Abstract
The seasonal allocation of carbohydrates in water hyacinth was evaluated to determine the physiological weak points in the life cycle of this plant in three large, moderately polluted freshwater canals north of the Nile Delta (Egypt). Monthly plant samples were divided into laminae, petioles, stolons, stem-bases and roots to determine the seasonal changes in water-soluble carbohydrates (WSC), starch and total non-structural carbohydrates (TNC) for each plant organ. Generally, water hyacinth allocated 2.2% of its total biomass to stolons, 4.5% to stem-bases, 19.0% to roots, 21.1% to laminae and 53.1% to petioles. The proportion of biomass allocated to the root system (stolons, stem-bases and roots) decreased from 38.5% in April to 17.2% in July, while that of the shoot system (laminae and petioles) increased from 61.5% to 82.8% during the same period. Stem-bases were found to contain the highest concentrations of WSC, starch and TNC throughout the water hyacinth’s life cycle. Starch represented the greatest part of the TNC pool, surpassing the concentration of WSC by 3.1- to 8.3-fold. The highest contents (g/m2) of WSC, starch and TNC were found in petioles. The period in the seasonal cycle when water hyacinth is expected to be most vulnerable to a control technique is when the carbohydrate contents are at the lowest. Based on the present study, this would be in April before complete mobilization of stored carbohydrates in the stem-bases and stolons have allowed expansion of the leaf material and the maximum growth rate.
1. Introduction
Eichhornia crassipes (C. Mart.) Solms (water hyacinth) is a free floating, stoloniferous, perennial aquatic plant that originated from the Amazon River Basin and has become distributed throughout the world (Global Invasive Species Database Citation2006). It is one of the worst nuisance aquatic plants globally (Holm et al. Citation1991). Water hyacinth interferes with water use by causing direct obstruction to navigation, degrading water quality for domestic use, impeding waterflow in irrigation canals and reducing outdoor recreation (Luu and Getsinger Citation1990a). It offers food and habitat for several harmful insects and for vectors of diseases including filariasis, encephalitis and malaria (Sucharit et al. Citation1981). Water hyacinth accelerates evapo-transpiration and loss of water, with estimates varying from 2.7-fold (Lallana et al. Citation1987) to 3.2-fold (Penfound and Earle Citation1948) greater from a water hyacinth mat in contrast to open water. This action is of significance in zones that sustain seasonal or chronic droughts (e.g. wet–dry tropics or the Mediterranean). The impacts of water hyacinth have been most severe in Africa, where large rivers, lakes and dams, vital for the economic development of the continent have been rendered unusable (Coetzee et al. Citation2009). Thus, manual, mechanical, chemical and biological control measures have been tried to manage and control water hyacinth (Zahran Citation2009). On the other hand, water hyacinth functions in water pollution removal, animal feed, mulch, manure and the production of pulp, paper and biogas (Gopal Citation1987; Rezania et al. Citation2015).
Carbohydrates can be divided into two main clusters: total structural carbohydrates (TSC) and total non-structural carbohydrates (TNC). TSC include permanent structural substances, such as cellulose, hemicellulose and other complex polymers. TSC provides the physical structure of the plant and usually remain where they are synthesized. In contrast, TNC can be converted to simple sugars and cycled in physiological processes. The TNC can be separated into two fractions: WSC (monosaccharides and disaccharides) and reserves (polysaccharides; Pesacreta and Luu Citation1988). WSC are readily available for metabolism, and their amounts vary in plant tissues. The reserve carbohydrate components of plants are stored in various organs (e.g. stem-bases, tubers, turions, rhizomes and roots) and are later metabolized during respiration and growth. Moreover, the reserve carbohydrates are the fundamental supply of energy during periods of growth in early spring (Smith Citation1975), and reserves aid recovery from stress, herbivory or disturbance (Madsen et al. Citation1993).
Quantitative studies that determine carbohydrate allocation patterns and biomass budgets throughout the life cycle contribute to our understanding of plants, their life history and strategies for controlling them (Asaeda and Karunaratne Citation2000; Asaeda et al. Citation2005; Asaeda et al. Citation2006; Asaeda et al. Citation2008; Eid Citation2010; Eid and Shaltout Citation2016; Eid et al. Citation2016). A physiological weak point in the life cycle of a plant is when it is least likely to recover from the application of a control method (Luu and Getsinger Citation1990a). Many studies have determined the physiological weak points within the life cycle of many macrophyte species based primarily on seasonal carbohydrate allocation patterns and have demonstrated that timing the control approach to the target species’ weak point in carbohydrate storage can improve the effectiveness of control (Madsen Citation1993a, Citation1997; Katovich et al. Citation1998; Madsen and Owens Citation1998; Owens and Madsen Citation1998; Woolf and Madsen Citation2003; Wersal et al. Citation2011; Wersal, Madsen, and Cheshier Citation2013; Eid and Shaltout Citation2016).
While considerable information exists on the ecology and biology of water hyacinth, little information is available regarding carbohydrate allocation in this species (Luu and Getsinger Citation1990a, Citation1990b; Madsen Citation1993b). The few published reports of carbohydrate levels in water hyacinth have dealt largely with carbohydrates as an indicator of potential methane gas production or animal feed (Penfound and Earle Citation1948; Tucker Citation1981a, Citation1981b; Tucker and DeBusk Citation1981). According to the author’s knowledge, no published studies have elucidated potential physiological weak points within the life cycle of the water hyacinth based on seasonal carbohydrate allocation patterns under the Egyptian natural conditions where it is considered a disastrously invasive aquatic plant. Hence, the goals of this study were to evaluate the seasonal allocation of carbohydrates in the various organs of water hyacinth in three large, moderately polluted freshwater canals in the north of the Nile Delta (Egypt), and to identify the potential physiological weak points (based on seasonal carbohydrate allocation patterns) in its life cycle to suggest the best time to begin control management on this invasive species.
2. Material and methods
The study area was located in the Nile Delta, Egypt, which encompasses the two branches of the Nile: Damietta to the east and Rosetta to the west. The mean daily solar radiation in 2014 for the study area ranged from 9.9 MJ/m2 in December to 27.9 MJ/m2 in June, while the mean daily air temperature ranged from 15.6 °C in February to 28.0 °C in August (NASA-POWER Citation2015).
The present study is an extension of research that was executed in 2014 at three large (>10 m in width), moderately polluted irrigation canals () of Kafr El-Sheikh Province, north of the Nile Delta (Egypt) and published by Eid and Shaltout (Citation2017a, Citation2017b). Sampling was achieved in monospecific and homogeneous water hyacinth stands at each irrigation canal (31° 03′ 32.29′′ N, 30° 58′ 37.57′′ E; 31° 03′ 28.03′′ N, 30° 57′ 21.57′′ E; and 31° 03′ 28.70′′ N, 30° 55′ 55.68′′ E), and water hyacinth biomass was sampled at monthly intervals from April 2014 to November 2014 using five randomly distributed quadrats (each 0.5 × 0.5 m). All the individual water hyacinths in the quadrat were collected and drained for 5 min; then, the plant materials were separated into laminae and petioles for the shoot system and stolons, stem-bases and roots for the root system. They were carried in polyethylene bags to the laboratory.
Table 1. Characteristics of water hyacinth stands in three large moderately polluted freshwater canals in the north of the Nile Delta, where the present study was carried out (after Eid and Shaltout Citation2017b).
Tap water was used to wash the collected samples in the laboratory using a 4-mm mesh sieve to avoid material loss, and then the samples were oven dried at 85 °C for one week and weighed to determine biomass in grams dry matter per square meter (g DM/m2). Afterwards, using a metal-free plastic mill, plant materials were ground into particles less than 0.4 mm for carbohydrate analysis. Water-soluble carbohydrates (WSC) were extracted from 0.25 g of ground material with hot distilled water, whereas TNC were extracted from another 0.25 g of ground material using diluted H2SO4 following the method of Smith et al. (Citation1964). WSC and TNC concentrations (mg/g DM) in the extracted solutions were measured by spectrophotometry using the phenol–H2SO4 colorimetric method as described in Granéli et al. (Citation1992), where the standard was glucose. The starch concentration was calculated as follows: starch concentration (mg/g DM) = 0.9 × (TNC concentration (mg/g DM) – WSC concentration (mg/g DM). Finally, the WSC, starch and TNC contents (g/m2) of the laminae, petioles, stolons, stem-bases and roots were calculated by multiplying the WSC, starch and TNC concentrations (mg/g DM) by the biomass of the respective parts (g DM/m2). Plant organ percentage of total biomass of water hyacinth was calculated as the biomass of a specific organ divided by the total biomass. Plant organ percentage of WSC, starch and TNC contents in organs of water hyacinth was calculated as the content of a specific organ divided by the total content.
The biomass data, as well as the WSC, starch and TNC data, did not differ significantly among studied irrigation canals (data not presented). Thus, the data of the three irrigation canals were combined, which resulted in 15 replicates at each sampling date per plant organ (laminae, petioles, stolons, stem-bases and roots). Before performing ANOVA (the analysis of variance), the data were tested for normality of distribution and homogeneity of variance and, when necessary, data were log-transformed. Repeated measures ANOVA were applied to the biomass data to test the differences over time. A Tukey’s HSD test with a significance threshold of p < 0.05 was applied to determine the differences among the eight months. The WSC, starch and TNC data for water hyacinth organs were subjected to two-way ANOVA to evaluate the differences among organs over time. The differences among means for every organ over eight months were identified using a Tukey’s HSD test with a significance threshold of p < 0.05. Statistica 7.1 was used to process all of the statistical analyses (StatSoft Citation2007).
3. Results
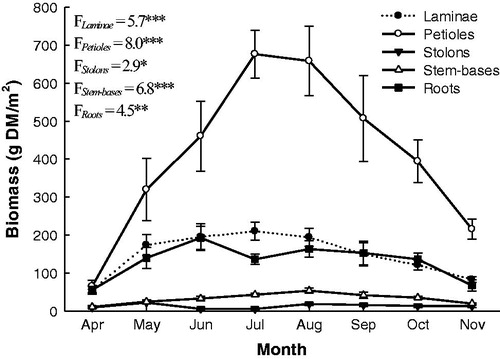
The seasonal biomass production of water hyacinth at the irrigation canals of this study is shown in . Shoot biomass (laminae and petioles) increased from 120.5 g DM/m2 in April to 886.8 g DM/m2 in July (736% of that in April) and then decreased to 298.6 g DM/m2 in November (34% of that in July) when the plants moved into the senescence stage. The root system (stolons, stem-bases and roots) biomass increased from 75.4 g DM/m2 in April to 231.5 g DM/m2 in June (i.e. increased by 307%), decreased to 184.6 g DM/m2 in July (80% of that in June), and then increased again to 235.2 g DM/m2 in August. It then decreased to 99.6 g DM/m2 in November ().
Figure 1. Monthly variation in organ biomass (g DM/m2) of the water hyacinth in three large moderately polluted freshwater canals in the north of the Nile Delta during one growing season (April–November 2014). Vertical bars indicate the standard errors of the means (n = 15). F-values represent the repeated measures ANOVA, df = 7. *p < 0.05. **p < 0.01. ***p < 0.001.
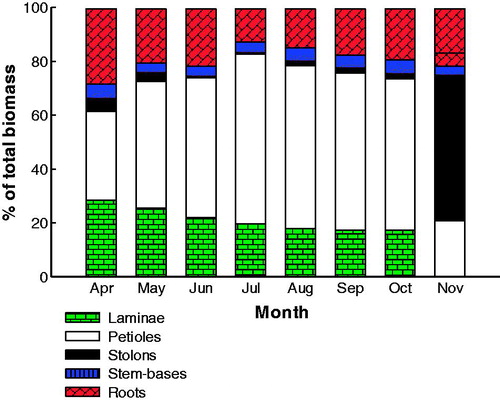
The proportions of dry weight allocated among water hyacinth organs followed distinct seasonal patterns (). Stolons and stem-base biomass made up only a small percentage of the total biomass. Generally, water hyacinth allocated 2.2% of its total biomass to stolons, 4.5% to stem-bases, 19.0% to roots, 21.1% to laminae and 53.1% to petioles. The proportion of biomass allocated to the root system decreased from 38.5% in April to 17.2% in July, while that of the shoot system increased from 61.5 to 82.8% through the same time ().
Figure 2. Plant organ percentage of the total biomass of water hyacinth in three large moderately polluted freshwater canals in the north of the Nile Delta during one growing season (April–November 2014).
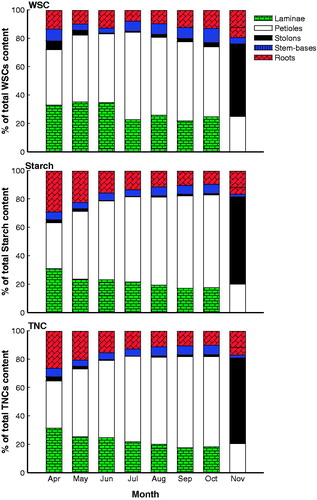
The annual patterns of WSC, starch and TNC concentrations (mg/g DM) of water hyacinth organs differed significantly among sampling months and among different organs (). Stem-bases were found to contain the highest concentrations of WSC, starch and TNC throughout the water hyacinth’s life cycle. The minimum WSC concentration was recorded in roots compared with other water hyacinth organs, whereas the minimum starch and TNC concentrations were recorded in the stolons. Stem-bases, stolons, laminae, petioles and roots showed a capacity to store up to 38.6, 32.5, 30.2, 21.5, and 15.7 mg/g DM WSC, respectively. The lowest WSC concentration in water hyacinth stem-bases and stolons occurred in June, and the lowest concentration in petioles occurred in August (). Starch represented the greatest part of the TNC pool, surpassing the concentration of WSC by 3.1- to 8.3-fold. Laminae showed a significant increase in starch concentration from 102.0 mg/g DM in April to 165.5 mg/g DM in August before decreasing, while stem-bases, petioles and stolons increased from 91.0, 91.4, and 46.3 mg/g DM in April to 195.6, 174.1, and 104.7 mg/g DM, respectively, by September before decreasing. Concentrations of TNC followed similar patterns to those observed for starch ().
Table 2. Monthly variation (mean ± standard error, n = 15) in the water-soluble carbohydrate (WSC), starch and total non-structural carbohydrate (TNC) concentrations (mg/g DM) in organs of the water hyacinth in three large moderately polluted freshwater canals in the north of the Nile Delta during one growing season (April–November 2014).
The results indicated significant variation in the annual patterns of WSC, starch and TNC content (g/m2) among the sampling months and among different organs of water hyacinth (). The highest content of WSC, starch and TNC was found in the petioles. It is evident that the water hyacinth population increased by five times the WSC content in one growing season, where the WSC content of all plant organs started at 4.4 g/m2 in April and peaked at 23.8 g/m2 in July (). The total content of starch and TNC began at 18.4 and 24.8 g/m2 in April and increased to 167.0 and 205.0 g/m2 in August, a 9.1- and 8.3-fold increase, respectively ().
Table 3. Monthly variation (mean ± standard error, n = 15) in the water-soluble carbohydrate (WSC), starch and total non-structural carbohydrate (TNC) contents (g/m2) in organs of the water hyacinth in three large-moderately polluted freshwater canals in the north of the Nile Delta during one growing season (April–November 2014).
An analysis of the percentage accumulation of WSC in water hyacinth organs showed that the shoot system contained approximately 71.8% of WSC reserves in April which increased to 84.2% in July, whereas the root system accounted for 28.2% in April which decreased to 15.8% in July (). In the current investigation, 36.9% of the starch reserves were found in the root system during April, while 63.1% were found in the shoot system. During July, 81.5% of the starch reserves were found in the shoot system and 18.5% were found in the root system. In addition, the root system contributed 35.4% of TNC reserves at the start of the growing season (April) to 18.2% in July, while the shoot system accounted for 64.6% of TNC reserves in April, and then increased to 81.8% in July ().
4. Discussion
The carbohydrate weak point is defined as a seasonal minimum of stored carbohydrates. The carbohydrate weak point occurs when carbohydrate utilization from storage exceeds carbohydrate production (Madsen Citation1997). At this point, the plant is the least able to recover from stress or disturbance, indicating the point where the success of management efforts will be maximized (Woolf and Madsen Citation2003). Carbohydrate allocation has been used to determine vulnerable points in the life cycles of many macrophytes, and these weak points are used as control points to suppress spring growth or eradicate the target species (Hydrilla verticillate, Madsen and Owens Citation1998; Owens and Madsen Citation1998; Myriophyllum spicatum, Madsen Citation1997; Potamogeton crispus, Woolf and Madsen Citation2003; Typha angustifolia, Linde et al. Citation1976; Typha domingensis, Eid and Shaltout Citation2016).
Based on the present study, the life cycle of the water hyacinth in moderately polluted freshwater canals of the Nile Delta could be summarized by the following: as the temperature warmed in the spring, young ramets emerged from over-wintering stem-bases, and biomass slowly increased. The biomass continued to increase during May and June, a period marked by maximum vegetative reproduction (Eid and Shaltout Citation2017b). The maximum water hyacinth biomass occurred in July and the water hyacinths flowered from June to July (personal observation). Although ramets were still being produced, the rate of production had slowed (Eid and Shaltout Citation2017b). Crowded conditions began to occur at this time due to the growth of individual plants. By August, crowded conditions resulted in the shading and senescence of many lower leaves. Throughout September, there was a general senescence of old, tall leaves, a production of new ramets and a decline in biomass. The onset of cold weather triggered a general senescence of all plants in October, and a considerable reduction of biomass occurred by November. The same pattern was reported for water hyacinth in subtropical Florida, USA (Center and Spencer Citation1981).
Stem-bases and stolons were minor components of the total biomass at any one time of the water hyacinth life cycle. This amount indicates a small contribution to vegetative propagation, since stem-bases (over-wintering and regrowth) and stolons (contributing to vegetative propagation) are all involved in propagation. Perennial macrophytes such as water hyacinth, which do not die back, generally contribute less to propagation, since their partitioning strategy calls for a strong, vegetatively mature plant (Madsen et al. Citation1993). Moreover, the present investigation proposed that water hyacinth had the capacity to distribute a higher portion of biomass to the shoot system (laminae and petioles), thus gaining additional energy (extra photosynthesis organ) for its rapid growth (Madsen et al. Citation1993; Xie et al. Citation2004). Additionally, Villamagna and Murphy (Citation2010) reported that greater allocation to the shoot system may increase the water hyacinth’s ability to shade out algae in the water column and other aquatic plants, demonstrating that biomass allocation pattern changes may be a competitive advantage for water hyacinths with altered resource levels in natural environments. During the growing season, the root system biomass of the water hyacinth in the current work reached 17.2–38.5% of the total biomass, while that of the shoot system reached 61.5–82.8% (72% of the leaf biomass was petioles and 28% was laminae). These percentages are in accordance with the outcomes of outdoor tank studies by Luu and Getsinger (Citation1990a) at Vicksburg (Mississippi, USA) and Madsen et al. (Citation1993) at Lewisville (Texas, USA).
Seasonal changes in storage organ (e.g. stem-bases, stolons, and rhizomes) biomass can be caused by the translocation of carbohydrates to or from other parts of the plant, mortality and metabolism of non-structural carbohydrate reserves (Chapin et al. Citation1990; Eid Citation2010). In the current study, the proportion of the biomass allocated to the root system decreased from 38.5% in April (at the beginning of vegetative development) to 17.2% in July. The decrease in root system biomass allocation was perhaps because of the upward translocation of reserves to the new shoots or to support rapid growth and expansion (Madsen and Owens Citation1998). A similar pattern has also been reported for Phragmites australis (Granéli et al. Citation1992; Asaeda et al. Citation2006; Eid et al. Citation2010), Pistia stratiotes (Eid et al. Citation2016; Eid Citation2017), Typha angustifolia (Asaeda et al. Citation2008) and Typha domingensis (Eid et al. Citation2012). Translocation during this period is also indicated by the decreases in WSC concentrations in stem-bases and stolons. Generally, plants rely on stored energy to initiate the growth of plant tissues until photosynthesis can begin (Madsen Citation1997). The root system biomass allocation starts to increase in August after spring translocation to new shoots, and this represents a shift in resource allocation to root system organs. A reloading of root system organs in August is also indicated by increased WSC, starch and TNC concentrations of the stem-bases and stolons. Moreover, the August drop in WSC concentrations for petioles, reported in the current research, can probably be attributed to flower formation (Eid and Shaltout Citation2017b). This finding was supported by the results of Linde et al. (Citation1976), who stated that the heavy flowering puts an extra drain on the carbohydrate reserves of Typha angustifolia.
The concentrations of WSC, starch and TNC in different organs of water hyacinth underwent seasonal dynamics in the present study. Stem-bases were found to contain the highest concentrations of WSC, starch and TNC throughout the water hyacinth’s life cycle. A similar trend was reported for water hyacinth in outdoor tanks in Vicksburg, USA (Luu and Getsinger Citation1990b). Stem-bases are the over-wintering structures of water hyacinth, and they play an important role in the seasonal carbohydrate cycle of the plant by providing energy for dormant buds and new growth in the spring. These high levels of carbohydrate concentrations in stem-bases indicate that stem-bases are strong carbohydrate sinks during the life cycle of the water hyacinth. Stem-bases accumulated up to 38.6 mg/g DM WSC, 195.6 mg/g DM starch, and 252.3 mg/g DM TNC by the July November period. After stem-bases, stolons contained the highest concentrations of WSC from September to November. This period of high WSC in stolons coincided with fall ramet production and indicates that stolons play a role in conducting WSC to stem-bases in young ramets (Luu and Getsinger Citation1990a). Stolons are active channels of carbohydrates that are freely shared among parent and daughter plants (Alpert et al. Citation1991), allowing the spread of the water hyacinth mat through the production of new daughter plants (Madsen et al. Citation1993). It is possible that some stored carbohydrates in the stem-bases were mobilized to support the development of new ramets during late fall (Luu and Getsinger Citation1990a), and since stolons are the only connecting route between stem-bases and newly produced ramets, the enrichment of these structures with WSC is reasonable. In the current work, the minimum WSC concentration was recorded in the roots. Similarly, Penfound and Earle (Citation1948) observed the lowest amount of carbohydrates in the roots compared with other organs of the water hyacinth. The roots of the water hyacinth are an actively respiring tissue and have no adaptations for carbohydrate storage; therefore, carbohydrates should not accumulate in this organ (Madsen et al. Citation1993).
Starch is one of the most common storage carbohydrates and is converted readily to WSC when needed for growth (Loescher et al. Citation1990). TNC are important in initiating regrowth when photosynthetic tissues are non-existent or are inadequate to supply both respiration and growth demands (Rashid et al. Citation2017). In the current project, starch and TNC concentrations varied seasonally. Moreover, as dry matter productivity increased in warm weather, starch and TNC concentrations increased. Those changes appeared to be related to seasonal differences in growth rates (Tucker and DeBusk Citation1981). The high levels of carbohydrates (starch and TNC) in the laminae and petioles during September suggest that the water hyacinth was vigorously photosynthesizing to accumulate carbohydrates for fall vegetative reproduction and for food reserves in stem-bases (Luu and Getsinger Citation1990a). The reduction of carbohydrate reserves (starch and TNC) in the stem-bases, stolons, laminae and petioles after September resulted primarily from the production of new ramets in the water hyacinth population, as well as due to the general senescence of tall leaves.
In water hyacinth, the carbohydrate contents (g/m2) reveal the contribution of component organs to the overall carbohydrate production of the population and emphasize the increase in carbohydrate production throughout the growing season. The contents of WSC, starch and TNC were the highest in petioles > laminae > roots > stem-bases > stolons. Unlike storage organs (stem-bases and stolons), WSC, starch and TNC concentrations in petioles are relatively low; but the high biomass of petioles results in large pools of WSC, starch and TNC, which means that biomass was the decisive factor in determining the quantity of carbohydrates per unit area of stand.
5. Conclusions
The time when water hyacinth is expected to be the most liable to a management approach is at the period in the seasonal cycle when sequestered carbohydrates are lowest; this will be efficient in minimizing its growth. Without sufficient stored carbohydrates, the plant will recover more slowly, and management techniques may provide more effective control. Therefore, control tactics should be applied when carbohydrate stores are at their lowest. Based on the present study, water hyacinth in large, moderately polluted freshwater canals in the north of the Nile Delta is most susceptible in early spring (April) before complete mobilization of stored carbohydrates in the stem-bases and stolons have allowed expansion of the leaf material and maximum growth rate. Since the shoot system (laminae and petioles) contains a significant proportion of whole plant WSC, starch and TNC contents, damage or death to the shoot system results in a decline in the plants’ ability to recover. Therefore, combining light herbicide applications with biocontrol efforts to slow growth rates (Center and Durden Citation1986; Haag et al. Citation1988) may be an effective technique from a physiological viewpoint. The destruction of the shoot system will delay further growth and carbohydrate production and remove a large pool of carbohydrates from the water hyacinth populations.
Acknowledgements
The author thanks two anonymous reviewers for their useful comments on an earlier version. The author extends his appreciation to the Deanship of Scientific Research at King Khalid University for funding this work.
Disclosure statement
This is to acknowledge any financial interest or benefit that has arisen from the direct applications of my research.
Additional information
Funding
Notes on contributors
Ebrahem M. Eid
Ebrahem M. Eid is an associate professor of Plant Ecology, Botany Department, Faculty of Science, Kafr El-Sheikh University, Kafr El-Sheikh, Egypt. He obtained his PhD degree in Plant Ecology in 2009 from the Faculty of Science, Tanta University, Tanta, Egypt. His fields of research experience include plant population ecology, aquatic plant biology, ecological modelling, phytoremediation and carbon sequestration. He has attended many training courses and scholarships in Egypt, Germany, the Netherlands, Hungary, Finland, Estonia, Japan and the Czech Republic, as well as several national and international symposia and conferences in Egypt and abroad. He has published 40 manuscripts in national and international specialized journals, covering many aspects of plant ecology.
References
- Alpert P, Warembourg FR, Roy J. 1991. Transport of carbon among connected ramets of Eichhornia crassipes (Pontederiaceae) at normal and high levels of CO2. Am J Bot. 78:1459–1466.
- Asaeda T, Karunaratne S. 2000. Dynamic modeling of the growth of Phragmites australis: model description. Aquat Bot. 67:301–318.
- Asaeda T, Manatunge J, Fujino T. 2005. Morphological adaptations of emergent plants to water flow: a case study with Typha angustifolia, Zizania latifolia and Phragmites australis. Freshw Biol. 50:1991–2001.
- Asaeda T, Manatunge J, Roberts J, Hai DN. 2006. Seasonal dynamics of resource translocation between the aboveground organs and age-specific rhizome segments of Phragmites australis. Environ Exp Bot. 57: 9–18.
- Asaeda T, Sharma P, Rajapakse L. 2008. Seasonal patterns of carbohydrate translocation and synthesis of structural carbon components in Typha angustifolia. Hydrobiologia. 607:87–101.
- Center TD, Durden WC. 1986. Variation in waterhyacinth/weevil interactions resulting from temporal differences in weed control efforts. J Aquat Plant Manage. 24:28–38.
- Center TD, Spencer NR. 1981. The phenology and growth of water hyacinth Eichhornia crassipes (Mart.) Solms in an eutrophic north-central Florida lake. Aquat Bot. 10:1–32.
- Chapin FS, Schultze E-D, Mooney HA. 1990. The ecology and economics of storage in plants. Ann Rev Ecol System. 21:423–447.
- Coetzee JA, Hill MP, Julien MH, Center TD, Cordo HA. 2009. Eichhornia crassipes (Mart.) Solms–Laub. (Pontederiaceae). In: Muniappan R, Reddy GVP, Raman A, editors. Biological control of tropical weeds using arthropods. Cambridge: Cambridge University Press; p. 183–210.
- Eid EM. 2010. Dynamics of carbohydrate translocation between the above- and under-ground organs of Phragmites australis (Cav.) Trin. ex Steudel in Lake Burullus, Egypt. Egypt J Aquat Res. 36:107–113.
- Eid EM. 2017. Verification of a numerical growth model of Pistia stratiotes L. using field data from tropical and subtropical sites. J Freshw Ecol. 32:391–403.
- Eid EM, Shaltout KH. 2016. Seasonal allocation of carbohydrates between above- and below-ground organs of Typha domingensis. Feddes Repert. 127:55–64.
- Eid EM, Shaltout KH. 2017a. Growth dynamics of water hyacinth (Eichhornia crassipes): a modelling approach. Rendiconti Fisiche Accademia Lincei 28:169–181.
- Eid EM, Shaltout KH. 2017b. Population dynamics of Eichhornia crassipes (C. Mart.) Solms in Nile Delta, Egypt. Plant Spec Biol. 32:279–291.
- Eid EM, Shaltout KH, Asaeda T. 2012. Modeling growth dynamics of Typha domingensis (Pers.) Poir. ex Steud. in Lake Burullus, Egypt. Ecol Model. 243:63–72.
- Eid EM, Shaltout KH, Al-Sodany YM, Soetaert K, Jensen K. 2010. Modeling growth, carbon allocation and nutrient budget of Phragmites australis in Lake Burullus, Egypt. Wetlands. 30:240–251.
- Eid EM, Galal TM, Dakhil MA, Hassan LM. 2016. Modeling the growth dynamics of Pistia stratiotes L. populations along the water courses of south Nile Delta, Egypt. Rendiconti Fisiche Accademia Lincei. 27:375–382.
- Global Invasive Species Database. 2006. Eichhornia crassipes (aquatic plant). International Union for the Conservation of Nature (IUCN), Gland [accessed 2015 June 15]. Available from: http://www.issg.org/database/species/ecology.asp?si=70.
- Gopal B. 1987. Water hyacinth (aquatic plant studies). Amsterdam: Elsevier.
- Granéli W, Weisner SEB, Sytsma MD. 1992. Rhizome dynamics and resource storage in Phragmites australis. Wetlands Ecol Manage. 1:239–247.
- Haag KM, Glenn MS, Jordan JC. 1988. Selective patterns of herbicide application for improved biological control of waterhyacinth. J Aquat Plant Manage. 26:17–19.
- Holm LG, Plucknett DL, Pancho JV, Herberger JP. 1991. The world’s worst weeds: distribution and biology. Malabar: Krieger Publishing Co.
- Katovich EJS, Becker RL, Sheaffer CC, Halgerson JL. 1998. Seasonal fluctuations of carbohydrate levels in roots and crowns of purple loosestrife (Lythrum salicaria). Weed Sci. 46:540–544.
- Lallana VH, Sabattini RA, Md Lallana C. 1987. Evapotranspiration from Eichhornia crassipes, Pistia stratiotes, Salvinia herzogii, and Azolla caroliniana during summer in Argentina. J Aquat Plant Manage. 25:48–50.
- Linde AF, Janisch T, Smith D. 1976. Cattail – the significance of its growth, phenology and carbohydrate storage to its control and management. Technical Bulletin No. 94, Madison: Wisconsin Department of Natural Resources.
- Loescher WH, McCamant T, Keller JD. 1990. Carbohydrate reserves, translocation, and storage in woody plant roots. HortSci. 25:274–281.
- Luu KT, Getsinger KD. 1990a. Seasonal biomass and carbohydrate distribution in waterhyacinth: small-scale evaluation. Technical Report No. A-90-1. Vicksburg (MS): US Army Engineer Waterways Experiment Station.
- Luu KT, Getsinger KD. 1990b. Seasonal biomass and carbohydrate allocation in water hyacinth. J Aquat Plant Manage. 28:3–10.
- Madsen JD. 1993a. Control points in the phenological cycle of Eurasian watermilfoil. Aquatic Plant Control Research Program, Vol. A-93-1. Vicksburg (MS): US Army Engineer Waterways Experiment Station.
- Madsen JD. 1993b. Allocation of biomass and carbohydrates in waterhyacinth (Eichhornia crassipes): pond-scale verification. Information Exchange Bulletin No. A-93-3, Vicksburg (MS): U.S. Army Engineer Waterways Experiment Station.
- Madsen JD. 1997. Seasonal biomass and carbohydrate allocation in a southern population of Eurasian watermilfoil. J Aquat Plant Manage. 35:15–21.
- Madsen JD, Owens CS. 1998. Seasonal biomass and carbohydrate allocation in dioecious Hydrilla. J Aquat Plant Manage. 36:138–145.
- Madsen JD, Luu KT, Getsinger KD. 1993. Allocation of biomass and carbohydrates in water hyacinth (Eichhornia crassipes): pond-scale verification. Technical Report No. A-93-3, Vicksburg (MS): US Army Engineer Waterways Experiment Station.
- NASA-POWER. 2015. Climatology resource for agroclimatology. NASA prediction of worldwide energy [accessed 2015 June 20]. Available from: http://power.larc.nasa.gov/cgi-bin/cgiwrap/solar/agro.cgi/.
- Owens CS, Madsen JD. 1998. Phenological studies of carbohydrate allocation in Hydrilla. J Aquat Plant Manage. 36:40–44.
- Penfound WT, Earle TT. 1948. The biology of the water hyacinth. Ecol Monogr. 18:447–472.
- Pesacreta GJ, Luu KT. 1988. Feasibility of relating phenology and carbohydrate partitioning to improve aquatic plant control. Miscellaneous Paper No. A-88-7. Vicksburg (MS): US Army Engineer Waterways Experiment Station.
- Rashid MH, Uddin MN, Asaeda T, Robinson RW. 2017. Seasonal variations of carbohydrates in Pueraria lobata related to growth and phenology. Weed Biol Manage. 17:103–111.
- Rezania S, Ponraj M, Talaiekhozani A, Mohamad SE, Md Din MF, Taib SM, Sabbagh F, Md Sairan F. 2015. Perspectives of phytoremediation using water hyacinth for removal of heavy metals, organic and inorganic pollutants in wastewater. J Environ Manage. 163:125–133.
- Smith D. 1975. Forage management in the north. Dubuque: Kendall/Hunt Publishing Company.
- Smith D, Paulsen GM, Raguse CA. 1964. Extraction of total available carbohydrates from grass and legume tissue. Plant Physiol. 39:960–962.
- StatSoft. 2007. Statistica Version 7.1. Tulsa (OK): StatSoft Inc.
- Sucharit S, Harinasuta C, Deesin T, Vutikes S. 1981. Studies of aquatic plants and grasses as breeding hosts for mosquitoes. Southeast Asian J Trop Med Public Health. 12:464–465.
- Tucker CS. 1981a. Relationships between culture density and the composition of three floating aquatic macrophytes. Hydrobiologia. 85:73–76.
- Tucker CS. 1981b. The effect of ionic form and level of nitrogen on the growth and composition of Eichhornia crassipes (Mart.) Solms. Hydrobiologia. 83:517–522.
- Tucker CS, DeBusk TA. 1981. Seasonal growth of Eichhornia crassipes (Mart.) Solms: relationship to protein, fiber, and available carbohydrate content. Aquat Bot. 11:137–141.
- Villamagna AM, Murphy BR. 2010. Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): a review. Freshw Biol. 55:282–298.
- Wersal RM, Cheshier JC, Madsen JD, Gerard PD. 2011. Phenology, starch allocation, and environmental effects on Myriophyllum aquaticum. Aquat Bot. 95:194–199.
- Wersal RM, Madsen JD, Cheshier JC. 2013. Seasonal biomass and starch allocation of common reed (Phragmites australis) (haplotype I) in Southern Alabama, USA. Invas Plant Sci Manage. 6:140–146.
- Woolf TE, Madsen JD. 2003. Seasonal biomass and carbohydrate allocation patterns in Southern Minnesota curlyleaf pondweed populations. J Aquat Plant Manage. 41:113–118.
- Xie Y, Wen MZ, Yu D, Li YK. 2004. Growth and resource allocation of water hyacinth as affected by gradually increasing nutrient concentrations. Aquat Bot. 79: 257–266.
- Zahran MA. 2009. Hydrophytes of the Nile in Egypt. In Dumont HJ, editor. The Nile: origin, environments, limnology and human use. Wageningen: Springer; p. 463–478.