Abstract
Environmental changes are generally known to influence the distribution and abundance of schistosome intermediate host snails (IHs). However, the influence of hydrologic changes per ser on the length of survival of schistosome IHs is not fully understood. To explore how desiccation may influence the survival of Bulinus globosus, the main IHs of Schistosoma haematobium in southern Africa, we conducted a study under laboratory conditions where snails were subjected to periods of desiccation and their survival evaluated. Desiccation period from 28 to 49 days post-draining of water was associated with an increase in mortality of 33.2 and 42.4% in large (mean shell height 7.81 ± 0.44 mm) and small (mean shell height 5.94 ± 0.68 mm) B. globosus snails, respectively. Although the duration of desiccation had no effect on the depth of burrowing, large size snails burrowed deeper into the soil than small size snails. The LT50 and LT90 of snails designated as large (7.81 ± 0.44 mm) were 73.35 ± 10.32 and 110.61 ± 21.03 days, respectively. On the other hand, LT50 and LT90 for snails designated as small (5.94 ± 0.68 mm) were 59.64 ± 8.56 and 84.19 ± 12.09 days, respectively. The survival of B. globosus during desiccation depended on the size/age of the snail where large size snails aestivate and survive for a longer period by burrowing deeper into the soil. We therefore conclude that adult B. globosus may play a significant role in habitat recolonization after a period of drought which is a common phenomenon in schistosomiasis endemic areas when population crashes.
Introduction
Schistosoma intermediate host snails (IHs) are vulnerable to climatic changes (Yang et al. Citation2007; Morley and Lewis Citation2013). Given their role in schistosomiasis transmission, it is important to evaluate their responses to predicted future changes in aquatic habitats due to climate change. Furthermore, increased anthropogenic activities may negatively affect aquatic and freshwater ecosystems (Lytle and Poff Citation2004; Tisseuil et al. Citation2012) resulting in altered functional diversity, integrity of the freshwater ecosystems (Konar et al. Citation2013) and abundance of freshwater organisms (Kingsford Citation2011; Lund et al. Citation2016). Increasing evidence also suggest that differences in the response of IHs to climate change may lead to changes in their interspecific and timing of interactions (Paull et al. Citation2012), distribution and abundance (Zhou et al. Citation2008; Stensgaard et al. Citation2013). Therefore, it is important to investigate the adaptation strategies of IHs to abiotic stresses such as water reduction and desiccation in order to predict their future potential responses to climate change.
Bulinus globosus (Gastropoda: Planorbidae) is the major IH snail of Schistosoma haematobium in southern Africa (Appleton and Madsen Citation2012). The snail is aquatic and breeds in fresh water habitats and can reproduce through selfing or outcrosing depending on the environmental conditions (Jarne et al. Citation1992). Hence, Bulinus globosus is considered to have a high biotic potential and can breed throughout the year in aquatic environments (O'keeffe Citation1985). Its rapid growth and re-population rate of habitats even after population crashes due to drought (Harrison and Shiff Citation1966; O'keeffe Citation1985; Marti Citation1986) makes it critical to gather information on the behavior of the specie during desiccation in search of strategies to enhance the effectiveness of vector-control in schistosomiasis control programs.
Despite considerable efforts to control schistosomiasis, changes in climate and land use practices have led to the creation of more suitable habitats for IHs with subsequent increase in disease transmission (Yang et al. Citation2007; Zhou et al. Citation2008; McCreesh et al. Citation2015; Stanton et al. Citation2017; Yigezu et al. Citation2018). In addition, experimental (Paull and Johnson Citation2011; Kalinda et al. Citation2017a), field studies (Marti Citation1986), and predictive models (Manyangadze et al. Citation2016b; Kalinda et al. Citation2018) concluded that a rise in temperature may lead to an increase in snail fecundity and hence abundance. This may in-turn increase the risks of transmission of schistosomiasis due to the abundance of IHs. Nevertheless, efforts are being advocated to significantly reduce morbidity due to schistosomiasis through mass drug administration programmes (MDA) (Fenwick et al. Citation2009; Wang et al. Citation2009).
In the climate change-schistosomiasis theory, much emphasis has been placed on the effect of temperature on IHs (Yang et al. Citation2007; Kalinda et al. Citation2017a, Citation2017b) and disease dynamics (Stensgaard et al. Citation2013; McCreesh et al. Citation2015; Ngarakana-Gwasira et al. Citation2016), however, studies on the effect of abiotic disturbances such as desiccation through long term and seasonal droughts are limited. Ephemeral rivers and seasonal water ponds which are the common habitat for IHs of Schistosoma parasites are subject to water level alterations and drying due to normal seasonal cycles, climatic and eco-hydrologic factors (Darby et al. Citation2008; Whitehead et al. Citation2009). Furthermore, hydro-ecological models have also predicted a potential increase in the frequency and intensity of droughts (Van Vliet and Zwolsman Citation2008). This may have long-term implications on the population structure and abundance of IHs of parasites of medical and veterinary importance (Woolhouse and Taylor Citation1990; Bavia et al. Citation1999).
In the context of predicted climate shifts, correctly identifying the mechanisms through which desiccation and resumption of favorable conditions may affect IHs will improve the accuracy of predicting the potential impact of climate change on schistosomiasis. Process-based experiments may be essential in providing significant cues on the potential impact of desiccation on freshwater snails. This will significantly contribute to critical knowledge needed for an integrated approach to eradicate schistosomiasis and other snail-borne diseases, especially in sub-Saharan Africa where snails are now found in habitats initially thought to be unfavorable (Stanton et al. Citation2017). In view of this, the present study examined the survival of Bulinus globosus during substratum drying under laboratory simulated desiccation conditions.
Methods
Breeding of experimental animals
Bulinus globosus snails were collected from a habitat in Ingwavuma (latitude: −26.9965, longitude: 32.27257) in uMkhanyakude district of KwaZulu-Natal province of South Africa (Manyangadze et al. Citation2016b; Kalinda et al. Citation2017a). Snails were allowed to breed to give F1 generation snails that were used in the study. To create two distinct age groups, the laying of egg masses was staggered by creating a three 3 weeks age difference. This allowed us to have two groups of snails with age difference of 3 weeks. Snails were allocated to experimental jars when the young snails were 4 weeks old (average shell height of 4.03 ± 0.56 mm) and the large snails were 7 weeks old (average shell height of 6.32 ± 0.71 mm). The F1 generation was maintained in 2 L cubical plastic aquaria (size: 18 × 18 × 10) cm3 which were filled with filtered pond water and maintained at room temperature (24.0–25.0 °C). The snails were fed ad libitum on blanched lettuce and Tetramin tropical fish food (Tetra®). The experimental room had a photoperiod of 12-hour light/12-hour dark.
Experimental design
Snails were assigned to two groups; large size snails comprised of 7 weeks old F1 generation and small snails comprised of 4 weeks old young F1 generation. This was purposely designed to evaluate the influence of snail size/age on survival during desiccation. Four hundred and eighty snails split in two groups (experimental and control groups) were randomly allocated to 48 2-L cubical plastic aquaria, with each aquarium containing 10 snails. Two control groups with the same shell height range and age as the experimental groups were also included. Each aquarium was filled with a 5 cm layer of soil (to give sufficient depth for burrowing) collected from a natural breeding site of B. globosus (Manyangadze et al. Citation2016a; Kalinda et al. Citation2017a). Prior to its use, the soil was washed in hot boiling water to kill any organisms within it and a composite sample was profiled using the sedimentation method. Before snails were added to the experimental 2-L cubical plastic aquaria, each aquarium was filled with 400 mL of filtered pond water until the soil was saturated and the water level was 4 cm above the surface of the soil. After 48 hrs, B. globosus F1 generation snails from each group were randomly allocated to each aquarium. The snails were acclimatized to the new environment for 7 days (Poznańska et al. Citation2015) before dewatering was initiated ().
Figure 1. The timeline of experimental events; (A) acclimatization period, (B) water draining period to induce aestivation, (C) desiccation period to allow soil to dry and (D) desiccation endpoints at which snail survival was assessed in selected aquaria.
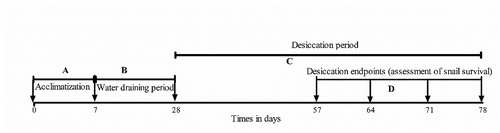
On day 8 after acclimatization, water was gradually drained from the aquaria of the experimental groups only. Three times a week (every after 2 days), 30 mL (cumulatively, 90 mL a week) of water was drained from each experimental aquarium using a graduated pipette (Koprivnikar et al. Citation2014; Poznańska et al. Citation2017). This process was done for a period of 3 weeks. In this period, 270 ml of water was drained from each aquarium. Feeding of snails in the experimental groups was stopped at day 21 from the beginning of the experiment while snails in the control group continued to be fed ad libitum. After water was drained in the experimental group, the soil was left to dry whereas water continued to be changed twice a week in the control group.
Measurement of water parameters, snail parameters and soil moisture
Water temperature, pH and conductivity in the experimental and control groups were monitored throughout the study period using the Hanna thermometer (Hanna Instruments HI 98129 PH/EC/TDS/°C Combo pocket instrument). The measurement of water quality parameters was stopped when the water dried off in the experimental group.
The study pre-determined the desiccation endpoints to be at day 57, 64, 71, and 78 (Betterton et al. Citation1988; Poznańska et al. Citation2015). At each desiccation endpoint (), 12 aquaria were randomly selected to determine snail survival and revival. This included six aquaria from the experimental and six aquaria from the control groups. For the experimental aquaria, we carefully dug through the soil layers using a spatula in order to retrieve the snails buried in the soil without damaging their shells. The height of soil in each aquarium had been marked in order to distinctly determine the depth burrowing. Furthermore, the drying soil was carefully removed along its developing cracks to avoid damaging the shells of any snails. Since most snails would be attached to the soil at the time of digging, the undisturbed soils were collected, and the depth taken depending on the locations of the snails which were often visible. The depth at which each individual snail within the aquaria was found was measured and recorded. This was done at each desiccation endpoint () and the shell height of the snails was also measured.
We checked snail survival in the control experiments daily while in the experimental aquaria, this was done at each desiccation endpoint (). To determine the status of snails dug at each desiccation endpoint (whether dead or alive), each snail was put in a small cup which had been filled with filtered pond water. The snails were then observed for three hours. Snails that opened their opercula with extension of their soft bodies were recorded as ‘alive’ and those that did not were recorded as ‘dead’.
Determination of soil moisture was done using the gravimetric method (Reynolds Citation1970) at each desiccation endpoint. For the control aquaria, water was decanted from the aquaria and muddy soil was collected for determination of moisture content. For the soil from experimental aquaria, drying soil samples were also collected and three samples of 50 g of soil from each aquarium were oven dried at 105 °C in an incubator (EcoTherm Labotec) for 24 hours. After oven-drying, the soil was re-measured to obtain the soil water content. The experiment was run until day 79 when it was terminated. Although snails in the control aquaria were still alive, all the snails in experimental aquaria had died and hence it was not possible to make comparisons with survival of the control snails.
Data analysis
We summarized the water quality parameters as means and standard deviation. We also compared shell height of the snails among groups at each desiccation endpoint using a one-way analysis of variance (ANOVA) after carrying out parametric tests of normality and homogeneity of variance to satisfy ANOVA assumptions.
A generalized linear model with a Poisson link function was used to determine the influence of desiccation time on the number of snails that died after determining the number of snails that had resuscitated at each desiccation endpoint. The number of snails that died during the course of the experiment and those that were resuscitated was expressed as a proportion.
Probit regression model was also used to determine the lethal time (LT) of snail mortality of 50% (LT50) and 90% (LT90) for both large and small snails using the R package Mass (Ripley Citation2015). Data analysis was done using R 3.4.2 version (R Core Team. R: A language and environment for statistical computing, 2013).
Results
Water quality parameters
The soil that was used comprised 42% sand, 44% silt, and 14% clay. Water temperature (±SD) in the four treatments ranged from 23.02 ± 0.12 °C to 23.12 ± 0.03 °C while pH was generally neutral (7.58 ± 0.05 to 7.59 ± 0.01) in all experimental groups including the control. The mean total dissolved solids ranged from 604.7 ± 70.64 to 851.9 ± 20.81 604 in all experimental groups including the control.
Snail growth
Length of desiccation had no effect on the shell height of both control and experimental snails (F3,32=2.84, p = 0.0536). At desiccation endpoints day 57 and 78, the mean (±SD) shell height of small size control group was 6.50 ± 0.65 and 7.71 ± 0.72 mm, respectively. On the other hand, shell height of snails in the small size experimental group at desiccation endpoints day 57 and 78 was 5.27 ± 0.52 and 5.98 ± 0.56 mm, respectively (). Significant differences in the height of snails was observed at desiccation endpoints day 64 (t = 3.06 p = 0.028) and 78 (t = 2.72, p = 0.042). The interaction between initial size of the snail and desiccation time led to no significant difference in the growth of snails in the small size control and experimental groups (F3,16=0.48, p = 0.699).
Figure 2. Cumulative shell height of B. globosus snails at different sampling points (desiccation end points). (A) = Shell height of experimental small snails and control at day 7 (initial measurements) and day 57, 64, 71, and 78 (Desiccation endpoints) of the experiment. (B) = Shell height of experimental large snails and control at day 7 (initial measurements) and day 57, 64, 71, and 78 (Desiccation endpoints) of the experiment.
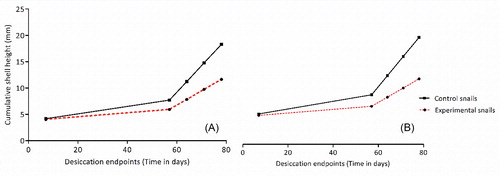
The shell height of snails in the large size control group at desiccation endpoints day 57 and 78 was 8.90 ± 0.41 and 10.3 ± 0.69 mm, respectively. The shell length of snails in the experimental group at desiccation endpoints day 57 and 78 was 7.8 ± 0.35 and 7.9 ± 0.44 mm, respectively (). The effect of the interaction between the initial size of the snail and desiccation time had no effect on the growth of snails in the large size control and experimental groups (F3,16=3.03, p = 0.0653). Nevertheless, significant differences in the shell height of snails was observed at desiccation endpoints day 64 (t = 3.05, p = 0.0379), 71 (t = 2.89, p = 0.044) and 78 (t = 3.40, p = 0.0272).
Snail size and depth of burrowing into the soil
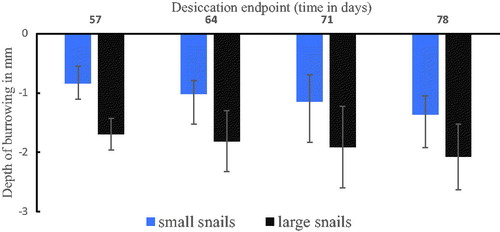
We found a significant size effect on the depth of burrowing (F1,16=26.08, p = 0.001) such that large size snails burrowed deeper compared to small size snails (). It was further observed that length of desiccation (F3,16=1.91, p = 0.168) and the interaction between snail size and length of desiccation (F3,16=1.01, p = 0.417) had no effect on depth of burrowing. On average, large size snails sampled on desiccation endpoints day 57 and 78 burrowed to the depth of 1.69 ± 0.27 and 2.09 ± 0.55 cm below the soil surface, respectively. On the other hand, small size snails sampled at desiccation endpoints day 57 and 78 burrowed to 0.84 ± 0.23 mm and 1.37 ± 0.33 cm below the soil surface.
Figure 3. Depth of burrowing by large and small B. globosus snails at different desiccation periods.
There was a reduction in the amount of moisture content in the soil as the desiccation period increased (). The number of snails that were resuscitated decreased with an increase in desiccation period (Coeff: −0.0338, z = −2.71, p = 0.007; ). Furthermore, snail mortality increased linearly with an increase in the length of desiccation (Coeff: 0.0314, z = 3.52, p < 0.001; ).
Figure 4. Soil moisture content measured at different time points (A) proportion of B. globosus snails that resuscitated at different desiccation time points (B) proportion of snails that died at different desiccation time points (C).
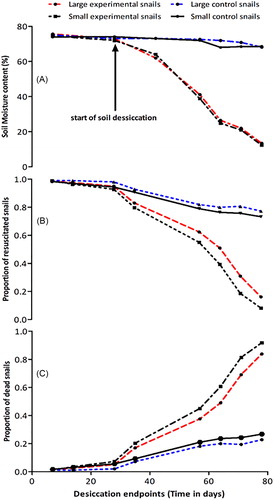
At day 57 and 78 of desiccation, the proportion of large experimental snails that had died was 0.341 (95% CI: 0.066–0.786) and 0.839 (95% CI: 0.399–1.219), respectively while 0.463 (95% CI: 0.183–1.066) and 0.918 (95% CI: 0.363–1.446) small experimental snails had died at day 57 and 78, respectively. On the other hand, 0.181 (95% CI 0.156–0.317) and 0.228 (95% CI: 0.193–0.722) control large size snails had died by 57 and 78, respectively. For small size control snails, 0.210 (95% CI: 0.112–0.604) and 0.268 (95% CI: 0.142–0.836) had died by day 57 and 78, respectively (, ).
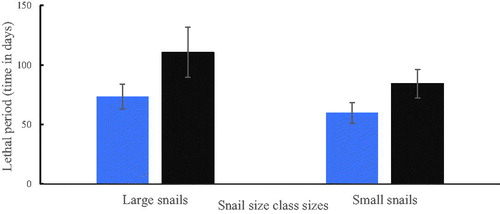
Results show that large size snails were more resilient to desiccation than small size snails (). The lethal time (LT) at which 50% (LT50) of the experimental large size snails died was 73.35 ± 10.32 days while it was 59.64 ± 8.56 days for the small snails. Furthermore, LT90 for large size snails was 110.61 ± 21.03 days while it was 84.19 ± 12.09 days for the small size snails.
Discussion
This study was designed to mimic conditions to which snails are exposed to in their natural habitats. It was observed that B. globosus was tolerant to drying conditions and capable of surviving and resuscitation after being exposed to desiccating conditions for 50 days. The observed resilience of these snails to drying and their eventual resuscitation at the resumption of favorable conditions may potentially lead to repopulating habitats. These findings are in consonant with the conclusions made by Harrison and Shiff (Citation1966) who suggested that B. globosus can rapidly colonize habitats and re-build its population density following resumption of favorable conditions. The study further showed that soil moisture is an important determinant of snail survival during desiccations. According to Stubbington and Datry (Citation2013), IHs can remain alive in the soil as long as it remains moist. The results from the current study, in a broader sense, agree with earlier studies done by Shiff (Citation1960) and Woolhouse and Taylor (Citation1990). Snail size was associated with high tolerance to desiccation and reduced mortality. Although earlier studies had exposed snails to damp mud/soil (Shiff Citation1960; Chu et al. Citation1967; Woolhouse and Taylor Citation1990) to evaluate their resistance to desiccation, the inclusion of water and its gradual reduction in this study allowed us to explicitly observe the response of B. globosus snails to progressive desiccation.
Both large and small size snails burrowed into the soil, nevertheless, large size B. globosus snails burrowed deeper in search of moisture and this increased its survival. According to Poznańska et al. (Citation2015), IHs burrow into the soil as they follow the decreasing water levels and this increases chances of survival. Furthermore, the current study indicates that soil moisture content is a vital factor determining the length of snail survival and the number of snails that resuscitate. Our findings are corroborated by Barlow (Citation1935) and Betterton et al. (Citation1988) who suggested that desiccation rate affects both the survival and resuscitation of snails negatively. In the current study, the differences in the number of snails that resuscitated between the two groups may be particularly important in the repopulation of snail habitats. Large size snails with the shell height of 8 mm burrowed deeper into the soil. This collaborates with the finding of Betterton et al. (Citation1988) who found that B. globosus with the shell height of about 9 mm burrowed deeper into the soils. Furthermore, Hira (Citation1968) also observed that the survival of B. globosus was higher for snails with the shell height of 8.1–12.9 mm. On the other hand, Chu et al. (Citation1967) indicated that Bulinus truncatus snails with the shell height of 4–8 mm survived far longer than those with the shell height of 8–12 mm. Collectively, our results suggest that tolerance to desiccation may be strongly associated with the size or age of the snail at the time of desiccation. Furthermore, our results suggest that adult B. globosus may play a significant role in habitat recolonization after a period of drought which is a common phenomenon in schistosomiasis endemic areas when population crushes.
Although studies focusing on the desiccation capacity and burrowing of IHs have largely been neglected, their importance may increase as the need to incorporate the ecology of snails in schistosomiasis control programs rise. Our study found that the survival of snails reduced linearly with an increase in desiccation time. The proportion of snails that may resuscitate at the end of the desiccation period may directly be influenced by the duration of the desiccation period. According to Paraense (Citation1975), desiccation period longer than five months led to a complete eradication of Biomphalaria glabrate. On the other hand, Woolhouse and Taylor (Citation1990) observed that B. globosus was resilient to desiccation. This may have been enhanced by its ability burrow into the soil (Rubaba et al. Citation2016). A study by Sturrock (Citation1970) further suggested that desiccation can reduce the population density of IHs and alter the age structure of resuscitating snails. Furthermore, Whitehead et al. (Citation2009) suggested that desiccation at schistosomiasis transmission sites disrupts the population build-up of snails and increase snail mortality thus reduce the risks of schistosomiasis transmission. This process had earlier been used in Zimbabwe by Chandiwana et al. (Citation1988) who demonstrated that effective management of water in irrigation schemes could reduce snail population. Results from the current study and studies done by others demonstrate the importance of manipulating snail habitats in combination with MDA for increased success of reducing the prevalence of schistosomiasis.
Our study provides insights into the relationship between snail survival, population extinctions, and recolonization of habitats. Field and experimental work has shown that the availability and persistence of water at transmission sites may increase snail population and potentially increase transmission of schistosomiasis. For example, water projects such as irrigation schemes were observed to enhance the creation of suitable site for IHs (Yang et al. Citation2005, Steinmann et al. Citation2006), increasing the potential risk of transmission of schistosomiasis. Our data showed that a reduction in soil moisture led to an increase in snail mortality. Predictive models have continued to project altered rainfall and water availability in certain parts of sub-Saharan Africa where B. globosus is the main IHs of S. haematobium. Results from our study indicate that such changes will be vital in the recolonization of snail breeding sites. Recolonization of habitats by IHs after resumption of favorable conditions such as water availability is consistent with results from other field experiments (Betterton et al. Citation1988; Manyangadze et al. Citation2016b). Bulinus globosus is a highly fecund snail (Marti Citation1986; Kalinda et al. Citation2017a) and rapidly recovers to populate habitats quickly (Harrison and Shiff Citation1966).
The current study focused on the potential influence of habitat change through water reduction on B. globosus population dynamics in terms of mortality and recovery. Burrowing and snail size played an important role in the survival of B. globosus snails. Increased snail survival especially at transmission sites increases the probability of recolonization and the risks of schistosomiasis transmission. Taking B. globosus as a model snail in the fight against schistosomiasis, increased duration of desiccation and potentially application of molluscicides at the inception of rains may further reduce snail population size and habitat recolonization.
Ethics approval
All experimental protocols and procedures of this study were reviewed and approved by the Animals Ethics Committee of the University of KwaZulu-Natal (UKZN) (Ref: REC/052/017PD) in accordance with the South African national guidelines on animal care, handling and use for biomedical research.
Availability of data and material
The data supporting the writing of this manuscript can be accessed from the project Centre of the Tackling Infection to Benefit Africa (TIBA), University of KwaZulu-Natal. Data can be requested by following the guidelines laid out in the Data Access Policy of the University of KwaZulu-Natal.
Authors' contributions
KC, SM, and MJC conceptualized the study. KC and PM performed the experiments and collected the data. KC analyzed the data and wrote the manuscript. MJC and SM read and edited the manuscript. All the authors approved the final version and agree to be accountable for any aspects of the work.
Acknowledgments
This research was commissioned by the National Institute of Health Research using Official Development Assistance (ODA) funding. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute of Health Research, or the Department of Health. The College of Health Sciences, University of KwaZulu-Natal co-funded the study through the postdoctoral fellowship that was awarded to Chester Kalinda. The authors also acknowledge the precious inputs from the editors and anonymous reviewers who helped improve the content and quality of the paper.
Disclosure Statement
The authors declare that they have no competing interests
Additional information
Funding
Notes on contributors
Chester Kalinda
Chester Kalinda has a PhD degree Public Health from the University of KwaZulu-Natal in Durban, South Africa. He is broadly interested in studying how cross-ecosystem interactions may influence infectious disease risk. Much of his research focuses on how climate change may affect intermediate hosts and vectors and transmission of parasites especially those of neglected tropical diseases.
Moses J. Chimbari
Moses Chimbari is a Professor of Public Health at the University of KwaZulu-Natal. He is also currently serving as the Deputy Director of the project called Tackling Infection to Benefit Africa (TIBA) and the vice president of ecohealth international. He has expertise in climate change, freshwater ecology, entomology and limnology. His research interests focus on neglected tropical diseases (Schistosomiasis and Soil Transmitted Helminths) and Malaria.
Mokgadi P. Malatji
Mokgadi P. Malatji is a PhD student at the University of KwaZulu-Natal. Her research focuses on host-parasite interactions and how these interactions influence infectious disease risks. Her research interests also include molecular biology; medical and veterinary parasitology and tropical diseases Currently, she is studying the taxonomy of Lymnaeidae species and Fasciola species.
Samson Mukaratirwa
Samson Mukaratirwa is a Professor of Parasitology at the University of KwaZulu-Natal. He is an expert in Neglected Parasitic Zoonoses. Besides focusing on parasitic diseases, Prof Mukaratirwa also strives to understand the ecology, pathogenesis and pathophysiology of mono and co-infections of various infections affecting the resource-poor communities in Africa.
References
- Appleton C, Madsen H. 2012. Human schistosomiasis in wetlands in southern Africa. Wetl Ecol Manag. 20(3):253–269.
- Barlow CH. 1935. Further studies of the revival, after drying, of the snail hosts of the human schistosomes of Egypt. Am J Hyg. 22(2):376–391.
- Bavia ME, Hale LF, Malone JB, Braud DH, Shane SM. 1999. Geographic information systems and the environmental risk of schistosomiasis in Bahia, Brazil. Am J Trop Med Hyg. 60(4):566–572.
- Betterton C, Ndifon G, Tan R. 1988. Schistosomiasis in Kano State, Nigeria: II. Field studies on aestivation in Bulinus rohlfsi (Clessin) and B. globosus (Morelet) and their susceptibility to local strains of Schistosoma haematobium (Bilharz). Ann Trop Med Parasitol. 82(6):571–579.
- Chandiwana SK, Taylor P, Chimbari M, Ndhlovu P, Makura O, Bradley M, Gondo P. 1988. Control of schistosomiasis transmission in newly established smallholder irrigation schemes. Trans R Soc Trop Med Hyg. 82(6):874–880.
- Chu K, Arfaa F, Massoud J. 1967. The survival of Bulinus truncatus buried in mud under experimental outdoor conditions. Ann Trop Med Parasitol. 61(1):6–10.
- Darby PC, Bennetts RE, Percival HF. 2008. Dry down impacts on apple snail (Pomacea paludosa) demography: implications for wetland water management. Wetlands. 28(1):204–214. English.
- Fenwick A, Webster JP, Bosque-Oliva E, Blair L, Fleming F, Zhang Y, Garba A, Stothard J, Gabrielli AF, Clements A. 2009. The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002–2008. Parasitology. 136(13):1719–1730.
- Harrison AD, Shiff CJ. 1966. Factors influencing the distribution of some species of aquatic snails. S Afr J Sci. 62(25):3–258.
- Hira P. 1968. Studies on the capability of the snail transmitting urinary schistosomiasis in Western Nigeria to survive dry conditions. West Afr Med J Niger Pract. 17(5):153–160.
- Jarne P, Delay B, Bellec C, Roizes G, Cuny G. 1992. Analysis of mating systems in the schistosome-vector hermaphrodite snail Bulinus globosus by DNA fingerprinting. Heredity. 68(2):141.
- Kalinda C, Chimbari M, Mukaratirwa S. 2017a. Effect of temperature on the Bulinus globosus – Schistosoma haematobium system. Infect Dis Poverty. 6:57.
- Kalinda C, Chimbari M, Mukaratirwa S. 2017b. Implications of changing temperatures on the growth, fecundity and survival of intermediate host snails of schistosomiasis: a systematic review. Int J Environ Res Public Health. 14(1):80.
- Kalinda C, Chimbari MJ, Grant WE, Wang H-H, Odhiambo JN, Mukaratirwa S. 2018. Simulation of population dynamics of Bulinus globosus: Effects of environmental temperature on production of Schistosoma haematobium cercariae. PLoS Negl Trop Dis. 12(8):e0006651.
- Kingsford RT. 2011. Conservation management of rivers and wetlands under climate change–a synthesis. Mar Freshw Res. 62(3):217–222.
- Konar M, Jason Todd M, Muneepeerakul R, Rinaldo A, Rodriguez-Iturbe I. 2013. Hydrology as a driver of biodiversity: controls on carrying capacity, niche formation, and dispersal. Adv Water Resour. 51:317–325.
- Koprivnikar J, Paull SH, Johnson PT. 2014. Combined influence of hydroperiod and parasitism on larval amphibian development. Freshwr Sci. 33(3):941–949.
- Lund JO, Wissinger SA, Peckarsky BL. 2016. Caddisfly behavioral responses to drying cues in temporary ponds: implications for effects of climate change. Freshw Sci. 35(2):619–630.
- Lytle DA, Poff NL. 2004. Adaptation to natural flow regimes. Trends Ecol Evol (Amst.). 19(2):94–100.
- Manyangadze T, Chimbari MJ, Gebreslasie M, Mukaratirwa S. 2016a. Risk factors and micro-geographical heterogeneity of Schistosoma haematobium in Ndumo area, uMkhanyakude district, KwaZulu-Natal, South Africa. Acta Tropica. 159:176–184.
- Manyangadze T, Chimbari MJ, Gebreslasie M, Pietro C, Mukaratirwa S. 2016b. Modelling the spatial and seasonal distribution of suitable habitats of schistosomiasis intermediate host snails using MAXENT in Ndumo area, KwaZulu-Natal Province, South Africa. Parasites Vectors. 9(1):572.
- Marti H. 1986. Field observations on the population dynamics of Bulinus globosus, the intermediate host of Schistosoma haematobium in the Ifakara area, Tanzania . J Parasitol. 72(1):119–124.
- McCreesh N, Nikulin G, Booth M. 2015. Predicting the effects of climate change on Schistosoma mansoni transmission in eastern Africa. Parasites Vectors. 8(1):1–9.
- Morley N, Lewis J. 2013. Thermodynamics of cercarial development and emergence in trematodes. Parasitology. 140(10):1211–1224.
- Ngarakana-Gwasira E, Bhunu C, Masocha M, Mashonjowa E. 2016. Transmission dynamics of schistosomiasis in Zimbabwe: A mathematical and GIS Approach. Commun Nonlinear Sci Numer Simul. 35:137–147.
- O'keeffe J. 1985. Population biology of the freshwater snail Bulinus globosus on the Kenya coast. I. Population fluctuations in relation to climate. J Appl Ecol. 22(1):73–84.
- Paraense W. 1975. The distribution of the molluscan vectors of schistosomiasis in the Americas. Brasilia Med. 11:11–14.
- Paull SH, Johnson PTJ. 2011. High temperature enhances host pathology in a snail–trematode system: possible consequences of climate change for the emergence of disease. Freshw Biol. 56(4):767–778.
- Paull SH, LaFonte BE, Johnson PTJ. 2012. Temperature‐driven shifts in a host‐parasite interaction drive nonlinear changes in disease risk. Glob Change Biol. 18(12):3558–3567.
- Poznańska M, Goleniewska D, Gulanicz T, Kakareko T, Jermacz Ł, Kobak J. 2015. Effect of substratum drying on the survival and migrations of a freshwater pulmonate snail Planorbarius corneus (Linnaeus, 1758). Hydrobiologia. 747(1):177–188.
- Poznańska M, Werner D, Jabłońska-Barna I, Kakareko T, Duong KU, Dzierżyńska-Białończyk A, Kobak J. 2017. The survival and behavioural responses of a near-shore chironomid and oligochaete to declining water levels and sandy substratum drying. Hydrobiologia. 788(1):231–244.
- Reynolds S. 1970. The gravimetric method of soil moisture determination. Part I. A study of equipment, and methodological problems. J Hydrol. 11(3):258–273.
- Ripley B. 2015. MASS: Support Functions and Datasets for Venables and Ripley’s MASS. R package version 7.3-45.
- Rubaba O, Chimbari M, Mukaratirwa S. 2016. The role of snail aestivation in transmission of schistosomiasis in changing climatic conditions. Afr J Aquat Sci. 41:1–8.
- Shiff CJ. 1960. Observations on the capability of freshwater vector snails to survive dry conditions. J Trop Med Hyg. 63(4):89–92.
- Stanton MC, Adriko M, Arinaitwe M, Howell A, Davies J, Allison G, LaCourse EJ, Muheki E, Kabatereine NB, Stothard JR. 2017. Intestinal schistosomiasis in Uganda at high altitude (>1400 m): malacological and epidemiological surveys on Mount Elgon and in Fort Portal crater lakes reveal extra preventive chemotherapy needs. Infect Dis Poverty. 6(1):34.
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. 2006. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 6(7):411–425.
- Stensgaard AS, Utzinger J, Vounatsou P, Hurlimann E, Schur N, Saarnak CFL, Simoonga C, Mubita P, Kabatereine NB, Tchuente LAT, et al. 2013. Large-scale determinants of intestinal schistosomiasis and intermediate host snail distribution across Africa: does climate matter? Acta Tropica. 128(2):378–390.
- Stubbington R, Datry T. 2013. The macroinvertebrate seedbank promotes community persistence in temporary rivers across climate zones. Freshw Biol. 58(6):1202–1220.
- Sturrock R. 1970. An investigation of some factors influencing the survival of St. Lucian Biomphalaria glabrata deprived of water. Ann Trop Med Parasitol. 64(3):365–371.
- Tisseuil C, Vrac M, Grenouillet G, Wade AJ, Gevrey M, Oberdorff T, Grodwohl J-B, Lek S. 2012. Strengthening the link between climate, hydrological and species distribution modeling to assess the impacts of climate change on freshwater biodiversity. Sci Total Environ. 424:193–201.
- Van Vliet M, Zwolsman J. 2008. Impact of summer droughts on the water quality of the Meuse river. J Hydrol. 353(1):1–17.
- Wang L-D, Chen H-G, Guo J-G, Zeng X-J, Hong X-L, Xiong J-J, Wu X-H, Wang X-H, Wang L-Y, Xia G. 2009. A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med. 360(2):121–128.
- Whitehead P, Wilby R, Battarbee R, Kernan M, Wade AJ. 2009. A review of the potential impacts of climate change on surface water quality. Hydrol Sci J. 54(1):101–123.
- Woolhouse M, Taylor P. 1990. Survival rates of Bulinus globosus during aestivation. Ann Trop Med Parasitol. 84(3):293–294.
- Yang G-J, Utzinger J, Sun L-P, Hong Q-B, Vounatsou P, Tanner M, Zhou X-N. 2007. Effect of temperature on the development of Schistosoma japonicum within Oncomelania hupensis, and hibernation of O. hupensis. Parasitol Res. 100(4):695–700.
- Yang G, Vounatsou P, Zhou X, Tanner M, Utzinger J. 2005. A potential impact of climate change and water resource development on the transmission of Schistosoma japonicum in China. Parassitologia. 47(1):127–134.
- Yigezu G, Mandefro B, Mengesha Y, Yewhalaw D, Beyene A, Ahmednur M, Abdie Y, Kloos H, Mereta ST. 2018. Habitat suitability modelling for predicting potential habitats of freshwater snail intermediate hosts in Omo-Gibe river basin, Southwest Ethiopia. Ecol Inform. 45:70–80.
- Zhou XN, Yang G-J, Yang K, Wang X-H, Hong Q-B, Sun L-P, Malone JB, Kristensen TK, Bergquist NR, Utzinger J. 2008. Potential impact of climate change on schistosomiasis transmission in China. Am J Trop Med Hyg. 78(2):188–194.