Abstract
Invasive Creole Painted Crayfish (Orconectes palmeri) have spread throughout the Flint River and are currently moving from there into a tributary, Muckalee Creek. The negative behavioral impact of the invasive is a concern for the endemic Muckalee Crayfish (Procambarus gibbus). Tests examining residence, size, and cover effects on aggression demonstrated that O. palmeri is a dominant species in these encounters. This may explain the success of O. palmeri in the Flint River with Procambarus spiculifer, which is a close relative of P. gibbus, and it may also mean the endemic P. gibbus is in danger of extirpation. A survey of the Muckalee showed O. palmeri has already entered the tributary and are likely spreading upstream.
Introduction
Most aquatic ecosystems have several invasive species which can directly affect the environment, and crayfish in particular can create a significant impact (Jackson et al. Citation2014). Orconectes palmeri has not been listed as a common invasive on the level of Procambarus clarkii or Orconectes rusticus (Larson and Olden Citation2010) but has spread extensively in the Flint River (Sargent et al. Citation2011) and here is seen to be an aggressive species. One useful tool with aggressive invasive species is the use of prior residence trials to determine their competitive impact on native species (Lorenz et al. Citation2011).
In October 1999, O. palmeri was collected in the Flint River 18 km south of Albany, GA, USA (Skelton Citation2010). Since then, O. palmeri has established itself throughout the middle and lower Flint River in southwest Georgia, where habitats have been greatly altered due to the production of upstream dams and reduction of water flow (Sargent et al. Citation2011). This species can be found in the Flint River between Lake Seminole and Lake Blackshear, and is a tertiary burrower found in rapid and sluggish freshwater streams (Crandall et al. Citation2001; Hobbs and Lodge Citation2010). O. palmeri creolanus (the likely subspecies introduced) is native to the Lake Pontchartrain drainage in Louisiana, the Pascagoula River system of eastern Mississippi, and the Pearl River system of western Alabama (Penn Citation1957).
O. palmeri has been displacing the native White Tubercled Crayfish (Procambarus spiculifer) along the Flint River and can be considered a potential threat to other native fauna (Sargent et al. Citation2011). In addition, crayfish frequently spread by moving upstream, and because O. palmeri is already established in the Flint, it is just a matter of time before they work their way up into all the tributaries (Sargent et al. Citation2011). Because of this possibility, the threat of O. palmeri to the Muckalee Crayfish (Procambarus gibbus) has become a great concern. P. gibbus is endemic to tributaries of the Flint River (Muckalee Creek and Coolewahee Creek) (Hobbs Citation1981). Recent surveys by Georgia Southwestern State University have shown O. palmeri present 3 miles into the Muckalee Creek system.
We have observed a rough ratio of 25 O. palmeri to every native P. spiculifer at the locations we collected O. palmeri for this experiment (Highway 32 bridge and just south of the Lake Blackshear Dam). This impact is not surprising, given that species of the genus Orconectes are considered aggressive in both intraspecific and interspecific behaviors (Capelli and Munjal Citation1982). P. spiculifer was the dominant crayfish species in surveys before this invasion (Hobbs Citation1981). Crayfish have been shown to competitively exclude natives and increase their predation (Hill and Lodge Citation1999) and we presume that is what is occurring here. There has been no research on the potential interactions of O. palmeri and P. gibbus, so here we examine what could happen as O. palmeri spreads further into the tributaries occupied by P. gibbus.
In this study, we observed interspecific interactions between invasive O. palmeri and native P. gibbus under laboratory conditions to determine the levels of aggression and the amount of time spent near cover for each crayfish. Intraspecific and interspecific trials were designed with both species for comparison purposes. Lastly, to examine habitat effects, residence trials were also performed with one and with multiple sources of cover.
We predict that (1) O. palmeri is more aggressive and will spend more time near cover, regardless of whether it is a resident or an invasive. (2) That there will be more time spent fighting with interspecific competition than intraspecific competition. (3) That size will play a role and smaller O. palmeri will not spend as much time near cover as larger O. palmeri. And (4) That an increase in shelters will decrease aggression as witnessed by a lower fighting time.
Methods and materials
For all studies performed, P. gibbus crayfish were collected from Muckalee Creek, Americus, GA, USA and O. palmeri crayfish were collected from the Flint River, between the Lake Blackshear Dam and Albany, GA, USA. Crayfish were captured using Frabill crayfish traps baited with hot dogs. Any collected individuals missing one or both chelae were not used for the experiment. Crayfish were placed in 416 liter holding pools filtered by an air-driven sponge. Each holding pool had an assortment of rocks, bricks, and pots for shelter. All collected crayfish were identified as O. palmeri or P. gibbus and placed into separate holding tanks by species. The crayfish were fed algae wafers every three days. Both species were very similar in size, ranging from 20 to 46 mm carapace length. The largest O. palmeri was 46 mm and the largest P. gibbus was 45 mm.
Aquarium trials
Each aquarium trial was done in 75-l aquaria with sandy substrate and a 10 cm long section of 5 cm diameter PVC pipe for shelter. Sand from the Georgia Southwestern State University Lake was rinsed 2–3 times initially and used to form a base in each container. The aquarium room was set to a 12 hour day–12 hour night cycle. No crayfish were re-used because they can recognize the social status of other crayfish and can also tell if the opponent has ‘won’ or ‘lost’ previous fights through the use of chemical signaling (Tierney et al. Citation1984; Moore Citation2007). Water changes were also done after every trial because the chemical signals produced by the crayfish can stay in the water for extended periods of time and this could affect crayfish behavior for later trials (Bergman and Moore Citation2003). For each trial, the sponge filter was removed to ensure that there was only one shelter (PVC) available (or more shelters for the multiple shelter treatment). This PVC shelter was randomly placed on one side of the aquarium. The resident for each trial was placed in the trial tank and was given 24 h to acclimate to the PVC and establish a sense of ‘prior residence’. After 24 h of prior residence, the other individual was introduced to the opposite side of the tank. Trials were video recorded for 30 minutes using two digital video cameras, a Sony Handycam (Model #: HDR-XR150), and a JVC Everio Camcorder (Model #: GZ-HM300BU). No humans were allowed in the room to eliminate observer effects. After each trial, crayfish were taken out of the tanks with a net and measured to the nearest millimeter for carapace length and to the nearest 100th of a gram for mass. No crayfish were re-used for any trial and all pairs were size matched (unless otherwise noted) so that they had a carapace length within 10% of each other (crayfish were roughly measured before trials for size matching).
All videos for the interspecific and intraspecific trials were examined for the first 10 minutes to record the amount of time spent fighting and the amount of time each resident and invasive crayfish was within one body length of the shelter (as a measure of holding territory).
20 trials examining intraspecific behavior of O. palmeri
19 trials examining intraspecific behavior of P. gibbus
20 trials examining interspecific behavior, P. gibbus as resident
20 trials examining interspecific behavior, P. gibbus as larger resident (10%–30% larger carapace)
14 trials examining interspecific behavior, O. palmeri as resident
20 trials examining interspecific behavior, P gibbus as resident (multiple shelters)
The only difference with the multiple shelter treatment was the addition of more PVC pipe pieces for shelter. An apparatus containing five different-sized PVC pipes was added to simulate a more complex, natural environment. The apparatus was constructed by cutting five PVC pipes (with diameters of 2.54 cm, 3.175 cm, 3.81 cm, 5.08 cm, and 7.62 cm) into 10 cm segments. The tubes were then arranged randomly side by side with their openings facing one direction and glued to each other using nontoxic silicone. After 24 hours of letting the silicone dry, they were lowered into one corner of the tank with the openings of each shelter viewable from the video camera. Data from the intra-versus interspecific differences were normal and were tested with parametric tests (t-tests) performed on Systat. The multiple cover data was not normally distributed and was tested by a Mann-Whitley U test.
Results
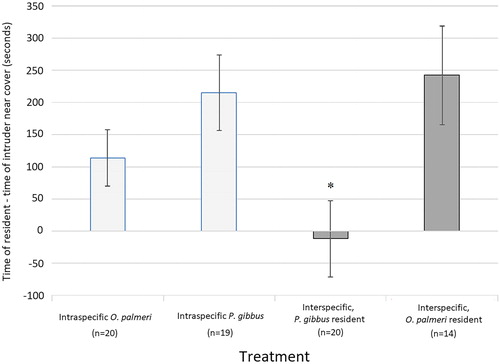
Intraspecific trials showed both species to be more successful at holding territory when residents, and both species were not significantly different from each other in this aspect (one-tailed t test, t(34) = −1.409, p = 0.084). For both species, residents held territory for more seconds than the intruders (an average of 215.16 seconds longer for resident P. gibbus and 114.05 seconds more for resident O. palmeri).
Interspecific trials differed depending on which crayfish species was the resident. When P. gibbus was the resident, it held territory for a relatively shorter time (−11.85 seconds ‘more’ than intruding O. palmeri) than when P. gibbus was a resident in the intraspecific trials (one-tailed t test, t(27) = −2.602, p = 0.007). When O. palmeri was the resident, it held territory for a similar time (242.36 seconds) to the residents of both intraspecific trials and for a significantly longer time than P. gibbus as an interspecific resident (one-tailed t test, t(37) = −2.750, p = 0.004) ().
Figure 1. This shows the differences in the time of the resident – the time of the intruder for time spent near cover. P. gibbus spent less time at cover in both interspecific trials as compared to the intraspecific trial with other P. gibbus. * denotes significance of p < 0.05.
Interspecific trials also differed from intraspecific trials with amount of time spent fighting. For intraspecific trials, P. gibbus spent 71.8 seconds fighting and O. palmeri spent 57.5 seconds fighting. For interspecific trials the amount of fighting was 131.8 seconds with P. gibbus as the resident and 128.5 seconds with O. palmeri as the resident. The only significant result in these comparisons, however, was that there was less time fighting with intraspecific O. palmeri when compared to the amount of time fighting when O. palmeri was invading resident P. gibbus (one-tailed t test, t(28) = −1.733, p = 0.047).
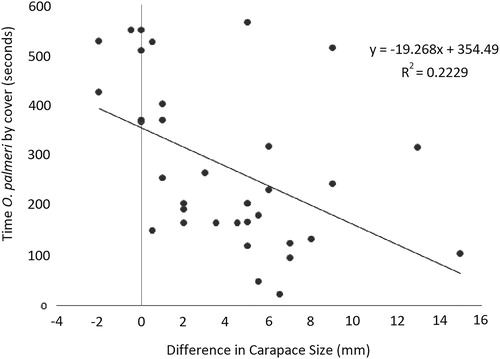
Smaller invading O. palmeri fought less and held territory less when compared to invading O. palmeri that were matched for size with P. gibbus residents. The time spent fighting was not significantly different (p = 0.068). The differences in the time near cover was best shown as a negative relationship between time near cover and invading O. palmeri size difference compared to resident P. gibbus (R2 = 0.2229, p = 0.004) ().
Figure 2. Linear regression examining relationship between invader size and time spent at cover. There is a negative relationship, with smaller O. palmeri spending less time at cover then larger O. palmeri.
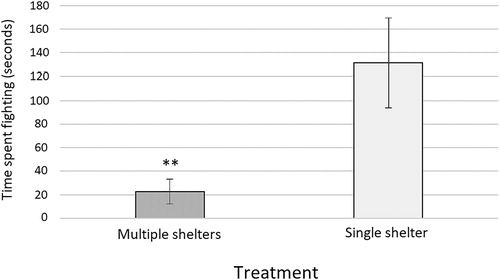
There was decreased aggression with multiple shelters. The PVC pipes took up most of the aquarium and both species ended up having large times for time spent near cover (551.2 seconds for O. palmeri and 427.9 seconds for P. gibbus). The amount of time spent fighting was less than the invading O. palmeri trials with one cover (22.6 seconds fighting versus 131.8 seconds), Mann-Whitney U test (U = 308.5, p = 0.001236) ().
Discussion
There was a clear aggressive interaction between the species with a competitive advantage of O. palmeri over P. gibbus. Our hypothesis of O. palmeri spending more time near cover was demonstrated in multiple instances. The only time where P. gibbus spent significantly more time than its partner in an experiment was when the other crayfish was P. gibbus. If O. palmeri was present, it held the territory longer than P. gibbus, whether it was the resident or the invader (). Our hypothesis of time spent fighting was weakly supported by data that was barely significant or almost significant, interspecific trials involved more fighting than intraspecific trials. Our hypothesis on size being a factor was also supported. There was a clear effect of size difference and time spent near cover for O. palmeri. Smaller O. palmeri spent less time at cover (). Lastly, the effect of multiple shelters was a supported hypothesis, with single cover trials having an average of two more minutes of fighting than multiple covers ().
In addition to being successful invaders, O. palmeri also held the territory well once they were residents. This sets up a scenario where O. palmeri not only successfully takes the territory, but does not relinquish it. This could be an explanation for the displacement of P. spiculifer in the Flint River and could be disastrous for P. gibbus in Muckalee Creek. Other successful invasions have shown similar results of aggressive invaders. P. clarkii actually did not prefer shelters but actively prevented native and similarly sized P. acutus from using shelter (Gherardi and Daniels Citation2004). Nakata and Goshima (Citation2003) observed invasive Pacifastacus leniusculus to be superior residents when defending territory against native Cambaroides japonicus, and size was seen as an overriding factor there as it was here.
More time spent fighting and not holding habitat may have negative influences on survival. Fighting also uses energy that could be used for growth and reproduction and also incurs the cost of potential injury. These costs have actually been observed in crustaceans (Hobbs Citation2016) and clearly indicate the value of the territory to the individual that is willing to incur these costs. The value of cover for crayfish may be for feeding or to avoid predation, as they are prey for a variety of species.
Habitat complexity in part addresses this, making each object of cover less valuable when they outnumber the crayfish involved. This is possibly a more natural perspective on the nature of these aggressive interactions and potential management (Bergman and Moore Citation2003). It is not surprising that we found less fighting and more territory holding for these species when there was more habitat. But it does indicate the battles are most likely for limited habitats.
This invasive O. palmeri has had clear negative interactions with P. spiculifer in the Flint River (Sargent et al. Citation2011). Now O. palmeri is found a few miles into Muckalee Creek from the Flint River populations (as seen in surveys done by Georgia Southwestern University researchers in 2015). This is a major concern because, unlike the crayfish species of the Flint River, P. gibbus has a very limited range and is a state threatened species already just because of this.
Reducing crayfish numbers is possible, with extirpation nearly impossible (Gherardi et al. Citation2011). Physical collection of invasive crayfish (Hein et al. Citation2007), monitoring or management of species near barriers/dams (Kerby et al. Citation2005), and even habitat and predator management may aid in reducing the impact of this species and the potential extinction of the endemic P. gibbus.
Notes on contributors
O. Thomas Lorenz Tom Lorenz is an associate professor of biology at Georgia Southwestern State University, where he conducts research on invasive species and behavior in crayfishes, reptiles, and fishes.
Elizabeth Craddock graduated from Georgia Southwestern State University and continued to do crayfish research at Georgia Southern University.
Cameron Baxter graduated from Georgia Southwestern State University and is now a Naval Aviator.
Alejandra Palacio completed her B.Sc. from Georgia Southwestern State University and is now a Navy Officer.
Acknowledgments
Support and animal housing for these studies was provided by Georgia Southwestern State University (GSW). Student advising was provided by Dr Ian Brown and the equipment was in part provided by the biology department of GSW and by the Cofer Fund at GSW.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bergman DA, Moore PA. 2003. Field observations of intraspecific agonistic behavior of two crayfish species, Orconectes rusticus and Orconectes virilis, in different habitats. Biol Bull. 205:26–35.
- Capelli GM, Munjal BL. 1982. Aggressive interactions and resource competition in relation to species displacement among crayfish of the genus Orconectes. J Crustacean Biol. 2:486–492.
- Crandall, KA, Fetzner JW, Hobbs HH. 2001. Orconectes (Buannulifictus) palmeri creolanus Creaser 1933. Gainesville, FL: USGS Nonindigenous Aquatic Species Database.
- Gherardi F, Daniels WH. 2004. Agonism and shelter competition between invasive and indigenous crayfish species. Can J Zool. 82:1923–1932.
- Gherardi F, Aquiloni L, Diéguez-Uribeondo J, Tricarico E. 2011. Managing invasive crayfish: is there a hope? Aquat Sci. 73:185–200.
- Hein CL, Zanden MJV, Magnuson JJ. 2007. Intensive trapping and increased fish predation cause massive population decline of an invasive crayfish. Freshwater Biol. 52:1134–1146.
- Hill AM, Lodge DM. 1999. Replacement of resident crayfishes by an exotic crayfish: the roles of competition and predation. Ecol Appl. 9:678.
- Hobbs HH. 1981. The crayfishes of Georgia. Smithson Contr Zool. 318:1–549.
- Hobbs H, Lodge D. 2010. Decapoda. In: Thorp JH, Covich AP, editor(s). Ecology and classification of North American freshwater invertebrates. New York: Academic Press; p. 901–967.
- Hobbs, NVS. 2016. Agonistic behavior and costs of aggression in decapod crustaceans. Kingston, RI: University of Rhode Island.
- Jackson MC, Jones T, Milligan M, Sheath D, Taylor J, Ellis A, England J, Grey J. 2014. Niche differentiation among invasive crayfish and their impacts on ecosystem structure and functioning. Freshwater Biol. 59:1123–1135.
- Kerby JL, Riley SP, Kats LB, Wilson P. 2005. Barriers and flow as limiting factors in the spread of an invasive crayfish (Procambarus clarkii) in southern California streams. Biol Conserv. 126:402–409.
- Larson ER, Olden JD. 2010. Latent extinction and invasion risk of crayfishes in the Southeastern United States. Conserv. Biol. 24:1099–1110.
- Lorenz OT, O’Connell MT, Schofield PJ. 2011. Aggressive interactions between the invasive Rio Grande cichlid (Herichthys cyanoguttatus) and native bluegill (Lepomis macrochirus), with notes on redspotted sunfish (Lepomis miniatus). J Ethol. 29:39–46.
- Moore PA. 2007. Agonistic behavior in freshwater crayfish. In Duffy JE, Thiel M, editors. Evolutionary ecology of social and sexual systems. Oxford: Oxford University Press, p. 90–114.
- Nakata K, Goshima S. 2003. Competition for shelter of preferred sizes between the native crayfish species Cambaroides japonicus and the alien crayfish species Pacifastacus leniusculus in Japan in relation to prior residence, sex difference, and body size. J Crustacean Biol. 23:897–907.
- Penn, GH. 1957. Variation and subspecies of the crawfish Orconectes palmeri. Tulane Stud Zool, 5(10): 231–262, figures 1–30.
- Sargent LW, Golladay SW, Covich AP, Opsahl SP. 2011. Physicochemical habitat association of a native and a non-native crayfish in the lower Flint river, Georgia: implications for invasion success. Biol Invasions. 13:499–511.
- Skelton CE. 2010. History, status, and conservation of Georgia crayfishes. Southeas Nat. 9:127–138.
- Tierney AJ, Thompson CS, Dunham DW. 1984. Site of pheromone reception in the crayfish Orconectes propinquus (Decapoda, Cambaridae). J Crustacean Biol. 4:554–559.