 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The non-indigenous round goby (Neogobius melanostomus) entered the Flint River, Michigan, USA around 1996 while zebra mussels (Dreissena polymorpha) invaded in late 1998. We wanted to identify if there were round goby or darter (Etheostoma/Percina spp.) diet or density alterations by comparing 1998 data with our 2002 data after mussel colonization. Chironomids dominated the round goby’s pre-zebra mussel diet in August 1998 (89% by number), followed by hydropsychids (10%). After zebra mussels arrived, diets diversified; chironomids declined to 52%, hydropsychids stayed the same, gastropods were more prominent (22%) and 4% were zebra mussels. Data from a site upriver (with no round gobies or zebra mussels) showed darters consumed mostly chironomids (49%), mayflies (11%) and hydropsychids (9%), suggesting reliance on chironomid prey and other interactions compromised the ability of darters to coexist with round gobies downstream, since only one of three species present during 1998 was collected in 2002. Benthic assemblages on rocks changed dramatically (R-values =0.95) from 1998 to 2002. Blackside darter (Percina maculata) density in the presence of round gobies increased eightfold compared with 1998. We hypothesized zebra mussels fostered the growth of macrophytes, resulting in odonates composing 42% by volume of blackside darter diets in 2002 compared with 10% in 1998. Diet overlaps between small and large round gobies and blackside darters were high (Schoener Index =0.55–0.59, R-values =0.11–0.24), suggesting potential negative competitive interactions. Zebra mussel-mediated ecosystem changes may have decreased interspecific competition for food with blackside darters, allowing coexistence with round gobies. Native darters with varied diets feeding in mid-water, are most likely to coexist with round gobies, especially where dreissenids potentially mediate competitive interactions.
1. Introduction
Round gobies (Neogobius melanostomus), native to the Black and Caspian seas, were first found in Michigan in 1990 (Jude et al. Citation1991) and are spreading throughout the Laurentian Great Lakes into Michigan’s inland waterways and the Mississippi River where they can dramatically change aquatic food webs (Janssen and Jude Citation2001; Jude Citation2001; Poos et al. Citation2010). Similar range extensions have been documented in their native habitats in Europe (Charlebois et al. Citation1997). The round goby poses a threat to native fishes, since they can prey on native benthic fish larvae (Weimer and Sowinski Citation1999; French and Jude Citation2001) and have caused extirpation of some species, such as mottled sculpin (Cottus bairdi), Johnny darters (Etheostoma nigrum) and logperch (Percina caprodes) (Dubs and Corkum Citation1996; Lauer and McComish 2004; Balshine et al. Citation2005; Krakowiak and Pennuto Citation2008; Bergstrom and Mensinger Citation2009; Stauffer et al. Citation2016). They also can affect native unionid mussels through direct predation and reduction in mandatory fish hosts (Poos et al. Citation2010). As round gobies have become integrated into the Great Lakes and European ecosystems (e.g. Hensler et al. Citation2008), round goby proliferations have implications for modifying toxic substance bioaccumulation patterns in aquatic food webs (Johnson et al. Citation2005; Ng et al. Citation2008) and promoting Type E botulism by poisoning fishes and birds (Somers et al. Citation2003) because of their consumption of contaminated zebra mussels (Dreissena polymorpha) (Domske and Obert Citation2001; Yule et al. Citation2006). Since these non-indigenous species have also colonized river systems connected to the Mississippi River (Irons et al. Citation2006), which have a high diversity of darters, and are expanding in Europe with concomitant effects on their native fishes, information on warm-water river systems is essential to understand their interactions with small benthic fishes in river systems never exposed to these species (Kornis et al. Citation2013).
In 1996, the round goby was found at two inland sites in Michigan: the Flint and Shiawassee rivers, which drain into the Saginaw River and eventually Saginaw Bay, Lake Huron. This was the first recorded appearance of this species at an inland site, far away from the Great Lakes and connecting channels. This finding prompted us to initiate this follow-up study, since relatively little was known about interactions with native, non-sport fishes in warm-water river systems. We only sampled the Flint River because (1) the only other inland river site with a known round goby invasion was the Shiawassee River and abundance was low there, (2) prior diet and density information was only available from this site (Carman et al. Citation2006) and (3) we had a unique opportunity to evaluate changes after zebra mussels colonized the site. Most studies since this one were conducted in tributaries either with or without zebra mussels; none made a comparison of changes before and after zebra mussel colonization. Carman et al. (Citation2006) studied round goby diets in the Flint River during 1998, just as zebra mussels were observed upriver of our site (based on several canoe trips from the Holloway Dam to the study site (see ) and (Leonardi Citation2004). Colonization by zebra mussels of the study site after 1998 may have disrupted trophic dynamics. Zebra mussels were first found in the St. Clair River in 1988 (O’Niell and Dextrase Citation1994) and rapidly spread throughout the Great Lakes because their planktonic veliger offspring drifted in rivers and lakes and were transported by freighters. They are voracious filter feeders, removing algae from the water column, increasing water clarity and negatively affecting Diporeia in all the Great Lakes south of Lake Superior (Nalepa et al. Citation1998). They have been implicated in drastic trophic cascades in many of the Great Lakes especially Lake Huron, which resulted in the eventual loss of the salmon fishery there (Bunnell et al. Citation2014). Riverine round gobies in their native habitats prefer mollusks, especially zebra mussels where available (Charlebois et al. Citation1997). However, in some cases (see Carman et al. Citation2006; Diggins et al. Citation2002), they can be mostly insectivorous. Hence a shift toward zebra mussels as a primary food source was hypothesized for large round gobies in the Flint River. With this shift, food previously eaten by round gobies may have become available to native fishes, which could have reduced resource competition and changed food web dynamics. There is abundant evidence (Lederer et al. Citation2006; Brush et al. Citation2012) of the depressing effect of round gobies on benthos. We also wondered if round gobies would eat large quantities of fishes, as they have done in native habitats (3–42% of round goby diets was fish: Charlebois et al. Citation1997), thus harming native species, such as darters (Etheostoma and Percina spp.).
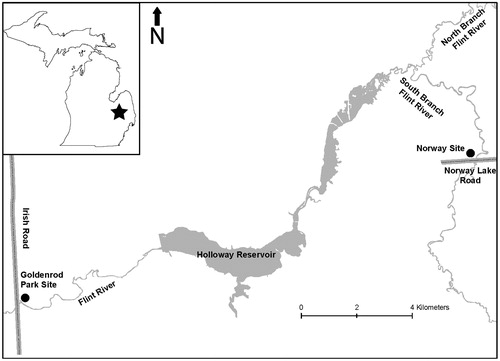
Figure 1. Map of the Flint River showing location in Michigan (black star) and the two sampling sites (black circles), Goldenrod Park (with round gobies and zebra mussels) and the Norway site (without round gobies and zebra mussels).
Because of information that round gobies have had detrimental impacts on benthic fishes (Reid and Mandrak Citation2008; Duncan et al. Citation2011), we focused on darters (Etheostoma/Percina spp.), since there were no mottled sculpins in our study area. We tested three hypotheses:
Hypothesis 1: round gobies compete with darters for resources causing diet overlap, which may have caused decreased abundance of darters.
Hypothesis 2: round gobies undergo a diet shift from being insectivorous to include more driessenids, which may allow better survival of darters because of increased benthic prey and reduced resource competition and diet overlap.
Hypothesis 3: Availability of more dreissenids should reduce predation on small fishes by round gobies.
To test these hypotheses we documented predation, diet overlap and density changes from 1998 to 2002 for round gobies and darters, and elucidated predator selection of insect prey to better understand potential impacts on benthos and potential competitive interactions between round gobies and darters, especially blackside darters (Percina maculata).
2. Methods
2.1. Study areas
This study was conducted in the Flint River, a warm-water stream in central Michigan, located in the Saginaw Bay, Lake Huron watershed (, Leonardi and Gruhn Citation2001; Leonardi Citation2004). The Goldenrod Park study site was at the intersection of the Flint River and Irish Road in Genesee County (43°06′13.36″ N, 83°33′24.12″ W), 3.9 river-km downstream from Holloway Dam and 3.1 river-km upstream from Mott Dam. The study site was primarily riffle habitat with river depths to 1.2 m and a mean width of 30 m. Substrate was primarily cobble on sand. Water clarity was high during 2002 based on personal observations, photographs taken at the site in 1998 compared with 2002 and increases in Secchi disc readings in the reservoir above from 1998 to 2003 (Leonardi Citation2004). During 1998 there were very few macrophytes in the area we seined, while during 2002, there were extensive aquatic macrophyte beds (Potamogeton crispus, P. filiformis and P. amplifolius). Discharge at the Goldenrod Park site (data from a USGS gauging station number 04147500 2.8 km upstream) was 2.9 m3/s during 29–30 August 2002, while it was 2.0 m3/s during 23–24 August 1998, and was controlled by the upstream dam. This dam is operated by the city of Flint, Michigan for a water supply and operates in a run-of-the-river mode.
An upriver site (Norway), which was the nearest location in the Flint River to Goldenrod Park that contained no round gobies or zebra mussels, was upstream of Goldenrod Park and the Holloway Dam off Norway Lake Road, Lapeer County (43°08′42.77″ N, 83°21′12.28″ W). The site was composed of a riffle area similar to habitat found at Goldenrod Park; however, discharge was 0.7 m3/s. Some sparse aquatic vegetation was observed.
2.2. Fish collection and handling
During 2002, fishes were collected from the Flint River east of Flint, Michigan at Goldenrod Park, Genesee County, using a 5-m-long bag seine (Jude and DeBoe Citation1996) five times over a 24-h period on 29–30 August 2002: before dawn (06:20 h), mid-day (13:15 h), before dusk (19:00 h), after dusk (21:10 h) and mid-night (23:25 h). A before-dawn sample was also collected on 2 September 2002 at 04:00 h. We kick-seined and sometimes pulled the seine along the bottom until around 70–80 fish/period were captured. Sampling was done to replicate the Carman study, so as to provide direct comparisons of diet and densities before and after zebra mussel colonization. The Norway site upstream () was sampled at 15:20 h on 29 August 2002 once during the day, to provide information on darter ecology in a nearby site unaffected by round gobies and zebra mussels. Blackside darters were the only darter species we collected during 2002 at Goldenrod Park, but no diet data were available from blackside darters during 1998. To compare blackside darter diets between years, all blackside darters collected during August 1998 from the Carman study (N = 13) were analyzed for stomach contents.
The Carman et al. (Citation2006) study site was identical to ours (Goldenrod park/downstream—see ); sampling occurred on 16 May, 28 June, 30 July and 22 August 1998. Fishes were collected seven times over a 24-h period, whereas we sampled only six times during this study. The same seine was used in both studies.
Area swept while seining was noted for samples collected during 1998 and 2002 so fish density could be estimated and compared. All round gobies and darters were preserved immediately in the field in ethyl alcohol. Other fishes were identified and their numbers estimated in the field before being released.
We only sampled during August 2002 and made our comparisons with similar 1998 August data (Carman et al. Citation2006). Our goal was to take advantage of this unique opportunity to demonstrate whether diet, density, or environmental changes had occurred after the dreissenid invasion. There is general support from the literature (French and Jude Citation2001; Carman et al. Citation2006) for the assertion that the diet of round gobies was mainly insectivorous, with a shift toward more mollusks (especially zebra mussels) with larger individuals, but with little change throughout the year. Therefore, we believed August data were adequate to demonstrate whether there were any major changes occurring in the area when compared with the Carman study.
2.3. Fish diet analysis
In the laboratory, fishes were identified to species, weighed to the nearest 0.1 g wet weight and measured (total length) to the nearest 1 mm. Stomachs were removed and their contents identified to the lowest taxonomic level, enumerated and volume measured using volumetric displacement (French and Jude Citation2001). When stomach contents were too small to measure, percent composition was estimated visually and volume was estimated to be 0.05 mL. All fishes, including those with empty stomachs (round gobies—14%, blackside darters—12%) were used in analyses. Carman et al. (Citation2006) analyzed round goby diets using numbers of organisms consumed, so direct diet comparisons could only be achieved using numbers. We used volume for all other diet comparisons in this study, including the diet analyses for all blackside darters, because we believed volume provided a more valid estimation of diet.
2.4. Benthos collections
We collected three rocks (approximately 20 × 20 cm) each from the riffle areas of Goldenrod Park and Norway after seining on 30 August and four rocks on 27 September 2002 for a total of seven rocks sampled per site. Rocks were randomly selected and carefully removed into a 0.25-m-diameter, 363-micron-mesh plankton net placed downstream of the rock. All rocks, except four collected at the Goldenrod Park site on 27 September, were placed in a large white enamel pan and benthic organisms were removed on site and preserved in ethanol. The other four samples were taken to the laboratory and processed the next day. Insects were identified at least to family. Other methods for assessing benthos (e.g. Surber sampler) may have given a better representation of the benthos, but we chose methods that were consistent with the Carman study.
2.5. Data analysis
We divided round gobies into two size groups: small (<54 mm TL; N = 371, mean =36, range 18–53, SE =0.4) and large (≥ 54 mm TL; N = 110, mean =75, range 54–104, SE =0.9), based on previous research indicating that large round gobies consumed almost exclusively dreissenid mussels, while their smaller counterparts ate mostly insects (Jude et al. Citation1995; French and Jude Citation2001; Andraso et al. Citation2011). Student’s t-tests (P = 0.05) with Levene’s tests for equality of variances were performed to determine diet differences for round gobies ≥54 mm TL between 1998 and 2002, and to detect changes in mean densities of round gobies and blackside darters at the Goldenrod Park riffle between 1998 and 2002. The Schoener Index (SI) (Schoener Citation1970) was used to assess diet overlap and species composition overlap of aquatic macroinvertebrates.
Where Px,i and Py,i are the proportions of food category i in the diets of species X and species Y, respectively and N = number of food categories. This index measures diet similarity and ranges from 0 (no overlap, completely different) to 1 (identical diet). Values of 0.5 or greater indicate substantial overlap (Hartman and Brandt Citation1995; Zahn Seegert et al. Citation2014). The Strauss Index (Strauss L) (Strauss Citation1979) was used to calculate prey selectivity (diet composition versus abundance of insects on rocks) for round gobies and blackside darters using the formula: L = ri – pi where ri is the relative abundance (r) of food item (i) in the stomach and pi is the relative abundance of food in the environment. The index ranges from –1 (strong avoidance) to +1 (strong preference).
We compared benthos results of Carman (N = 135 rocks sampled from May to August 1998—no significant differences among months) with 2002 data (Goldenrod site) and the Goldenrod site with the Norway site using t-tests with Bonferroni correction. Since there were different mean numbers of benthic organisms found per rock between 1998 and 2002, we standardized the counts to 100 organisms per rock. These data were also used to determine diet preferences of round gobies and darters.
To garner further support for benthos and diet assemblage differences, we used R–vegan (Oksanen et al. Citation2017) to calculate an Analysis of Similarity (ANOSIM). ANOSIM is a nonparametric permutation applied to a rank similarity matrix of samples (Clarke and Warwick Citation2001). Diet and benthos data were converted to proportions, square-root transformed using EXCEL and R created a Bray-Curtis similarity matrix which was used in the ANOSIM analysis. R-values from ANOSIM measure the absolute separation of diet and benthos assemblages in the site, year and fish comparisons we made and are usually more useful than P-values derived from the analysis (Clarke and Gorley Citation2001). R-values range between –1 and +1 with values <0.25 indicating little separation, those 0.5–0.75 indicating some overlap between groups and those >0.75 indicate clear separation between groups (Clarke and Gorley Citation2001).
3. Results
3.1. Fish density changes and community composition
Overall, 30 species of fishes were collected at the Norway and Goldenrod Park sites (data not shown); Leonardi and Gruhn (Citation2001) collected 28 and 25 species respectively above and below Goldenrod Park in 1997, including five darter species. During 2002, round gobies and blackside darters accounted for 69% and 9%, respectively, of the fish community at Goldenrod Park (N = 824), while blackside darters and other darters (greenside Etheostoma blennioides, Johnny and rainbow E. caeruleum darters) represented 5 and 13% of the community at the upriver site (N = 121 fishes) during sampling in August and September 2002. Four darter species were present at Norwood during 2002, while at Golden Rod Park there were three darter species in 1998, which were reduced to one species found during 2002 ().
Table 1. Density (no./100 m2) of darter species and round gobies at Goldenrod Park (1998 and 2002) in the Flint River, Michigan and at the Norway site upriver of the study site where round gobies and zebra mussels were absent.
Blackside darter densities during August at the Goldenrod Park riffle increased nearly eight fold from 1998 to 2002 (1.2/100 m2 versus 10.2/100 m2); differences were significant (p = 0.0092, t = 3.15, d.f. = 11) (). Round goby abundance also increased significantly from 1998 to 2002 (29/100 m2 versus 77/100 m2—p = 0.0225, t = 2.65, d.f. = 11). Densities of blackside, greenside, Johnny and rainbow darters at the Norway site were 3, 2, 6 and 1 fish/100 m2, respectively, much lower than those of either round gobies or blackside darters at the Goldenrod Park riffle.
Diets and diet overlaps of round goby and blackside darter
Large round goby diets in August changed substantially from 1998 to 2002 after zebra mussels invaded the Goldenrod Park site (), but diet assemblages showed minimal overlap (SI =0.27) and little separation (R = 0.15). Large round goby diets at Goldenrod Park during 2002 were more diverse than those of 1998 and were dominated by chironomid larvae and pupae (37% by number), hydropsychid larvae (9%), gastropods (22%), zygopterans (6%), ostracods (5%) and zebra mussels (4%) (). The large round goby diet during 2002 also contained fish (6% by volume) including juvenile spotfin shiners (Cyprinella spiloptera), bluegills (Lepomis macrochirus), and pumpkinseeds (Lepomis gibbosus). In contrast, large round gobies from 1998 ate predominantly chironomids (89% by number) and hydropsychids (10%). Large round goby diets overlapped substantially with small round goby diets (SI =0.65, R = 0.15—no separation), and diets of both sizes of round gobies overlapped with blackside darters (SI =0.55–0.59, R = 0.11–0.24—no separation). Small round gobies ate more trichopterans by volume and almost twice as many chironomids as blackside darters, while blackside darters ate more ephemeropterans, plecopterans and three times as many odonates as the other two groups (). Large round gobies ate gastropods and zebra mussels (12 and 9% respectively of the diet), while blackside darters ate none.
Table 2. Percent composition by number (N) and volume (V) of diets of large (LG) and small (SM) round gobies (RG) Neogobius melanostomus and blackside darters (BD) Percina maculata collected during August 1998 and late August - September 2002 from the Flint River, Michigan at Goldenrod Park (GR) and the Norway site (NW).
Table 3. Schoener Index (SI - diet overlap) and Analysis of Similarity (ANOSIM) values for various comparisons of benthos data on rocks (ANOSIM values only) and diet of round gobies (RG) and blackside darters (BD) of different sizes and from different years. GR = Goldenrod Park, LG = large, SM = small, na = not applicable.
During 2002, small round gobies ate more chironomids than did large round gobies (43 versus 19% by volume), while large round gobies ate more gastropods (22 versus 6%) (). Small and large round gobies during 2002 selected against hydropsychid prey (Strauss L = –0.17, –0.20), while large round gobies preferred gastropods and zygopterans (L = 0.12, 0.06).
To determine if blackside darter diets were influenced by the zebra mussel invasion at the study site, we compared their 2002 diets with 1998 diets. The change in blackside darter diets between 1998 and 2002, although pronounced, showed some overlap (SI =0.39) but little separation (R = 0.24). Blackside darters collected during 1998 at Goldenrod Park consumed mainly chironomids (53% by volume), ephemeropterans (32%) and zygopterans (10%) (). Blackside darters during 2002 consumed almost five-fold more odonates (zygopterans plus anisopterans) (48 versus 10% by volume) than in 1998, fewer chironomid larvae and pupae (21 versus 53%), fewer mayflies (4 versus 32%), but more hydropsychid larvae (12 versus 2%). Using the Strauss Index comparing benthos on rocks with diet, which may have missed pulses of prey in the water column or bottom, it was still clear that like round gobies, blackside darters selected against hydropsychid and also chironomid prey at the Goldenrod Park riffle (L = –0.15, –0.29), despite the fact that hydropsychids increased almost ninefold on the rocks compared with 1998 values (). Odonates, mostly zygopterans, appeared to be preferred prey, since they made up 48% by volume of the blackside darter diets during 2002 (L = 0.37—high preference), while composing 4.7% of the benthos on rocks. However, some may have been eaten from macrophytes skewing this comparison. Blackside darters at Goldenrod Park demonstrated no strong selection for or against other prey items.
Table 4. List of the mean number of benthic organisms (standardized to 100/rock) found on rocks during 1998 (n = 135, pooled data over 4 months) (Carman et al. Citation2006) and 2002 (n = 7 per site, this study). Rocks were removed from Goldenrod Park (Goldenrod) and the Norway site upriver in the Flint River, Michigan. Rocks from 2002 were collected on 30 August (n = 3 per site) and 27 September 2002 (n = 4 per site) and data pooled.
At the Norway site, diets of darters were exclusively invertebrates; diets varied by species, with blackside and rainbow darters having more diverse diets than greenside and Johnny darters (). Blackside, rainbow and greenside darters ate ephemenopteran and trichopteran larvae and chironomids in similar amounts; whereas, Johnny darter diets were composed of 97% chironomids by volume. Rainbow darter was the only darter species to eat Heptageniidae. Norway-site blackside darters ate almost twice as many chironomids and hydropsychids than at Goldenrod Park. In addition, Norway-site blackside darters uniquely ate amphipods and corixids, while Goldenrod Park fish ate more odonates. Blackside darters at Norway showed positive selection for amphipods (L = 0.20), chironomids (L = 0.32), corixids (L = 0.20) and selected against heptageniids (L = –0.15), hydropsychids (L = –0.32) and trichopterans (L = –0.17). Blackside darter diets at the Norway site were most similar to those of strongly benthic greenside darters (SI =0.60, significant), less similar to diets of Johnny darters (SI =0.42) and least similar to their mid-water-form counterpart rainbow darters (SI =0.30). Greenside darters ate more trichopterans and plecopterans by volume than blackside darters.
3.2. Benthos data
Seventeen taxa (pooled into 11 taxonomic groups) of benthic invertebrates were collected during 2002 at Goldenrod Park from the seven rocks sampled with a mean number of 98 per rock (). Chironomids were the most abundant taxa composing 50% of benthic organisms found, while Hydropsychidae composed 27%. Zebra mussels were not very abundant on rocks, since only ca. 7/rock (7%) were observed. A comparison of benthic groups on rocks from 1998 with 2002, showed clear separation of benthic assemblages between years (R-value =0.95). Chironomidae declined significantly (without the Bonferroni correction, but not with it) from 82% in 1998 to 50% during 2002 (p = 0.0035, t = 3.1549, d.f. = 140), Odonata significantly increased from 0.1 to 4.7% (p = 0.0003, t = 4.0420, d.f. = 140), while remaining comparisons were not significant. Comparing Goldenrod Park benthic groups during 2002 with the Norway site assemblage showed clear separation (R – value =0.92). Odonates were significantly (without the Bonferroni correction, but not with it) more abundant at Goldenrod Park (4.7%) than at Norwood (0.4%) (p = 0.0154, t = 2.8229, d.f. = 12). Remaining groups were not significantly different from each other, although Heptageniidae (10-fold), Hydropsychidae (2-fold) and Trichoptera minus Hydropsychidae (8-fold) were all higher at Norwood compared with the Goldenrod site. Two of these comparisons were almost significant.
4. Discussion
4.1. Diet overlap of round gobies with darters
We hypothesized resource competition as a possible negative interaction of round gobies with native darters and studies (Kuhns and Berg Citation1999; Raby et al. Citation2010) have shown round gobies can severely depress benthic organisms. Since round gobies composed around 70% of the riffle fish community during 1998 and 2002, their impact on native species of fishes and invertebrates was hypothesized to be great. As evidence of this, diets of blackside darters and round gobies at Goldenrod Park overlapped, since the diet of small and large round gobies was composed of 35% by volume Chironomidae, while blackside darter diets were 21% chironomids and even higher (40%) at the Norway site. Wynes and Wissing (Citation1982) reported that blackside and rainbow darters in an Ohio stream did not feed on the drift (because most drift occurred at night), but were almost obligate, daytime bottom feeders. This would place them in potential direct competition with round gobies, especially small ones that also concentrated their feeding in this benthic zone.
Diet information for Johnny and greenside darters at the Norway site was only a single sampling bout to provide data on what they would eat in the absence of round gobies. These data, along with literature data, suggest there would be a substantial diet overlap with round gobies, since darter diets were 97 and 45% chironomids by volume, respectively, a staple of round gobies. However, rainbow darters at the upstream site had a varied diet consisting of only 15% chironomids, suggesting they may be able to obtain alternate food resources if chironomids were in limited supply (see Stauffer et al. Citation2016 who found this), as blackside darters apparently have, in areas where round gobies were present. Darters are known to feed extensively on chironomids in other environments (Turner Citation1921; Hlohowskyj and White Citation1983). Since round gobies ate odonates in low quantities (9% of the diet) and the majority of odonate consumption occurred at night, we presume this predation was opportunistic while searching for chironomids. Conversely, blackside darters consumed large quantities of odonates (48% by volume of their diet) at the Goldenrod Park riffle mostly during the day, probably by picking resting or feeding odonates from rocks and plants. Most damselflies eaten were Argia spp., which are cryptic, day-active and common residents of riverine environments, especially in the presence of macrophytes. Benthos data showed odonate (mostly damselflies) percent composition on rocks was much lower in 1998 (<1%) than during 2002 (4.7%). Thus, blackside darters, whose population increased dramatically with appearance of macrophytes, appeared to be able to evade interspecific competition with round gobies by apparently feeding more at mid-levels in the water column, something noted for blackside darters by Greenberg (Citation1991). Additional support was provided by Malone (Citation2016), who studied tributaries to Lake Michigan with and without round gobies. They showed that blackside darters had similar densities in invaded and round goby-free tributaries.
Zebra mussels were less important in round goby diets than expected, but they appear to have caused extensive ecological changes in the Flint River. We observed proliferation of aquatic macrophytes, apparently induced by zebra mussels clearing the water column (Effler and Siegfried Citation1998; Schloesser and Manny Citation2007). Zebra mussels were first found in Holloway Reservoir above our site during 1995 and from 1998 to 2003, Carlson’s Trophic State Index increased, there was lower total phosphorus and chlorophyll a, and higher Secchi disc readings (Leonardi Citation2004). These changes were reflected at our river site and promoted greater sunlight penetration (Skubinna et al. Citation1995; Horgan and Mills Citation1999; Vanderploeg et al. Citation2002), which was followed by an increase in macrophytes, odonates and blackside darters. Blackside darters prefer clear to slightly turbid water (Becker Citation1983) and increased abundance of macrophytes is also ideal habitat for odonates. More odonates were found at the study site in 2002 on rocks than were found in 1998. One of the major food items for blackside darters at Goldenrod Park was odonates, which was not the case in 1998, nor were they important at the Norway site, where chironomids were predominantly eaten. Because odonates thrive in macrophytes, this may represent not only an ideal prey for blackside darters, but also a habitat shift allowing them to flourish in the presence of round gobies. Blackside darters are known to use vegetation for shelter (Becker Citation1983) and are midwater-dwelling fish (Trautman Citation1981). DeVanna et al. (Citation2011) proposed that Dreissena acted as ecosystem engineers and facilitators, with the Flint River ecosystem an example, since changes that occurred appear to have allowed expansion of blackside darters. This is exceptional, since other major studies in tributaries have found that round gobies depressed all darters (Phillips et al. Citation2003; Krakowiak and Pennuto Citation2008; Poos et al. Citation2010) except rainbow darters (Stauffer et al. Citation2016), but these systems did not have zebra mussels present.
4.2. Feeding behavior and invertebrate prey susceptibility
It is important to understand the functional feeding behavior of darters and round gobies and the behavior of their invertebrate prey to ascertain potential competitive interactions and mechanisms of potential impact among these groups (Matthews Citation1998). The round goby is a benthic species that feeds mainly on invertebrates and can affect any species with a similar niche, such as darters, madtoms Noturus spp., sculpins (Krakowiak and Pennuto Citation2008) and logperch (Stauffer et al. Citation2016). Round gobies can severely depress benthic populations (Djuricich and Janssen Citation2001; Ratti and Barton Citation2003; Pennuto et al. Citation2010). When we compared the overall diet composition of round gobies and blackside darters during 2002 with the overall benthos composition on rocks, we showed that zygopterans were preferred prey, very abundant at Goldenrod Park (almost 5% of the benthos population on the rocks and they were probably much more abundant in the macrophytes, which did not exist in 1998), and composed 5–7 and 42% by volume of the diets of round gobies and blackside darters respectively at Goldenrod Park.
Between 1998 and 2002, hydropsychids and odonates showed increased abundances on rocks. Round gobies and blackside darters generally increased their consumption of these two groups, showing availability is an important component of diet for both species.
Blackside darters may be more capable of partitioning resources in the presence of round gobies than other darters. One exception was rainbow darters, which seem to co-exist with round gobies (Burkett and Jude Citation2015; Stauffer et al. Citation2016). Many other studies have shown darters to be detrimentally affected by round gobies (summarized by Poos et al. Citation2010). Blackside darters in this study appeared to be an exception, since they had a more diverse diet in the presence of round gobies and zebra mussels than they did at the Norway site, possibly due to: (1) differences in habitat—Norway site was a riffle with cooler water, fewer macrophytes and lower discharge, and (2) differences in feeding mode (feeding higher in the water column) perhaps in response to predation (Schlosser Citation1987), competition with round gobies, or insect availability. Blackside darters forage mostly in mid-water during the day (Keast and Welsh Citation1968; Thomas Citation1970). As a result they are able to partition their food and habitat resources (Schoener Citation1974) with a presence in the upper water column and a more diverse diet than most other darters (and round gobies), which have no air bladders, have more prominent sub-terminal mouths and feed on the bottom (Smart and Gee Citation1979; Paine et al. Citation1982). Further evidence for mid-water feeding by blackside darters was their consumption of corixids and zooplankton. Corixids are usually associated with vegetation and can be found in the water column, a finding also noted by Smart and Gee (Citation1979). Daphnia spp. and harpacticoid copepods were found in the diet of large, but not small round gobies during 2002, which was also noted by Carman et al. (Citation2006) and Weimer and Sowinski (Citation1999). Zooplankton apparently originated from the Flint River upstream impoundment, which documents that large round gobies and blackside darters fed on drifting zooplankters. Chironomidae usually pupate at dusk and during the night (Palmen Citation1955) making them susceptible to predation during dusk, when blackside darters fed heavily on these dipterans.
Zebra mussels in low volumes were found in diets of small round gobies and were eaten in larger volumes (9% by volume) by large round gobies. This pattern is similar to findings in other studies (Jude et al. Citation1995; French and Jude Citation2001); however, zebra mussels made up substantially higher percentages of the diets in these studies than what we measured. We attributed this to the Flint River being in the early colonization period by zebra mussels and the smaller maximum sizes apparently reached by round gobies in small streams compared with those found at other Great Lakes sites. Once round gobies reached sizes of >100 mm they eat almost exclusively dreissenid mussels (Jude et al. Citation1995). Therefore, a diet shift toward zebra mussels may not yet be complete, or round gobies may prefer soft-bodied prey in this river system when they have a choice.
4.3. Impact of round gobies
Before round gobies became established in the Flint River, there were five species of darters (blackside, greenside, Johnny, fantail Etheostoma flabellare and rainbow) inhabiting the river in the immediate area (Leonardi and Gruhn Citation2001). The one closest station sampled by MDNR during 1997 was 8 km east of our site. They collected 27 species, 507 fishes and three different species of darters: blackside, Johnny and greenside. By 1998, after round gobies had invaded, three darter species remained at the Goldenrod Park site (blackside, Johnny and rainbow) (Carman et al. Citation2006), while during our study in 2002, only the blackside darter was captured at Goldenrod Park. However, it should be noted that effort was comparatively low during 2002 compared with 1998 resulting in the capture of 824 fish with sampling occurring six times at one site over a 24-h period.
Round gobies have already been implicated in the local extirpation of mottled sculpins (Janssen and Jude Citation2001), greenside darters (Jude Citation2001; Poos et al. Citation2010) and Johnny darters (Lauer et al. Citation2004; Krakowiak and Pennuto Citation2008). Logperch were detrimentally affected in the field (Stauffer et al. Citation2016) and in laboratory studies (Balshine et al. Citation2005). Round gobies may also detrimentally affect darters by eating their larvae (Weimer and Sowinski Citation1999; Abbett et al. Citation2013) and eggs, which was believed to have happened for greenside darters (Jude Citation2001), which are known to spawn on vegetation and algae and guard their nests (Winn Citation1958; Balon et al. Citation1977) in Lake Erie. Logperch also spawn on sand (Becker Citation1983) and have been extirpated locally on near shore Lake Erie reefs (Jude Citation2001, Stauffer et al. Citation2016). On the other hand, rainbow darters, which deposit their eggs on sand, seem to co-exist with round gobies in the St. Clair River in Michigan, as their population numbers remained unchanged after round gobies invaded that system in the early 1990s (French and Jude Citation2001), but populations were diminished, but not eliminated during a study comparing the 1994 populations with 2011 (Burkett and Jude Citation2015). Stauffer et al. (Citation2016) also found rainbow darters co existed with round gobies. Krakowiak and Pennuto (Citation2008) showed fish communities in streams with round gobies contained no rainbow darters, even though they were present prior to the round goby invasion. Johnny darters lay their eggs on the undersides of rocks in small crevices (Balon et al. Citation1977), which may be too small for spawning round gobies. However, they have still been extirpated in other locations, suggesting a different mechanism for their elimination (Lauer et al. Citation2004; Poos et al. Citation2010).
We hypothesized that fish consumption by round gobies would decline after zebra mussels colonized the river and acted as a buffer. Fish consumption by round gobies has been rarely observed in the Great Lakes region (French and Jude Citation2001), but does occur in their native Caspian Sea area (3–42% of diets were fish: Charlebois et al. Citation1997). However, Vâsek et al. (Citation2014) found no field evidence to support round goby fish predation in the Danube Basin. During this study, after zebra mussel colonization, large round gobies ate three species of fish (pumpkinseed, bluegill, spotfin shiner and unknown fish which composed 0.2% by number and 6% by volume of the diet, while Carman et al. (Citation2006) found them eating a stonecat (Noturus flavus) and a round goby (<1% by number). In other studies, Burkett and Jude (Citation2015) found fish made up 3% by volume of round goby diets and Poos et al. (Citation2010) discussed fish predation as a threat. Abbett et al. (Citation2013) documented consumption of larval fish thought to be fantail darters, which composed 11.2% of the round goby diet (dry weight). Jude (Citation2001) reported consumption of a large trout-perch (Percopsis omiscomaycus) in the St. Clair River and fathead minnows (Pimephales promelas) used as bait were found in Lake Michigan round gobies. A few eggs were eaten by round gobies in Lake Michigan (Mychek-Londer et al. Citation2013), while lake trout eggs and fry were eaten in a laboratory setting (Chotkowski and Marsden Citation1999). Fish predation has important ecological consequences, as it establishes that round gobies, when given an opportunity, will eat small fishes (and eggs) and opens another avenue, predation, to their ability to impact native species (see Ross Citation1991). More opportunity for predation may exist in warm-water environments, since they are more productive with generally higher species diversity, than most of the Great Lakes. The apparent disappearance of four species of darters documented during this study does not appear to be related to predation by round gobies, but by changing environmental conditions induced by zebra mussels or competition from round gobies for benthic prey, which is consistent with other studies (Krakowiak and Pennuto Citation2008; Poos et al. Citation2010).
Similar effects observed elsewhere and the Flint River may likewise be expected to occur in the Mississippi River watershed, since round gobies have already become established in the Illinois River (Irons et al. Citation2006) and are likely to spread downstream quickly, because their larvae exhibit diel vertical migration behavior (Hensler and Jude Citation2007). Their continued success will likely be enhanced, because of the presence of a diverse invertebrate fauna, including many mollusks, and because zebra mussels are already present along the length of the Mississippi River and in many of its tributaries. We expect round gobies may inflict the same types of ecological instabilities documented on Flint River native fish and invertebrate assemblages. However, presence of dreissenid mussels along the Mississippi River may buffer some negative impacts of round gobies on native benthic fishes by providing an alternative food source instead of native benthos and creating increased water clarity fostering macrophyte growth and more odonate prey. These changes would favor darters that feed higher in the water column, as we have found with blackside darters in the Flint River.
The Flint River is a typical, warm-water river in Michigan and demonstrates the effects that zebra mussels and round gobies could have on other inland waterways in the Great Lakes Basin and beyond. Loss of natural biodiversity spurred by these non-indigenous species can make ecosystems more difficult to manage. Already dreissenids have been implicated in sport fish (Francis et al. Citation1996) and invertebrate (Nalepa et al. Citation1998) declines, including a trophic cascade in Lake Huron (Evans et al. Citation2011).
In our case the blackside darter and perhaps other darters were probably depressed by colonization of the river by round gobies in 1996, but this effect appeared to be ameliorated for blackside darters after zebra mussels invaded. Because round gobies are known to penetrate tributaries (Phillips et al. Citation2003; Kornis and Vander Zanden Citation2009; Jude, unpublished data) and along with dreissenids are transported easily from one body of water to another, they are likely to become established in many lotic ecosystems with favorable habitats. Here they would be expected to depress invertebrate populations and displace or extirpate darter and other benthic fish species that are susceptible to round goby impacts or cannot utilize the mid-water environment to find prey, as we documented on the Flint River.
Acknowledgements
We thank Jason Jude for assistance with sampling for the 2 September collections and Lacey Mason for the figure. Stephanie Carman provided raw data. Jenna Voss and Russ Marlow assisted with benthos counting and identifications. Miles Luo and the University of Michigan Consulting for Statistics, Computing, and Analytics’ Michael Hornstein and James Henderson are thanked for running R. John Janssen, Paul Seelbach and Justin Mychek-Londer provided very helpful and important critiques on an earlier version as did anonymous reviewers.
Disclosure statement
There have been no financial interests or other benefits that derived from the conducting or publishing of this research.
Additional information
Funding
Notes on contributors
David J. Jude
David Jude, PhD is a fishery biologist and limnologist and a retired emeritus research scientist in the School of Natural Resources and Environment at the University of Michigan. He has over 40 years of Great Lakes research and teaching experiences. His research interests include invasive species (he discovered the first round gobies in North America), toxic substances, wetlands and fish population studies with emphasis on early life history stages. He has been down in a research submarine, the Johnson Sea Link II, worked with remotely operated vehicles, and participated in over 100 Great Lakes cruises aboard several ships, including the EPA’s Lake Guardian and the University of Michigan’s R/V Laurentian.
Stephen R. Hensler
Steven Hensler has a BS degree from Ohio State University and a MS degree from the University of Michigan where he worked on yellow perch and round goby early life history. He has done extensive research on the Great Lakes, worked for the U.S. Fish and Wildlife Service studying invasive species in the Great Lakes from 2006 to 2016, and more recently became executive director of a research foundation, the Cerulean Center, located in Traverse City, Michigan.
Meghan M. Murray
Meghan Miner graduated from the University of Michigan with a BS degree in Conservation Biology. She is a former NOAA fisheries observer and science communication specialist with COMPASS and is now a freelance science journalist in Kona, Hawaii.
References
- Abbett R, Waldt E, Johnson J, McKenna Jr J, Dittman, D. 2013. Interactions between invasive round goby (Neogobius melanstomus) and fantail darters (Ethoestoma flabellare) in a tributrary of the St. Lawrence River, New York, USA. J Freshw Ecol. 28:529–537.
- Andraso G, Cowles J, Colt R, Patel J, Campbell M. 2011. Ontogenetic changes in pharyngeal morphology correlate with a diet shift from arthropods to dreissenid mussels in round gobies (Neogobius melanostomus). J Great Lakes Res. 37:738–743.
- Balon E, Momot W, Regier H. 1977. Reproductive guilds of percids: results of the paleogeographical history and ecological succession. J Fish Res Board Can. 34:1910–1921.
- Balshine S, Verma A, Chant V, Theysmeyer, T. 2005. Competitive interaction between round gobies and logperch. J Great Lakes Res. 31:68–77.
- Becker, G. 1983. The fishes of Wisconsin. Madison (WI): University of Wisconsin Press.
- Bergstrom MA, Mensinger, AF. 2009. Interspecific resource competition between the invasive round goby and three native species: Logperch, slimy sculpin, and spoonhead sculpin. T Am Fish Soc. 138:1009–1017.
- Brush JM, Fisk AT, Hussey NE, Johnson TB. 2012. Spatial and seasonal variability in the diet of round goby (Neogobius melanostomus): Stable isotopes indicate that stomach contents overestimate the importance of dreissenids. Can J Fish Aquat Sci. 69:573–586.
- Burkett E, Jude D. 2015. Long-term impacts of invasive round goby Neogobius melanostomus on fish community diversity and diets in the St. Clair River, Michigan. J Great Lakes Res. 41:862–872.
- Bunnell, D., Barbiero, R., Ludsin, S., Madenjian, C., Warren, G., Dolan, D., Brenden, T., Briland, R., O. Gorman, R., He, Ji, Johengen, T., et al. 2014. Changing ecosystem dynamics in the Laurentian Great Lakes: Bottom-up and top-down regulation. Bioscience. 64:26–39.
- Carman S, Janssen J, Jude D, Berg M. 2006. Diel interactions between prey behaviour and feeding in an invasive fish, the round goby, in a North American river. Freshw Biol. 51(4):742–755.
- Charlebois PM, Marsden J, Goettel R, Wolfe K, Jude D, Rudnicka, S. 1997. The round goby, Neogobius melanostomus (Pallas): a Review of European and North American Literature. Illinois-Indiana Sea Grant Program and Illinois Natural History Survey. INHS Spec Publ. 20, p. 76.
- Chotkowski M, Marsden E. 1999. Round goby and mottled sculpin feed on lake trout eggs and fry: field predictions from laboratory experiments. J Great Lakes Res. 25:26–35.
- Clarke, K, Gorley, R. 2001. Primer v5: User manual/tutorial. 91 pp. Plymouth (UK): Primer-E-LTD.
- Clarke, K, Warwick, R. 2001. Changes in marine communities: An approach to statistical analysis and interpretation. Primer-E. 2nd ed. Plymouth (UK): Primer-E-LTD.
- DeVanna K, Bodamer B, Wellington C, Hammer E, Mayer C, Bossenbroek, J. 2011. An alternative hypothesis to invasional meltdown in the Laurentian Great Lakes region: general facilitation by Dreissena. J Great Lakes Res. 37(4):632–641.
- Diggins T, Kaur J, Chakraborti, DePinto, J. 2002. Diet choice by the exotic round goby (Neogobius melanostomus) as influenced by prey motility and environmental complexity. J Great Lakes Res. 28:411–420.
- Djuricich P, Janssen J 2001. Impact of round goby predation on zebra mussel size distribution at Calumet Harbor, Lake Michigan. J Great Lakes Res. 27: 312–318.
- Domske H, Obert E. 2001. Avian botulism in Lake Erie. Great Lakes Rev. 5:1–5.
- Dubs DO, Corkum L. 1996. Behavioral interactions between round gobies (Neogobius melanostomus) and mottled sculpins (Cottus bairdi). J Great Lakes Res. 22(4):838–844.
- Duncan JM, Marschner CA, González, MJ. 2011. Diet partitioning, habitat preferences and behavioral interactions between juvenile yellow perch and round goby in nearshore areas of Lake Erie. J Great Lakes Res. 37:101–110.
- Effler S, Siegfried C. 1998. Tributary water quality feedback from the spread of zebra mussels: Oswego River, New York. J Great Lakes Res. 24:453–463.
- Evans M, Fahnenstiel G, Scavia D. 2011. Incidental oligotrophication of North American Great Lakes. Environ Sci Technol. 45(8):3297–3303.
- Francis JT, Robillard S, Marsden E. 1996. Yellow perch management: a multi-jurisdictional challenge. Fisheries. 21(2):18–20.
- French JRP III, Jude D. 2001. Diet and diet overlap of non-indigenous gobies and small benthic native fishes co-inhabiting the St Clair River, MI. J Great Lakes Res. 7:300–311.
- Greenberg, L. 1991. Habitat use and feeding behavior of thirteen species of benthic stream fishes. Env Biol Fish. 31:389–401.
- Hartman K, Brandt S. 1995. Trophic resource partitioning, diets, and growth of sympatric estuarine predators. T Am Fish Soc. 124:520–537.
- Hensler SR, Jude D. 2007. Diel vertical migration of round goby larvae in the Great Lakes. J Great Lakes Res. 32:295–302.
- Hensler SR, Jude D, He J. 2008. Burbot growth and diets in lakes Michigan and Huron: an ongoing shift from native species to round gobies. In: Paragamian VL and Bennett DH, editors. Burbot: ecology, management, and culture American Fisheries Society, Symposium 59, Bethesda, MD; p. 91–107.
- Hlohowskyj I, White A. 1983. Food resource partitioning and selectivity by the greenside, fantail, and rainbow darters (Pisces: Percidae). Ohio J Sci. 83:201–208.
- Horgan MJ, Mills E. 1999. Zebra mussel filter feeding and food-limited production of Daphnia: recent changes in lower trophic level dynamics of Oneida Lake. Hydrobiologia. 411:79–88.
- Irons KS, McClelland M, Pegg M. 2006. Expansion of round goby in the Illinois waterway. Am Mid Nat. 156:198–200.
- Janssen J, Jude D. 2001. Recruitment failure of mottled sculpin Cottus bairdi in southern Lake Michigan induced by the newly introduced round goby Neogobius melanostomus. J Great Lakes Res. 27:319–328.
- Johnson TB, Bunnell D, Knight C. 2005. A potential new energy pathway in central Lake Erie: the round goby connection. J Great Lakes Res. 31(Suppl. 2):238–251.
- Jude DJ. 2001. Round and tubenose gobies: 10 years with the latest Great Lakes phantom menace. Dreissena 11:1–14.
- Jude DJ, DeBoe S. 1996. Possible impact of gobies and other introduced species on habitat restoration efforts. Can J Fish Aquat Sci. 53(Suppl.1):136–141.
- Jude DJ, Janssen J, Crawford G. 1995. Ecology, distribution, and impact of the newly introduced round and tubenose gobies on the biota of the St Clair and Detroit rivers. In: Munawar M, Edsall T, Leach J, editors. The Lake Huron ecosystem: ecology, fisheries, and management. Amsterdam: Academic Publishing.
- Jude DJ, Reider R, Smith G. 1991. Establishment of Gobiidae in the Great Lakes Basin. Can J Fish Aquat Sci. 49:416–421.
- Keast A, Welsh L. 1968. Daily feeding periodicities, food uptake rates and dietary changes with hour of day in some lake fishes. J Fish Res Board Can. 25:1133–1144.
- Kornis M, Vander Zanden J. 2009. Forecasting the distribution of the invasive round goby (Neogobius melanostomus) in Wisconsin tributaries to Lake Michigan. Can J Fish Aquat Sci. 67:553–562.
- Kornis M, Sharma S, Vander Zanden J. 2013. Invasion success and impact of an invasive fish, round goby, in Great Lakes tributaries. Div Dist. 19:184–198.
- Krakowiak PJ, Pennuto C. 2008. Fish and macroinvertebrate communities in tributary streams of eastern Lake Erie with and without round gobies (Neogobius melanostomus, Pallas 1814). J Great Lakes Res. 34:675–689.
- Kuhns LA, Berg M. 1999. Benthic invertebrate community responses to round goby (Neogobius melanostomus) and zebra mussel (Dreissena polymorpha) invasion in southern Lake Michigan. J Great Lakes Res. 25:910–917.
- Lauer T, Allen P, McComish T. 2004. Changes in mottled sculpin and Johnny darter trawl catches after the appearance of round gobies in the Indiana waters of Lake Michigan. T Am Fish Soc. 133:185–189.
- Lederer A, Massart J, Janssen J. 2006. Impact of round gobies (Neogobius melanostomus) on dreissenids (Dreissena polymoprha and Dreissena bugensis) and the associated macroinvertebrate community across an invasion front. J Great Lakes Res. 32:1–10.
- Leonardi JM. 2004. Holloway Reservoir. Status of the Fishery Resource Report. Available from Michigan Department of Natural Resources, Fisheries Division, Ann Arbor, MI.
- Leonardi JM, Gruhn W. 2001. Flint River Assessment. Special Report 17. Available from Michigan Department of Natural Resources, Fisheries Division, Ann Arbor, MI.
- Malone M. 2016. Early round goby (Neogobius melanostomus) invasion into Lake Michigan tributaries and competitive interactions with two native benthic fishes [M.S. thesis], 62 pp. Loyola University, Chicago (IL).
- Matthews WJ. 1998. Patterns in freshwater fish ecology. New York: Chapman and Hall.
- Mychek-Londer JG, Bunnell DB, Stott W, Diana JS, French JRP, Chriscinske MA. 2013. Using diets to reveal overlap and egg predation among benthivorous fishes in Lake Michigan. T Am Fish Soc. 142:492–504.
- Nalepa T, Hartson DJ, Fanslow D, Lang G, Lozano S. 1998. Declines in benthic macroinvertebrate populations in southern Lake Michigan, 1980-1993. Can J Fish Aquat Sci. 55:2402–2413.
- Ng C, Berg M, Jude D, Janssen J, Charlebois P, Amara L, Gray K. 2008. Chemical amplification in an invaded food web: seasonality and ontogeny in a high biomass, low diversity ecosystem. Environ Toxicol Chem Sci Tech. 27:2186–2195.
- Oksanen F, Blanchet G, Friendly M, Kindt P, Legendre P, McGinn D, Minchin P, O’Hara R, Simpson G, Solymos P, Stevens M, Szoecs E, Wagner H. 2017. vegan: Community Ecology Package. R package version 2.4–2 https://CRAN. R-project.org/package=vegan.
- O’Niell, C, Dextrase, A. 1994. The introduction and spread of the zebra mussel in North America . Proceedings of the 4th Zebra Mussel Conference. Madison (WI): University of Wisconsin Sea Grant.
- Paine M, Dodson J, Power G. 1982. Habitat and food resource partitioning in four species of darters (Percidae: Etheostoma) in a southern Ontario stream. Can J Zool. 60:1635–1641.
- Palmen E. 1955. Diel periodicity of pupal emergence in natural populations of some chironomids. Annales Zoologici Societatis Zoologicae Botanicae Fennicae ‘Vanamo’ 17:1–30.
- Pennuto CM, Krakowiak P, Janik C. 2010. Seasonal abundance, diet, and energy consumption of round gobies (Neogobius melanostomus) in Lake Erie tributary streams. Ecol Freshw Fish. 19:206–215.
- Phillips EC, Washek M, Hertel W, Niebel B. 2003. The round goby (Neogobius melanostomus) in Pennsylvania tributary streams of Lake Erie. J Great Lakes Res. 29 (1):34–40.
- Poos M, Dextrase A, Schwalb N, Ackermann J. 2010. Secondary invasion of the round goby into high diversity Great Lakes tributaries and species at risk hotspots: potential new concerns for endangered freshwater species. Biol Inv. 12:1269–1284.
- Raby G, Gutowsky L, Fox M. 2010. Diet composition and consumption rate in round goby (Neogobius melanostomus) in its expansion phase in the Trent River, Ontario. Environ Bio Fish. 89:143–150.
- Ratti C, Barton D. 2003. Decline in the diversity of benthic invertebrates in the wave-zone of Eastern Lake Erie, 1974–2001. J Great Lakes Res. 29:608–615.
- Reid SM, Mandrak NE. 2008. Historical changes in the distribution of threatened channel darter (Percina copelandi) in Lake Erie with general observations on the beach fish assemblage. J Great Lakes Res. 34:324–333.
- Ross ST. 1991. Mechanisms structuring stream fish assemblages – are there lessons from introduced species? Environ Bio Fish. 30:359–368.
- Schloesser D, Manny B. 2007. Restoration of wild celery, Vallisneria americana, in the lower Detroit River of the Lake Huron-Lake Erie corridor. J Great Lakes Res. 33(Suppl. 1):8–19.
- Schlosser I. 1987. The role of predation in age- and size-related habitat use by stream fishes. Ecology. 8:651–659.
- Schoener T. 1970. Non-synchronous spatial overlap of lizards in patchy habitats. Ecology. 54:408–418.
- Schoener T. 1974. Resource partitioning in ecological communities. Science. 185:27–39.
- Skubinna JP, Coon T, Batterson T. 1995. Increased abundance and depth of submersed macrophytes in response to decreased turbidity in Saginaw Bay, Lake Huron. J Great Lakes Res. 21:476–488.
- Smart HJ, Gee J. 1979. Coexistence and resource partitioning in two species of darters (Percidae), Etheostoma nigrum and Percina maculata. Can J Zool. 57:2061–2071.
- Somers C, Lozer M, Kjoss V, Quinn J. 2003. The invasive round goby (Neogobius melanostomus) in the diet of nestling double-crested cormorants (Phalacrocorax auritus) in Hamilton Harbour, Lake Ontario. J Great Lakes Res. 29:392–399.
- Stauffer Jr. J., Schmars J., Wilson C., Taylor R., Murray C. 2016. Status of round goby and tubenose goby in Pennsylvania. Northeast Nat. 23:395–407.
- Strauss RE. 1979. Reliability estimates for Ivlev’s electivity index, the forage ratio, and a proposed linear index of food selection. T Am Fish Soc. 108:344–352.
- Thomas L. 1970. An ecological study of four darters of the genus Percina (Percidae) in the Kaskasia River, Illinois. Illinois Natural History Survey Biological Survey Notes 70.
- Trautman MB. 1981. The Fishes of Ohio. Columbus (OH): Ohio State University Press.
- Turner C. 1921. Food of the Ohio common darters. Ohio J Sci. 22:41–62.
- Vanderploeg HA, Nalepa T, Jude D, Mills E, Holeck K, Liebig J, Grigorovich I, Ojaveer H. 2002. Dispersal and emerging ecological impacts of Ponto-Caspian species in the Laurentian Great Lakes. Can J Fish Aquat Sci. 59:1209–1228.
- Vâsek M, Veŝtiĉkova L, Roche K, Jurajda P. 2014. Diet of two invading gobiid species (Proterorhinus semilunaris and Neogobius melanostomus) during the breeding and hatching season: no field evidence of extensive predation on fish eggs and fry. Limnologica. 46:31–36.
- Weimer M, Sowinski M. 1999. Diet of the round goby (Neogobius melanostomus) in Lake Erie. Dreissena. 10:7–12.
- Winn, HE. 1958. Comparative reproductive behavior and ecology of fourteen species of darters (Pisces: Percidae). Ecol Monogr. 28:155–192.
- Wynes D, Wissing T. 1982. Resources sharing among darters in an Ohio stream. Am Mid Nat. 107:294–304.
- Yule A, Barker I, Austin J, Moccia R. 2006. Toxicity of Clostridium botulinum type E neurotoxin to Great Lakes fish: implications for avian botulism. J Wildl Dis. 42:479–493.
- Zahn Seegert SE, Rosi-Marshall EJ, Baxter CV, Kennedy TA, Hall, RO Jr, Cross WF. 2014. High diet overlap between native small-bodied fishes and non-native fathead minnow in the Colorado River, Grand Canyon, Arizona. T Am Fish Soc. 143:1072–1083.
