
To conduct vegetation recovery research, our project team built a vegetation restoration demonstration area in the Ruxi River Basin in Zhong County in March 2012. Originally, the land was a patch of abandoned terraces with a gradient of 26°, a slope aspect to the west, and an area of 0.133 km2. According to the rhythm of water-level fluctuations and the differences in the resistance to flooding of plants, different groups of artificial vegetation were constructed from 175 to 145 m ASL. Specifically, these groups included trees, shrubs, and herbaceous plants; shrubs and herbaceous plants; and herbaceous plants. T. distichum is one of the tree species that was planted in the area. Two-year-old seedlings of T. distichum, which grows rapidly from June to August and enters a period of relative dormancy in winter, were purchased from the nursery of the Chongqing Grand Theatre and planted at a line spacing of 1 × 1 m in the section at elevations of 165–175 m ASL in the vegetation recovery demonstration area of the TGR in March 2012.
Experimental design
According to the differences in flooding depth and duration within 1 flooding cycle, the sample plot was divided into three belt transects that correspond to 3 treatments: shallow submergence (SS; 175 m ASL), periodic moderate submergence (MS; 170 m ASL) and deep submergence (DS; 165 m ASL). The maximum flooding depth and number of flooding days in each belt transect are shown in . To minimize the effects of sunlight on the trees in the forest stand, five dominant trees were randomly selected in the three belt transects, and the roots, branches and leaves were sampled separately. At the same time, 10 trees were selected at random, and their growth status was recorded. The heights of the trees were measured with a height-measuring rod. The crown diameters were measured with a tapeline, and the base diameters were measured with a vernier caliper.
Table 1. Flooding depths and durations of the treatment groups at different elevations during 3 yearly flooding cycles.
Sampling
To explore the mechanisms of sustainable flooding tolerance employed by T. distichum, based on previous studies (Wang et al. Citation2016; He et al. Citation2018), samples of roots, branches and leaves were collected in situ in July 2015 after the trees had suffered three flooding cycles that extended from June to August in 2012, 2013 and 2014. The sampling occurred in the middle of the fourth growing season when the water level was at its lowest point of 145 m ASL in the study region in the WLFZ of the TGR. At the time of sampling, 3 and 4 months had elapsed since the T. distichum individuals at 165 m and 170 m ASL, respectively, had experienced exposure, and the trees were in the growing season at the time of sampling. From the initial stage of planting in March 2012 to the experimental sampling in July 2015, T. distichum experienced three annual cycles of periodic submergence (). A long-reach chainsaw was used to collect branch samples at random from the upper and medium layers of the canopy in four directions: east, west, south, and north. Branches (5–10 mm in diameter) and leaves of normal growth were evenly mixed as branch and leaf samples and sealed in Ziploc bags. Five duplicates were collected at each elevation. The plant bases were taken to represent the centers of circles, and a soil auger with a bore diameter of 100 mm was used to drill the plant root samples at an equidistant radius of 0.5 m; these samples were mixed and sealed in Ziploc bags. Five duplicates were collected for each elevation. The samples were placed in cold storage before being transported to the laboratory and rinsed with tap water and deionized water. All of the samples were placed in an oven. First, water was removed at 105 °C for 5 min, and the samples were then dried at 80 °C to a constant weight. The plant samples (roots, branches and leaves) were pulverized, passed through a 100-mesh sieve, and sealed for measurements of N, P and NSCs.
Figure 2. Water-level changes in the WLFZ of the TGR in Zhong County from January 2012 through January 2015. Source: Wang et al. (Citation2016).
Sample analysis
Measurements of N and P
The plant root, branch and leaf samples were dried, ground into powder, and sifted through a 1-mm sieve. The N contents were determined using a vario EL cube CHNOS elemental analyzer (Elementar, Hanau, Hessen, Germany). The samples were digested in a Speedwave MWS-4 microwave digestion system (Berghof, Eningen, Baden-Wurttemberg, Germany), and the P contents were measured using an iCAP 6000 inductively coupled plasma-optical emission spectrometer (Thermo, Waltham, MA, USA).
Measurement of NSCs
In this study, NSCs are defined as the sum of soluble sugars (glucose, sucrose and fructose) and starch (Hoch et al. Citation2002). The roots, branches and leaves of the plants were dried, ground into powder and sifted through a 1-mm sieve. The soluble sugar and starch contents were determined using the anthrone method (Li et al. Citation2008; Wei et al. Citation2014).
Statistical analysis
Duplicate root, branch and leaf samples were collected from each tree. The means were calculated as the averages of five trees. SPSS 20.0 and Microsoft 2010 software were used for all statistical analyses. General linear model (GLM) procedures were used to determine any significant overall differences among the treatments and plant tissues and the interactions between the treatments and tissues for all of the data. The effects of submergence on the NSC, N and P contents of T. distichum were established using one-way ANOVA. Tukey’s test was used to test for differences among the submergence treatments at the 0.05 level.
Results
Growth status of T. distichum
Taxodium distichum grew well and displayed a survival rate of 100% after it had undergone 3 yearly submergence–exposure cycles in the WLFZ (). Compared with the initial stage of planting, the plant height, basal diameter and canopy diameter had all increased (p < 0.05) by May 2015. After 3 years of growth, the growth indices of T. distichum growing at different elevations, which experienced different flooding depths and durations, were different. Compared with the plants at 175 m ASL, the heights and canopy diameters of the plants growing at an elevation of 170 and 165 m were obviously smaller. However, there was no significant difference in the basal diameters.
Table 2. Growth status of T. distichum.
Responses of the NSC, N and P contents
Contents of NSCs
The effects of the treatments, the plant tissue types and the interactions between the treatments and tissue types on the contents of soluble sugar, starch, soluble sugar/starch and NSCs are summarized in . Compared with the SS group, the contents of soluble sugar, soluble sugar/starch and NSCs increased in the MS and DS groups, and the starch content increased with DS. In particular, the soluble sugar contents under MS and DS and the starch and NSC contents under DS increased compared with the SS group, based on Tukey’s test at the 0.05 level ().
Table 3. Effects of the submergence treatments and the plant tissue types on the contents of NSC, N and P in T. distichum.
Table 4. Effects of submergence on the contents of NSC, N and P in T. distichum.
The soluble sugar contents were highest in the leaves, and the starch contents were highest in the roots (). In general, the roots displayed the highest contents of NSCs, followed by the leaves, which displayed higher contents of NSCs than the branches.
Figure 3. Contents of soluble sugar, starch and NSCs in T. distichum under shallow submergence (SS), moderate submergence (MS) and deep submergence (DS). (A) Soluble sugar contents. (B) Starch contents. (C) NSC contents. Different lowercase letters for the same tissue indicate significant differences among the different water levels at the 0.05 level based on Tukey’s test.
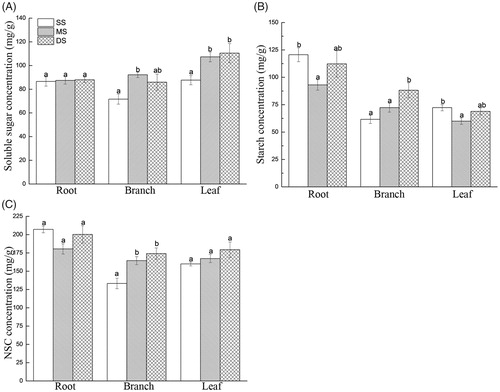
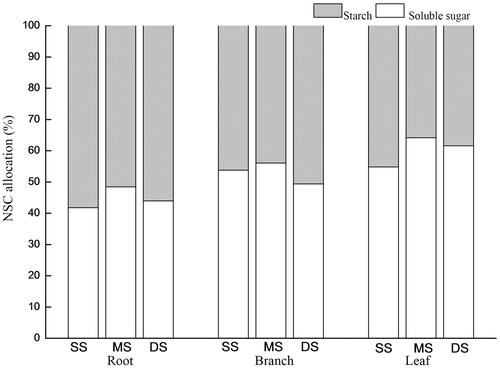
The content of soluble sugar in the roots did not change in response to increases in the flooding depth over time, whereas the soluble sugar contents in the branches and leaves increased (p < 0.05). The soluble sugar contents in the branches and leaves of the MS and DS groups were higher than that of the SS group (p < 0.05). The starch contents in the roots and leaves of the MS and DS groups were lower than that of the SS group, and the contents in the MS and SS groups were different (p < 0.05). The starch contents in the branches increased significantly under DS compared with SS. The contents of NSCs in the roots were similar to the soluble sugar contents, and no significant differences were noted among the submergence treatments. The NSC contents in the branches and leaves gradually increased with increasing flooding depth and time. The proportion of the NSC components in the roots, branches and leaves of T. distichum was altered by flooding (), resulting in increases in the proportion of soluble sugar.
Figure 4. Percentages of soluble sugar and starch in the NSCs in the roots, branches and leaves of T. distichum under shallow submergence (SS), moderate submergence (MS) and deep submergence (DS). Note: Values are the mean ± standard error (n = 5); different lowercase letters represent significant differences among the submergence treatments.
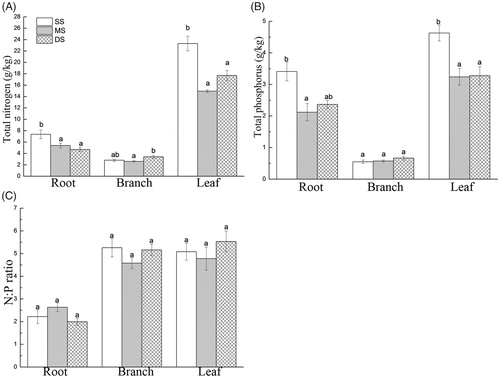
Contents of N and P and N/P ratios
The effects of the submergence treatments, the plant tissue types and the interactions between the treatments and the tissue types on the contents of N and P and the N/P ratio are presented in . The contents of N and P decreased under MS and DS compared with SS (p < 0.05), but the N/P ratio exhibited a non-significant change under submergence ().
As observed in , the contents of N in the roots and leaves of T. distichum decreased with increasing flooding depth and time (p < 0.05). The N contents in the roots and leaves of the MS and DS groups were lower than those of the SS group (p < 0.05). The N content followed the order of leaves > roots > branches. The P contents in the roots and leaves of the MS and DS groups decreased significantly compared to those in the SS group. However, there was no significant change in the P content of the branches. The content of P followed the order of leaves > roots > branches. There was no significant difference between the N/P ratios of the belowground (root) and aboveground (branch and leaf) parts among the submergence treatments, and the N/P ratios of these parts were 2.00–2.63, 4.58–5.26 and 4.78–5.53, respectively.
Figure 5. Contents of N and P and N/P ratios of T. distichum under shallow submergence (SS), moderate submergence (MS) and deep submergence (DS). Note: Values are the mean ± standard error (n = 5); different lowercase letters represent significant differences among the submergence treatments (p < 0.05). A, total nitrogen. B, total phosphorus. C, N/P ratio.
Effects of flooding on the stoichiometry of NSCs, N and P
The soluble sugar/N ratios in the roots, branches and leaves gradually increased significantly with increasing flooding depth and time (except for the branches of the DS group; ). The soluble sugar/N ratios of the MS and DS groups were higher than that of the SS group (p < 0.05). In trees affected by submergence, the starch/N ratios gradually increased in all of the tissue types. The starch/N ratios in the roots of the DS group were higher than those of the SS group (p < 0.05). The starch/N values in the leaves of the MS and DS groups were significantly higher than that of the SS group. The variation in the NSC/N ratios was similar to that of the starch/N ratios. Submergence resulted in increases in the NSC/N ratios of T. distichum.
Figure 6. Relationships of the N, P and NSC component stoichiometry in T. distichum under shallow submergence (SS), moderate submergence (MS) and deep submergence (DS). (A) Soluble sugar/N ratios. (B) Soluble sugar/P ratios. (C) Starch/N ratios. (D) Starch/P ratios. (E) NSC/N ratios. (F) NSC/P ratios. Note: Values are the mean ± standard error (n = 5); different lowercase letters represent significant differences among the submergence treatments (p < 0.05).
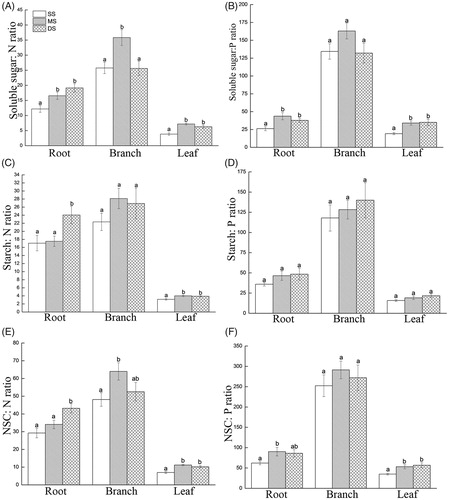
The soluble sugar/P ratios in the roots and leaves increased significantly with increasing flooding depth and time. The sugar/P ratios in the MS and DS groups were higher than those in the SS group (p < 0.05). There was no significant difference in the soluble sugar/P ratios in the branches. The starch/P ratios in the different tissue types reflected a non-significant increase. In the trees affected by submergence, the NSC/P ratios in the roots of the MS and DS groups increased compared with those of the SS group (p < 0.05). The NSC/P ratios in the branches underwent a non-significant increase. However, the NSC/P ratios in the leaves of the MS and DS groups were significantly higher than those of the SS group.
The output of NSC generated from inputs of N and P per unit mass increased. The soluble sugar/N and starch/N ratios increased in the roots, branches and leaves in the flooded groups compared with the control, indicating that the amounts of soluble sugar and starch generated from the input of a unit mass of N were not restricted as the N content decreased. Due to submergence, the soluble sugar/P ratios in the tissue types increased, whereas the starch/P ratio underwent a non-significant change. These observations indicate that the main output from the input of a unit mass of P was soluble sugar. The output of this substance was not increased by a decrease in P absorption, and the increase in the soluble sugar content inhibited starch synthesis (Yu et al. Citation2010). The NSC/N and NSC/P ratios of the flooded groups all increased, indicating that NSC synthesis was not inhibited, even though the absorption of N and P was inhibited. In the trees affected by submergence, the NSC output generated from the input of a unit mass of N and P increased.
Discussion
Environmental problems, such as decreasing biodiversity and the soil erosion caused by changes in water levels in the WLFZs of natural and artificial bodies of water around the world, have become global concerns. Understanding the mechanisms by which flood-tolerant species resist the effects of flooding is helpful in restoring vegetation and improve ecological service functions in WLFZs. Submergence and changes in environmental factors, such as light, temperature, oxygen content and the oxidation–reduction potential of soil, affect plant survival (Pezeshki and DeLaune Citation2012; Pucciariello et al. Citation2014). Due to the challenges and limitations of in situ testing, the primary stress factor, submergence, was considered in this study. Internationally, in-depth research has been conducted on the mechanisms by which plants tolerate flooding during the growing season (Wu et al. Citation2012; Pedersen et al. Citation2013; Song et al. Citation2013; Bailey-Serres and Colmer Citation2014; Pucciariello et al. Citation2014). However, little research has investigated the mechanisms by which plants tolerate submergence during the dormant period. Submergence stress in the WLFZ of the TGR usually occurs in autumn and winter, which correspond to the dormant period of T. distichum. In this context, the active response of T. distichum to submergence may relate to the ‘tolerance’ strategy in winter, corresponding to previous studies (Iwanaga et al. Citation2015), and the storage of material during exposure.
Carbohydrates are the main products of plant photosynthesis, and they play a very important role in maintaining normal physiological activity within plants (Li et al. Citation2008; Wu et al. Citation2010). In plants, carbohydrates are usually stored in the form of structural carbohydrates (SCs) that are mainly used to support the construction of the morphological elements of plants (Jiang et al. Citation2012); NSCs act as an important energy source in metabolism (Pan et al. Citation2002). Studies (Das et al. Citation2005; Tan et al. Citation2009) have shown that the flood tolerance of a plant and its later recovery and growth may be highly correlated with the levels of carbohydrate storage in the plant itself. NSCs are important substances that are involved in plant life processes (Pan et al. Citation2002; Yu et al. Citation2011). The NSC contents in plants generally reflect the overall supply of carbon to plants; moreover, they represent the growth status of plants, their ability to buffer the effects of external interference and stress, and their adaptation strategies (Wurth et al. Citation2005; Myers and Kitajima Citation2007). In the present study, the soluble sugar contents of the flooded groups increased compared with that of the SS group (p < 0.05; ), and the soluble sugar contents in the branches and leaves increased significantly after undergoing three annual submergence–exposure cycles (), which further indicates that soluble sugar can mobilized at any time to participate in metabolic activities in plants (Barbaroux et al. Citation2003; Chantuma et al. Citation2009). Additionally, the increase in the contents of this material demonstrates that the ability of T. distichum to resist stress increased after long-term adaptation to the environment of the WLFZ. The contents of starch in the roots and leaves decreased in the flooded groups (). However, the overall contents of NSCs remained stable in the roots and increased in the branches and leaves (). The NSC pools in the flooded groups tended to increase (), indicating that T. distichum with larger NSC pools could grow normally during exposure after undergoing submergence during the dormant period. From the perspective of plant growth, growth indices, such as the plant height and canopy diameter, of the flooded groups were lower than those of the SS group (). This result indicates that the growth mechanism was inhibited, and this inhibition was directly correlated with the reduced photosynthetic capacity and the decrease in assimilation products in T. distichum (Wang et al. Citation2016). Previous studies have shown that plants can store a certain amount of NSCs in their bodies to provide energy to resist external environmental stresses and maintain their normal physiological functions, and they can balance the storage of assimilation products and growth inputs to ensure their survival under adverse conditions (Ericsson et al. Citation1996). In agreement with the above results, T. distichum adjusted the proportions of the photosynthetic products allocated to growth and the NSC pool under winter submergence. Under conditions in which photosynthetic products are reduced, priority is given to guaranteeing NSC storage (Chen et al. Citation2013; Liu et al. Citation2015), which may be an important mechanism that T. distichum uses to successfully cope with winter submergence in the WLFZ.
Photosynthesis is closely related to the contents of nutrient elements, especially the contents of N and P in plant leaves. To a certain extent, higher N and P contents in leaves yield higher net photosynthetic rates (Domingues et al. Citation2010; Jin et al. Citation2013). The accumulation of nutrient elements in plants reflects the ability of plants to absorb nutrients under certain environmental conditions; thus, such accumulations can reveal the characteristics of plant species and reflect the relationships between plants and their environments (Jin et al. Citation2013). Under normal circumstances, plants absorb certain percentages of N and P, in accordance with their physiological structures and their requirements for material synthesis (Wu et al. Citation2010). The N and P contents in plants and their proportion can reflect the ability of plants to adapt to environmental conditions. However, in the present study, the contents of N and P decreased due to long-term submergence, and submergence inhibited the absorption of these elements (, ). The results of an early simulation experiment on the uptake of elements by T. distichum indicate that this species is strongly tolerant of flooding, and submergence does not have a significant influence on the uptake of elements by this plant (Pezeshki et al. Citation1999). These findings may result from relatively intensive in situ submergence. Flooding causes changes in the contents and availability of nutrients in the soil and consequently affects the uptake and transport of nutrients by plants (Pezeshki and DeLaune Citation2012; Han et al. Citation2016). However, the N/P ratios did not differ between the flooded groups and the SS group (; ), indicating that, although the absorption of N and P was inhibited, the N/P ratios remained stable and that the stoichiometry of N and P did not deviate from the standard nutrient values. Research has shown that the N/P ratio in leaves is an important ecological indicator (Yu et al. Citation2010): N/P ratios <14 generally indicate N limitation, whereas ratios >16 suggest P limitation. Ratios of 14–16 indicate that plant growth is jointly constrained by N and P. In the results of this study, the N/P ratios were all less than 14, indicating N limitation.
NSCs in plants are an important energy source for metabolism. The NSC, N and P contents in plants and their ratios reflect the material available to support the growth, NSC outputs, and input ratios of N and P of the plants, as well as their efficiency to a large extent. In addition, by means of the ratio of NSCs to N and P, one can understand the impacts of N and P on the contents of NSC components and the dynamic equilibrium in the metabolism of C by plants (Nilsson et al. Citation1997; Li et al. Citation2008). In the present study, the synthesis of soluble sugar and NSCs was not restricted as the N and P content decreased, and the increasing soluble sugar contents restricted the synthesis of starch as the P contents decreased (); these results are consistent with those of a previous study (Guo et al. Citation2015).
In the Zhong County vegetation restoration demonstration area, after 3 years of adaptive growth, T. distichum showed good adaptability to the habitat of the WLFZ and tended to use the limited N and P resources to add photosynthetic products to the NSC pool, thereby reducing the input into assimilation products for use in morphogenesis, consistent with its NSC contents and growth. These results were consistent with previous research showing that T. distichum is a candidate for vegetation in the WLFZ of the TGR (Wang et al. Citation2016).
This study of the characteristics of the contents of NSCs, N and P of T. distichum grown at different elevations reveals that flooding during the dormant period affects the storage of material and the uptake of nutrients by vegetation used for artificial restoration in the WLFZ of the TGR. T. distichum individuals grown at lower elevations increased their storage of NSCs and the soluble sugar content of the NSCs by actively adjusting the distribution of photosynthetic products to address the damage caused by flooding in winter. Flooding inhibited the absorption of N and P. However, the N/P ratios remained stable, reducing the impacts on photosynthetic production and NSC synthesis. The contents of NSCs, N, and P and their stoichiometry changed with increasing flooding depth. The ratios of NSCs to N and P increased, and the NSC output generated from the input of a unit mass of N and P increased.
In conclusion, T. distichum in a system of vegetation planted artificially in the WLFZ of the TGR showed good adaptability in terms of the physiological aspects of NSCs, N, and P compared with a control group after 3 years of flooding-exposure cycles. T. distichum may allocate its photosynthetic assimilation products between growth and its NSC pool to ensure that its carbohydrate requirements during the flooding period and the recovery and growth period after exposure are met.
Funding
The National Key Research and Development Program of China (2017YFC0505305), the Program for the Follow-on Work of the Three Gorges: Ecological and Biological Diversity Conservation in the Reservoir Region (5000002013BB5200002), and the Key Research Projects in Forestry of Chongqing (Yu Lin Ke Yan 2015-6).
Notes on contributors
Ting Wang is a postgraduate in ecology, who now works in forestry.
Dr. Hong Wei is a professor at South West University, where she has taught in the disciplines of ecology and biostatistics, and worked with the responses of plant physiology to the stress of heavy metal and submergence in Three Gorges Reservior.
Wenchao Ma, Cui Zhou, Hongchun Chen and Rui Li are the postgraduates in ecology, who exploring the plant physiology to the stress of heavy metal and submergence in Three Gorges Reservior.
Shuai Li is a postgraduate in ecology, who now works in forestry.
Disclosure Statement
No potential conflict of interest was reported by the authors.
References
- Bailey-Serres J, Colmer TD. 2014. Plant tolerance of flooding stress-recent advances. Plant Cell Environ. 37:2211–2215.
- Bailey-Serres J, Voesenek LACJ. 2008. Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol. 59:313–339.
- Barbaroux C, Breda N, Dufrene E. 2003. Distribution of above-ground and below-ground carbohydrate reserves in adult trees of two contrasting broad-leaved species (Quercus petraea and Fagus sylvatica). New Phytol. 157:605–615.
- Chantuma P, Lacointe A, Kasemsap P, Thanisawanyangkura S, Gohet E, Clement A, Guiliot A, Ameglio T, Thaler P. 2009. Carbohydrate storage in wood and bark of rubber trees submitted to different level of C demand induced by latex tapping. Tree Physiol. 29:1021–1031.
- Chen HJ, Zamorano MF, Ivanoff D. 2013. Effect of deep flooding on nutrients and non-structural carbohydrates of mature Typha domingensis and its post-flooding recovery. Ecol Eng. 53:267–274.
- Conner WH, McLeod KW, McCarron JK. 1997. Flooding and salinity effects on growth and survival of four common forested wetland species. Wetl Ecol Manag. 5:99–109.
- Das KK, Sarkar RK, Ismail AM. 2005. Elongation ability and non-structural carbohydrate levels in relation to submergence tolerance in rice. Plant Sci. 168:131–136.
- Domingues TF, Meir P, Feldpausch TR, Saiz G, Veenendaal EM, Schrodt F, Bird M, Djagbletey G, Hien F, Compaore H, et al. 2010 Co-limitation of photosynthetic capacity by nitrogen and phosphorus in West Africa woodlands. Plant Cell Environ. 33:959–980.
- Elcan JM, Pezeshki SR. 2002. Effects of flooding on susceptibility of Taxodium distichum L. seedlings to drought. Photosynthetica 40:177–182.
- Ericsson T, Rytter L, Vapaavuori E. 1996. Physiology of carbon allocation in trees. Biomass Bioenerg. 11:115–127.
- Fan DY, Xiong GM, Zhang AY, Liu X, Xie ZQ, Li ZJ. 2015. Effect of water-lever regulation on species selection for ecological restoration practice in the water-level fluctuation zone of Three Gorges Reservoir. Chin J Plant Ecol. 39:416–432.
- Gomez-Aparicio L, Zamora R, Gomez JM, Hodar JA, Castro J, Baraza E. 2004. Applying plant facilitation to forest restoration: A meta-analysis of the use of shrubs as nurse plants. Ecol Appl. 14:1128–1138.
- Guo ZW, Hu JJ, Yang QP, Li YC, Chen SL, Chen WJ. 2015. Influence of mulching management on the relationships between foliar non-structural carbohydrates and N, P concentrations in Phyllostachys violascens stand. Chin J Appl Ecol. 25:1064–1070.
- Han WJ, Bai LL, Li CX. 2016. Effect of flooding on photosynthesis, growth and nutrient content of Cynodon dactylon. Acta Prataculturae Sinica. 25:49–59.
- Han WX, Fang JY, Guo DL. Zhang Y. 2005. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 168:377–385.
- He YY, Wang CY, Yuan ZX, Li XX, Yang WH, Song H, Li CX. 2018. Photosynthetic characteristics of Taxodium ascendens and Taxodium distichum under different submergence in the hydro-fluctuation belt of the Three Gorges Reservoir. Acta Ecologica Sinica. 38:2722–2731.
- Hoch G, Popp M, Korner C. 2002. Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos 98:361–374.
- Iwanaga F, Tanaka K, Nakazato I, Yamamoto F. 2015. Effects of submergence on growth and survival of saplings of three wetland trees differing in adaptive mechanisms for flood tolerance. Forest Syst. 24:1.
- Jackson MB, Ishizawa K, Ito O. 2009. Evolution and mechanisms of plant tolerance to flooding stress. Ann Bot. 103:137–142.
- Jiang ZJ, Huang XP, Zhang JP. 2012. Effect of environmental stress on non-structural carbohydrates reserves and transfer in seagrasses. Acta Ecologica Sinica 32:6242–6250.
- Jie SL, Fan DY, Xie ZQ, Zhang XY, Xiong GM. 2012. Features of leaf photosynthesis and leaf nutrient traits in reservoir riparian region of Three Gorges Reservoir, China. Acta Ecologica Sinica 32:1723–1733.
- Jin Q, Wang R, Zhou XR, Zhou ZY, Lu X, Zhao P, Li JH, Zhou YY. 2013. Vegetative growth and nutrient element accumulation of Amorpha fruticose under different waterlogging stress condition. Pratacultural Sci. 30:904–909.
- Juan DLCJS, Liz PMF, Stanislav M. 2012. Plant responses to stress due to flooding. A review. Revista Colombiana de Ciencias Hortícolas. 6:96–109.
- Li C, Zhong Z, Geng Y, Schneider R. 2010. Comparative studies on physiological and biochemical adaptation of Taxodium distichum and Taxodium ascendens seedlings to different soil water regimes. Plant Soil 329:481–494.
- Li CX, Zhong ZC, Liu Y. 2005. Effect of soil water change on photosynthetic characteristics of Taxodium distichum seedlings in the hydro-fluctuation belt of the Three Gorges Reservoir Area. Acta Ecologica Sinica 25:1953–1959.
- Li CX, Zhong ZC. 2007. Influence of mimic soil water change on the contents of malic acid and shikimic acid and root-biomass of Taxodium distichum seedings in the hydro-fluctuation belt of the Three Gorges Reservoir region. Acta Ecologica Sinica 27:4394–4402.
- Li M, Xiao W, Shi P, Wang SG, Zhong YD, Liu XL, Wang XD, Cai XH, Shi ZM. 2008. Nitrogen and carbon source-sink relationships in trees at the Himalayan treelines compared with lower elevations. Plant Cell Environ. 31:1377–1387.
- Li M, Xiao W, Wang S, Cheng G, Cherubini P, Cai X, Wang X, Zhu W. 2008. Mobile carbohydrates in Himalayan treeline trees I. Evidence for carbon gain limitation but not for growth limitation. Tree Physiol. 28:1287–1296.
- Liu Z, Cheng R, Xiao W, Guo Q, Wang N. 2014. Effect of off-season flooding on growth, photosynthesis, carbohydrate partitioning, and nutrient uptake in Distylium chinense. PLoS One 9:e107636.
- Liu Z, Cheng R, Xiao W, Guo Q, Wang X, Feng X. 2013. Effects of submergence on the growth and photosynthetic characteristics of Rhizoma cyperi in hydro-fluctuation belt of Three Gorges Reservoir area, Southwest China. Shengtaixue Zazhi 32:2015–2022.
- Liu Z, Cheng R, Xiao W, Guo Q, Wang Y, Wang N, Wang Y. 2015. Leaf gas exchange, chlorophyll fluorescence, non-structural carbohydrate content and growth responses of Distylium chinense, during complete submergence and subaerial re-emergence. Aquat. Bot. 124:70–77.
- Liu ZB, Cheng RM, Xiao WF, Wang RL, Feng XH, Wang XR. 2013. Effect of waterlogging on photosynthetic and physioecological characteristics of plants. World Forest. Res. 26:33–38.
- Lü MQ, Wu SJ, Chen CD, Huang P. 2015. A review of studies on water level fluctuating zone (WLFZ) of the Three Gorges Reservoir (TGR) based on bibliometric perspective. Acta Ecologica Sinica. 35:3504–3518.
- Ma WC, Liu Y, Zhou C, Wang T, Wei H. 2017. Effect of water-level change on nutritional characteristic of Taxodium distichum in the Three Gorges Reservoir. Acta Ecologica Sinica. 37:1128–1136.
- McGroddy ME, Daufresne T, Hedin LO. 2008. Scaling of C: N: P stoichiometry in forests worldwide: implications of terrestrial Redfield-type ratios. Ecology 89:890.
- Millard P, Sommerkorn M, Grelet G. 2007. Environmental change and carbon limitation in trees: a biochemical, ecophysiological and ecosystem appraisal. New Phytol. 175:11–28.
- Myers JA, Kitajima K. 2007. Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J Ecol. 95:383–395.
- New T, Xie Z. 2008. Impacts of large dams on riparian vegetation: applying global experience to the case of China’s Three Gorges Dam. Biodivers. Conserv. 17:3149–3163.
- Nilsson C, Jansson R, Zinko U. 1997. Long-term responses of river-margin vegetation to water-level regulation. Science 276:798–800.
- Pan QM, Han XG, Bai YF, Yang JC. 2002. Advances in physiology and ecology studies on stored non-structure carbohydrates in plants. Chin Bullet Bot. 19:30–38.
- Panda D, Sarkar RK. 2014. Mechanism associated with nonstructural carbohydrate accumulation in submergence tolerant rice (Oryza sativa L.) cultivars. J Plant Interact. 9:62–68.
- Pedersen O, Colmer TD, Sand-Jensen K. 2013. Underwater photosynthesis of submerged plants—recent advances and methods. Front Plant Sci. 4:1–19.
- Perata P, Voesenek LACJ. 2007. Submergence tolerance in rice requires Sub1A, an ethylene-response-factor-like gene. Trends Plant Sci. 12:43–46.
- Pezeshki SR, DeLaune RD, Anderson PH. 1999. Effect of flooding on elemental uptake and biomass allocation in seedlings of three bottomland tree species. J Plant Nutr. 22:1481–1494.
- Pezeshki SR, DeLaune RD. 2012. Soil oxidation-reduction in wetlands and its impact on plant functioning. Biology 1:196–221.
- Pezeshki SR, Santos MI. 1998. Relationships among rhizosphere oxygen deficiency, root restriction, photosynthesis, and growth in baldcypress (Taxodium distichum L.) seedlings. Photosynthetica 35:381–390.
- Poorter L, Kitajima K. 2007. Carbohydrate storage and light requirements of tropical moist and dry forest tree species. Ecology 88:1000–1011.
- Pucciariello C, Voesenek LACJ, Perata P, Sasidharan R. 2014. Plant responses to flooding. Front Plant Sci. 5:1–2.
- Reich PB, Oleksyn J. 2004. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Natl Acad Sci USA. 101:11001–11006.
- Song PH, Zeng QW, Shang JZ, Ma B, Xiang ZH, He NJ. 2013. Research progress in plant responses to waterlogging stress. Sci Sericult. 39:160–165.
- Stiller V. 2009. Soil salinity and drought alter wood density and vulnerability to xylem cavitation of baldcypress (Taxodium distichum (L.) Rich.) seedlings. Environ Exp Bot. 67:164–171.
- Tan SD, Zhu MY, Dang HS, Wang Y, Zhang QF. 2009. Physiological responses of Bermudagrass(Cynodon dactylon(L.) Pers.) to deep submergence stress in the Three Gorges Reservoir Area. Acta Ecologica Sinica 29:3685–3691.
- Thomson CJ, Armstrong W, Waters I, Greenway H. 1990. Aerenchyma formation and associated oxygen movement in seminal and nodal roots of wheat. Plant Cell Environ. 13:395–403.
- Vann CD, Megonigal JP. 2002. Productivity responses of Acer rubrum and Taxodium distichum seedlings to elevated CO2 and flooding. Environ Pollut. 1161:S31–S36.
- Voesenek LACJ, Sasidharan R, Visser EJW, Bailey-Serres J. 2016. Flooding stress signaling through perturbations in oxygen, ethylene, nitric oxide and light. New Phytol. 209:39–43.
- Wang CY, Li CX, Wei H, Xie YZ, Han WJ. 2016. Effects of long-term periodic submergence on photosynthesis and growth of Taxodium distichum and Taxodium ascendens saplings in the hydro-fluctuation zone of the Three Gorges Reservoir of China. PLoS One 11:1–17.
- Wang XR, Cheng RM, Feng XH, Guo QS, Xiao WF. 2009. Characteristics of soil seed banks in backwater area of Three Gorges Reservoir water-level- fluctuating zone at initial stage of river-flooding. Chin J Appl Ecol. 20:2891–2897.
- Wei J, Wu CY, Jiang Y, Wang HL. 2014. Sample preparation optimization for determination of soluble sugar in red jujube fruits by anthrone method. Food Sci. 35:136–140.
- Wu JG, Huang JH, Han XG, Gao XM, He FL, Jiang MX, Jiang ZG, Primack RB, Shen ZH. 2004. The Three Gorges Dam: an ecological perspective. Front Ecol Environ. 2:241–248.
- Wu JT, Yang SY, Deng WC, Chang WY. 2010. Responses of tissue non-structural carbohydrates and leaf nitrogen contents to altitude in two dwarf bamboos in Wolong. Acta Ecologica Sinica. 30:610–618.
- Wu L, Zhang WW, Ge XM, Tang LZ. 2012. A review of the response mechanisms of plants to waterlogging stress. World Forest Res. 25:27–33.
- Wu W, He XD, Zhou Q X. 2010. Review on N:P stoichiometry in eco-system. J Desert Res. 30:296–302.
- Wurth M, Pelaez-Riedl S, Wright SJ, Korner C. 2005. Non-structural carbohydrate pools in a tropical forest. Oecologia 143:11–24.
- Ye XQ, Zeng B. 2013. Survival and carbohydrate storage in two tolerant plant species exposed to prolonged flooding in the Three Gorges Reservoir Region. Acta Hydrobiol Sin. 37:450–457.
- Yu LM, Wang CK, Wang XC. 2011. Allocation of nonstructural carbohydrates for three temperate tree species in Northeast China. Chin J Plant Ecol. 35:1245–1255.
- Yu Q, Chen Q, Elser JJ, He NP, Wu HH, Zhang GM, Wu JG, Bai YF, Han XG. 2010. Linking stoichiometric homoeostasis with ecosystem structure, functioning and stability. Ecol Lett. 13:1390–1399.
- Zhu N, Qin A, Guo Q, Zhu L, Xu G, Pei S. 2015. Spatial heterogeneity of plant community in Zigui and Wushan typical hydro-fluctuation belt of Three Gorges Reservoir areas. Forest Res. 28:109–115.