Abstract
The roles that adventitious roots play in flooding tolerance have been well-studied in willows, while which of the maximum height of adventitious roots and the abundance of adventitious roots in willows is more important for their flooding tolerance is not well known. In this study, we analyzed the effects of adventitious roots on the flooding tolerance of Salix cavaleriei by comparing the maximum height of adventitious roots and the abundance of adventitious roots in dead and live willows along a flooding gradient from 0 to 180 cm in Lake Erhai, China. The results showed that willow mortality increased drastically when the water depth suffered by willows exceeded 100 cm. Live willows developed more adventitious roots and produced them higher on the trees compared with dead willows, however, the pest infestation percentage of the dead willows was larger. Additionally, both the maximum height and the abundance of adventitious roots in live willows were significantly correlated with water depth, whereas in dead willows, these variables were not significantly correlated or only weakly correlated with water depth. The results indicate that producing adventitious roots higher on the trees may be more important than developing abundant adventitious roots lower on the trees when S. cavaleriei is subjected to high flooding levels. Our data highlight that a faster adventitious root response promotes this species’ survival under flood stress, although pest infestation accounts for a small percentage of willow mortality. Accordingly, we should choose those willows that can develop more abundant adventitious roots and higher on the stems to plant in regions with abrupt water-level fluctuations.
Introduction
Water-level fluctuations are strong and common in subtropical shallow lakes due to the large seasonal and inter-annual variability in rainfall and evapotranspiration. These fluctuations strongly affect the structure and function of lake ecosystems, as macrophytes are sensitive to water level change (Leira and Cantonati Citation2008; Webb et al. Citation2012). In lakes, flooding is characterized by its rate, duration, frequency, amplitude and timing (Blom and Voesenek Citation1996; Parad et al. Citation2013). At the initial stage of flooding, soil pores fill with water, and gas diffusion is severely inhibited, leading to a lower oxygen supply of roots and shoots and to light deprivation of shoots (Blom and Voesenek Citation1996; Bailey-Serres and Voesenek Citation2008). The flooded soil redox potential is sharply reduced when the remaining oxygen is gradually consumed by roots and soil organisms (Blom and Voesenek Citation1996). Nutrient availability decreases strongly due to the interruption of important microbial processes, such as nitrification (Blom and Voesenek Citation1996; Bailey-Serres and Voesenek Citation2008). Consequently, both plant tissue nitrogen concentration and total nitrogen content decrease following the decline in nitrate availability or nitrogen uptake by plants (Pezeshki Citation2001). A severe anoxia occurs in flooded soil once the remaining oxygen is consumed. At that point, the transformation of nitrate to nitrogen or ammonium ions and the formation of manganese and ferric oxides are greatly reduced (Blom and Voesenek Citation1996; Bailey-Serres and Voesenek Citation2008). These accumulated reduced ions, together with toxic substances originating from the anaerobic metabolism of plants and microorganisms, are toxic to plants (Blom and Voesenek Citation1996; Webb et al. Citation2012). On one hand, root formation and respiration are restricted, while root decay is promoted; on the other hand, leaf formation and expansion are inhibited, while leaf senescence and abscission are strengthened, resulting in a slow plant root and shoot growth (Kozlowski Citation1997; Pezeshki Citation2001; Mielke and Schaffer Citation2010). Riparian trees can survive this disadvantageous circumstance using their carbohydrate reserves to maintain the necessary metabolism (Parolin Citation2009). However, the trees will eventually reduce their photosynthetic capacity and deplete their carbohydrate reserves under conditions of chronic flooding stress (Pezeshki et al. Citation1996). Ultimately, flooded plants can be irreversibly damaged and die if flooding continues or increases.
Oxygen shortage and hypoxia are the overriding stressors suffered by flooded plants. To cope with flooding, flood-tolerant trees develop physiological and morphological adaptations to anaerobic conditions (Timoney and Argus Citation2006; Bailey-Serres and Voesenek Citation2008). The former include processes of carbohydrate metabolism, net assimilation, photosynthesis, respiratory activity, stomatal conductance and transpiration (Timoney and Argus Citation2006; Bailey-Serres and Voesenek Citation2008). The latter include developing shallow root systems, large and succulent water roots, adventitious roots, hypertrophied lenticels, hypertrophied stems, amphistomatic leaves, thicker cuticles and multiple trunks (Keeley Citation1979, Parolin et al. Citation2004; Ghahremaninejad et al. Citation2012). Adventitious roots are among the most conspicuous and essential morphological responses of flood-tolerant trees, since this tolerance is controlled by the rootstock other than the scion (Jackson Citation2008; Vidoz et al. Citation2010). These aerenchymatous roots usually grow on the shoot base, the hypocotyl, the top segment of the root and on stem nodes (Blom and Voesenek Citation1996). Ethylene and auxin are the key hormones involved in the development of adventitious roots, which can form within several days, depending on the species (Blom and Voesenek Citation1996). The potential production of adventitious roots is higher in winter, although this production changes seasonally (Houle and Babeux Citation1993). Adventitious roots mitigate anoxia by transporting oxygen from aerial parts of the plant to the roots (Pezeshki et al. Citation1998). In addition, adventitious roots facilitate continuous water and nutrient uptake by plants because their hydraulic conductivity is higher, and they can replace certain functions of the original root system (Islam and Macdonald Citation2004; Parad et al. Citation2013).
Willow species (genus Salix) are riparian specialists adapted to highly disturbed environments subjected to water level variation due to their rapid growth, prolific seed production, flexible branches, different root systems (such as extensive adventitious roots), clonal reproduction and fast vegetative regeneration (Keeley Citation1979; Karrenberg et al. Citation2002; Kuzovkina and Quigley Citation2005). For this reason, many willow species are used throughout the world to restore wetlands, control erosion, improve water quality, produce biological energy and provide sustainable harvesting opportunities (Kuzovkina and Quigley Citation2005; Radtke et al. Citation2012; Su et al. Citation2016). The roles that adventitious roots play in flooding tolerance have been well-studied in willows and other plant species, among different willow species, between young and old willow seedlings and even between different gender willows (Pezeshki et al. Citation1998; Timoney and Argus Citation2006; Pasquale et al. Citation2012; Su et al. Citation2016). Vidoz et al. (Citation2010) found that flooding tolerance was positively correlated with the number of adventitious roots, while other studies concluded that the flooding tolerance of trees was not related to their adventitious roots (Pereira and Kozlowski Citation1977; Timoney and Argus Citation2006). It is very possible that these authors did not discriminate between live and dead plants when analyzing the role of adventitious roots in flooding tolerance. In addition, the abundance of adventitious roots has attracted considerable attention in previous research, while the height of adventitious roots has been neglected (Pereira and Kozlowski Citation1977; Timoney and Argus Citation2006; Vidoz et al. Citation2010). Consequently, the effects of adventitious root height on the flooding tolerance of willows remains poorly documented; moreover, these roots can form at different heights of the stems and indicate an approximate flood depth (Blom and Voesenek Citation1996; Timoney and Argus Citation2006). It is well-known that mature willows are more flood tolerant than immature willows or seedlings (Timoney and Argus Citation2006; Gorla et al. Citation2015), although young willows are more capable of generating different root systems (Keeley Citation1979). The mortality rate of black willow (Salix nigra) posts is 80% in habitats exposed to prolonged, frequent flooding (Pezeshki et al Citation1998). Similarly, a widespread dieback of five common willow species (without regard to age) was correlated with flooding time, duration and amplitude in the Peace–Athabasca Delta over the period of 1993–2001 (Timoney and Argus Citation2006). However, the percent mortality in mature willows and the reasons why they survive better under flooding conditions are far from clear. To address these knowledge gaps, we determined the roles of adventitious roots in the survival of Salix cavaleriei by comparing the responses of S. cavaleriei trees to an abrupt water level increase in Lake Erhai from 2009 to 2012. We hypothesized that (1) live willows developed more adventitious roots compared to willows that died and (2) live willows produced adventitious roots higher on the trees compared with willows that died under the condition of an abrupt water level increase in Lake Erhai.
Materials and methods
Study site
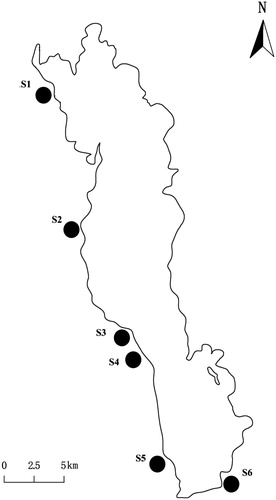
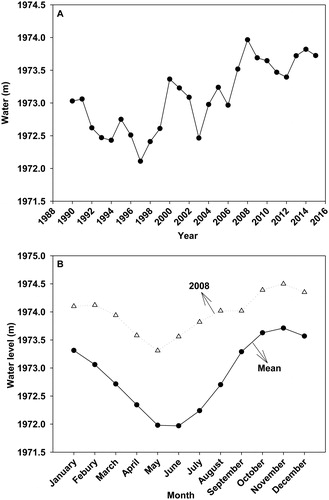
Lake Erhai (25°36′–25°58′ N, 100°06′–100°18′ E) is located in Yunnan Province, Southwest China. The plateau lake is 42.58 km long and 8.0 km wide with a 251.0 km2 water surface area and a 2,656 km2 watershed. The lake has a 10.8 m average depth and a 21.3 m maximum depth, corresponding to a 2.96 × 109 m3 water volume (). This lake has a warm plateau climate and the monsoon results in an annual precipitation of 1024 mm, primarily in summer. The mean annual temperature, sunshine duration and frost-free period are 15.6 °C, 2345 h and 228 days, respectively (Ye et al. Citation2018). It is rich in macrophytes and riparian trees (especially S. cavaleriei) although submersed plants are declining in recent years due to the rapid economy and population growth (Liu et al. Citation2012; Zhang et al. Citation2015). Lake Erhai is transitioning from mesotrophic to eutrophic conditions and is experiencing wetland vegetation destruction due to heavy nutrient loading from the lake basin (Liu et al. Citation2012; Zhang et al. Citation2015). The water level has fluctuated dramatically in recent decades, and the water depth is higher in autumn and winter than in spring and summer (). The water level in winter has increased from 1971 to 1972.61 m with the lowest water level and from 1974.2 to 1974.31 m with the highest water level for spring crop irrigation and nutrient concentration dilution to avoid algal blooms (Li Citation2008). The annual mean water level in 2008 (1973.97 m) was the highest in the past 26 years, and a continuous 30-day water level of 80 cm (1974.52 m) higher than the monthly water level was recorded in October (1973.62 m) and November (1973.71 m) in 2008 ().
Field survey
The mortality (or dieback) of S. cavaleriei was obvious in the last years since the water level was raised sharply in 2008 (). In August, 2009, we selected six transects along the shoreline of Lake Erhai, where S. cavaleriei dominated among the wood plants, to compare the adventitious root responses to flooding stress between live and dead willows (). In each transect, three to five 20 m × 20 m plots were randomly surveyed along the water table gradient. In each plot, willow heights (WH) were measured with a Senshi digital measuring pole and basal stem diameters (BSD) were measured with a caliper. The crown projection area (CPA) of each willow was calculated at the same time. The maximum height of the water level line marked on the willow stem after flooding receded was determined from the soil surface to the line; this maximum height was referred to as the water depth (WD) suffered by willows. As Timoney and Argus (Citation2006) suggested that adventitious roots could mark an approximate flood depth, the maximum height of the adventitious roots (MHR) was also measured from the soil surface to the highest place on the stem where adventitious roots developed from. In addition to quantitative analyses of the MHR and willow survival, an adventitious root class (ARC) analysis was also performed. Adventitious root classes were defined by the mean adventitious root measurements as follows: 1 = adventitious roots produced on 0 to 1 quarter of the willow stem circumference; 2 = 1 to 2 quarters; 3 = 2 to 3 quarters, 4 = 3 to 4 quarters. There will be a hole (holes), from which the adults come out, on the willow trunk if the willow is infected by a long-horned beetle (Anoplophora chinensis). And infection by the beetle was also carefully checked as natural enemies can negatively affect host resistance to other stresses (Mittler Citation2006; Nejat and Mantri Citation2017). The pest percentage (PP) of the live (dead) willows was calculated as the number of live (dead) infected S. cavaleriei in each plot divided by the total number of live (dead) S. cavaleriei in the plot. Live and dieback willows were counted; because of dormancy, dieback willows were considered dead only when they could not sprout after three years (in spring of 2012) (Lang et al. Citation1987). Due to the limited number of dead willows, we surveyed a number of dead willows outside our plots. A total of 317 live willows and 226 dead willows were recorded in our field survey.
Data analysis
Statistical data analyses and one-way or two-way analysis of variance (ANOVA) were conducted using the software package SPSS 17.0 (SPSS Inc., Chicago). The homogeneity of variances was tested using Levene’s test and differences among means were determined using Duncan’s test (p < 0.05). One way ANOVAs were used to analyze the differences in WH, BSD, CPA, PP, WD, MHR and ARC between live and dead willows. To analyze the responses of willows to different water depths quantitatively, three maximum heights of the water level line were established as <50, 50–100 and >100 cm. Survival rates (number of live willows divided by total number of willows) at the three WD were compared using a one-way ANOVA. A two-way ANOVA was used to determine the willow survival status (live vs. dead) and WD (<50, 50–100 and >100 cm) effects on MHR and ARC. Post-hoc comparisons among the three WD were made using Tukey’s honestly significant difference (HSD) test. Pearson correlation coefficients were determined to assess the relationships between MHR, ARC and WD.
Results
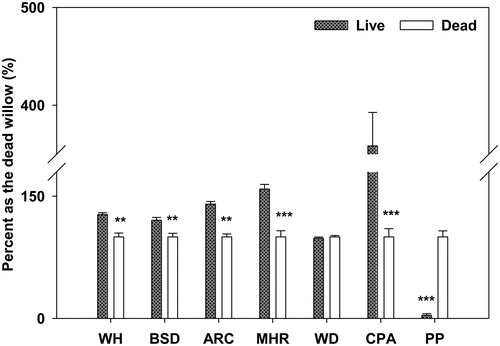
In general, the live willows were older and bigger than the dead ones. Live willows had higher WH, BSD and CSA values than dead willows (, ANOVA, p < 0.01), since there was no differences in WD between live and dead willows (, ANOVA, p > 0.05). Similarly, the MHR and ARC values of the live willows were higher than those of the dead willows (, ANOVA, p < 0.01). In contrast, the PP of the live willows was lower than that of the dead willows (, ANOVA, p < 0.01).
Figure 3. Measured willow height (WH), basal stem diameter (BSD), adventitious root class (ARC), the maximum height of adventitious roots (MHR), the maximum height of the water level line (WD), crown projection area (CPA) and pest percentage (PP) of the live and dead S. cavaleriei. ‘*’ means significant different at p < 0.05, ‘**’ means significant different at p < 0.01, ‘***’ means significant different at p < 0.001.
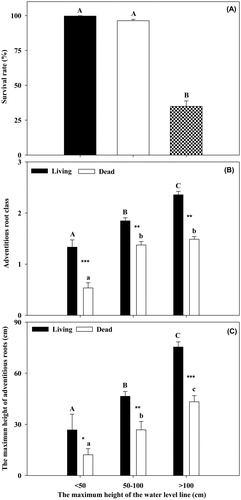
The survival rates at the two lower WD (<50 and 50–100 cm) were higher than that at the highest WD (>100 cm) (, ANOVA, p < 0.01). The MHR and ARC values of the live willows were higher than those of the dead willows at the three WD (, ANOVA, p < 0.05). Both the MHR and ARC values of the live willows were highest at the highest WD and lowest at the lowest WD (, ANOVA, p < 0.05). Similarly, the MHR value of the dead willows was highest at the highest WD and lowest at the lowest WD (, ANOVA, p < 0.05). However, the ARC value of the dead willows showed no differences at the two higher WD (, ANOVA, p > 0.05), although they were higher than that at the lowest WD (, ANOVA, p < 0.05).
Figure 4. The survival rate (A), adventitious root class (B) and the maximum height of adventitious roots (C) of the live and dead S. cavaleriei at different gradients of the maximum height of the water level line. Different bold letters mean significantly different of survival rate and the other two indices of the live S. cavaleriei, different lowercase letters mean significantly different for the dead S. cavaleriei, ‘*’ means significant different at p < 0.05, ‘**’ means significant different at p < 0.01, ‘***’ means significant different at p < 0.001.
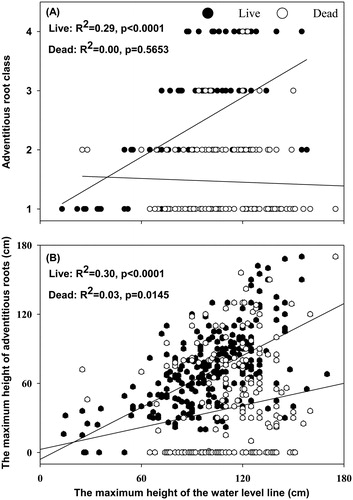
The MHR (R2 = 0.30, p < 0.0001) and ARC (R2 = 0.29, p < 0.0001) of the live willows were both significantly correlated with the WD. For the dead willows, the MHR relatively weakly correlated with the WD (R2 = 0.03, p < 0.0145), and the ARC was not significantly correlated with the WD (R2 = 0.00, p = 0.5653) ().
Discussion
We observed that S. cavaleriei mortality was not caused by a higher water depth experienced by the dead willows, as there was no difference in the maximum height of the water level line between live and dead willows (). In our study, large, old willows were more flood tolerant than small, young willows, which was in agreement with previous studies (Timoney and Argus Citation2006; Gorla et al. Citation2015). It is possible that larger, older willows have a bigger carbohydrate reserve than smaller, younger willows because plants use these reserves to maintain the necessary metabolism under flooding stress (Parolin Citation2009). A larger long-horned beetle infestation (prior to the death) percentage of dead willows (8.41% vs. 0.32% for live willow) may partially explain willow mortality because a herbivorous enemy can negatively affect its host’s resistance to other stresses (Mittler Citation2006; Nejat and Mantri Citation2017). These pests prefer larger, older willows as their hosts, possibly because these larger, older willows can provide more food for the beetle larvae. Nevertheless, willow mortality caused by this pest does not seem too high, as indicated above, and the number of deaths caused by this pest should be investigated in the future. Furthermore, we do not think that the death of the willows is caused by the lower water in the summer because the precipitation mostly concentrates in the summer months in Lake Erhai.
Our results highlight that adventitious roots play an important role in the flooding tolerance of willow, as adventitious roots are an essential response when trees suffer flooding stress (Jackson Citation2008; Vidoz et al. Citation2010). Adventitious roots can mitigate anoxia in the below-water parts of plants by transporting oxygen from aerial parts (Pezeshki et al. Citation1998). Furthermore, adventitious roots can substitute the original root system in assimilating water and nutrients to some extent (Islam and Macdonald Citation2004; Parad et al. Citation2013). In our study, the ARC and MHR increased with the WD for both live and dead willows, indicating that this willow species respond actively to flooding by developing more adventitious roots and producing them higher on the trees. Our results agree with previous findings (Jackson Citation2008; Vidoz et al. Citation2010). However, Timoney and Argus (Citation2006) observed that willow dieback was not prevented by their abundant adventitious roots. Similarly, the flooding tolerance of woody angiosperms was not well correlated with their capacity of producing adventitious roots (Pereira and Kozlowski Citation1977). As indicated by Timoney and Argus (Citation2006), drought stress accounted for a great deal of willow dieback in their study. Moreover, in these previous studies, dead and live trees were not discriminated when the correlation between flooding tolerance and adventitious roots was assessed, or the flood stress was sufficiently severe that adventitious roots could not touch the surface of the standing water in their research (Pereira and Kozlowski Citation1977; Timoney and Argus Citation2006). Additionally, these researchers did not measure the maximum height of the adventitious roots in their experiments. These differences may account for the different results observed in our study.
For the live willows, both ARC and MRH were significantly correlated with the WD, while for the dead willows, MHR was weakly correlated with the WD and ARC was not significantly correlated with the WD (). Furthermore, mortality increases quickly with water depth, and both the ARC and MHR values of the live willows were higher than those of the dead willows in the three water depths (). We speculate that it is very likely that the live willows responded faster than the dead willows in our study, as far as adventitious roots are concerned. The live willows developed more adventitious roots and produced them at higher places than the dead willows. This fast response of adventitious roots to flooding stress may drastically promote willow survival. Therefore, we can predict the flooding tolerance and survival of individual S. cavaleriei by analyzing their adventitious roots.
The data demonstrate that willow survival drops sharply when the maximum height of the water level line exceeds 100 cm (). We presume that S. cavaleriei can tolerate shallow flooding up to 100 cm, which is much higher than the degree of inundation tolerance (40–60 cm) of numerous other species of willow, although the tolerance varies among Salix species (Hosner Citation1958; Bell Citation1974; Jackson and Attwood Citation1996; Pezeshki et al. Citation1998; Amlin and Rood Citation2001; Timoney and Argus Citation2006; Vidoz et al. Citation2010; Su et al. Citation2016). The different results might be because the willow seedlings or shrubs examined in previous studies were smaller and younger than the mature willow trees in our study. When the maximum height of the water level line exceeded 100 cm, the dead willows could not develop more abundant adventitious roots but could produce them at higher places on the trees (). Similarly, for the dead willows, the WD was relatively weakly correlated with the MHR, and the WD was not significantly correlated with the ARC (). Consequently, producing adventitious roots higher up on the trees may be more important than developing abundant adventitious roots when S. cavaleriei is subjected to flooding levels above 100 cm. In addition, we can deduce that S. cavaleriei prefers allocating more resources to produce adventitious roots at higher places over developing more abundant adventitious roots at lower places because the latter cannot reach the water surface under severe flooding stress. This strategy maximizes the use of the limited resources in willows (Schultz et al. Citation2013). However, for the live willows, both ARC and MHR were significantly and positively correlated with the WD, and live willows could develop more abundant adventitious roots when the water depth exceeded 100 cm ( and ). This finding may be observed because most of the adventitious roots at lower places of the trees had developed prior to the abrupt flooding in 2008, while most of the adventitious roots at higher places of the trees were new.
Willows can provide diverse organisms with a rich habitat and food, which represents their high wildlife value (Kuzovkina and Quigley Citation2005). As riparian specialists, willows have many superior physiological and ecological characteristics suitable for wetland conservation, restoration and reconstruction, such as fast growth, efficient nutrient uptake, toxic metal accumulation, high evapotranspiration rate and vegetative propagation (Karrenberg et al. Citation2002; Kuzovkina and Quigley Citation2005). In addition, Salix species have advantages over herbaceous species for water quality improvement through phytoextraction because of their deeper root system and profitable biomass production, which increases income for farmers during restoration period (Eriksson and Ledin Citation1999; Kuzovkina and Quigley Citation2005). Nevertheless, a higher water level is not beneficial for the development of adventitious roots in willows, especially in the winter when adventitious root production is the most vigorous (Houle and Babeux Citation1993). We strongly suggest that the local government keep the water level of Lake Erhai below 1974 m to avoid subjecting willows to flood level above 100 cm or to transplant the willows growing in low-lying areas to higher areas.
In summary, large, old S. cavaleriei was more flooding-tolerant than small, young willow and willow mortality increased drastically when the water depth suffered by willows exceeded 100 cm. Both the maximum height and the abundance of adventitious roots in live willows were significantly correlated with water depth, whereas in dead willows, these variables were not significantly correlated or only weakly correlated with water depth. The results highlight that a faster adventitious root response promotes this species’ survival under flood stress, although pest infestation accounts for a small percentage of willow mortality.
Notes on contributors
Ai-ping Wu is an associate professor at Hunan Agricultural University, where he has taught in ecology, and worked with ecology of macrophytes and invasive species.
Ya-Xuan Zhao, Liang-Yu Qi and Wen Zhong are undergraduates in ecology, who now works in freshwater ecology.
DR. Fa-Lin Chen and Yun-shan Liang teach in ecology, who explore the agricultural ecology.
Te Cao and Guo-Rong Zhu are professors in ecology, who now works in freshwater ecology.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This study is financially supported by the National Natural Science Foundation of China (Nos. 31400402, 41401358 and 31200427), Natural Science Foundation of Hunan Province (No. 2018JJ2162) and the State Scholarship Found of China Scholarship Council (CSC) (No: 2016-3035).
References
- Amlin NA, Rood SB. 2001. Inundation tolerance of riparian willows and cottonwoods. J Am Water Resources Assoc. 37(6):1709–1720.
- Bailey-Serres J, Voesenek LACJ. 2008. Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol. 59:313–339.
- Bell DT. 1974. Tree stratum composition and distribution in the streamside forest. Am Midl Nat. 92(1):35–46.
- Blom CWPM, Voesenek LACJ. 1996. Flooding: the survival strategies of plants. Trends Ecol Evol (Amst). 11(7):290–295.
- Eriksson J, Ledin S. 1999. Changes in phytoavailability and concentration of cadmium in soil following long term Salix cropping. Water Air Soil Pollut. 114:171–184.
- Ghahremaninejad F, Khalili Z, Maassoumi AA, Mirzaie-Nodoushan H, Riahi M. 2012. Leaf epidermal features of Salix species (Salicaceae) and their systematic significance. Am J Bot. 99(4):769–777.
- Gorla L, Signarbieux C, Turberg P, Buttler A, Perona P. 2015. Transient response of Salix cuttings to changing water level regimes. Water Resour Res. 51:1758–1774.
- Hosner JF. 1958. The effects of complete inundation upon seedlings of six bottomland tree species. Ecology. 39(2):371–373.
- Houle G, Babeux P. 1993. Temporal variations in the rooting ability of cuttings of Populus balsamifera and Salix planifolia from natural clones – populations of subarctic Quebec. Can J Res. 23(12):2603–2608.
- Islam MA, Macdonald E. 2004. Ecophysiological adaptations of Black spruce (Picea mariana) and tamarack (Larix laricina) seedlings to flooding. Trees. 18(1):35–42.
- Jackson MB. 2008. Ethylene-promoted elongation: an adaptation to submergence stress. Ann Bot. 101(2):229–248.
- Jackson MB, Attwood PA. 1996. Roots of willow (Salix viminalis L.) show marked tolerance to oxygen shortage in flooded soils and in solution culture. Plant Soil. 187(1):37–45.
- Karrenberg S, Edwards PJ, Kollmann J. 2002. The life history of Salicaceae living in the active zone of floodplains. Freshw Biol. 47(4):733–748.
- Keeley JE. 1979. Population differentiation along a flood frequency gradient: physiological adaptations to flooding in Nyssa sylvatica. Ecol Monogr. 49(1):89–108.
- Kozlowski TT. 1997. Responses of woody plants to flooding and salinity. Tree Physiol Monogr. 1:1–29.
- Kuzovkina YA, Quigley MF. 2005. Willows beyond wetlands: uses of Salix L. species for environmental projects. Water Air Soil Pollut. 162(1–4):183–204.
- Lang GA, Early JD, Martin GC, Darnell RL. 1987. Endo-, para-, and ecodormancy: physiological terminology and classification for dormancy research. HortScience. 22:371–377.
- Leira M, Cantonati M. 2008. Effects of water-level fluctuations on lakes: an annotated bibliography. Hydrobiologia. 613(1):171–184.
- Li P. 2008. Analysis of water level management in Lake Erhai in Dali. Pearl River. 2:46–48 (in Chinese).
- Liu WZ, Li SY, Bu M, Zhang QF, Liu GH. 2012. Eutrophication in the Yunnan plateau lakes: the influence of lake morphology, watershed land use, and socioeconomic factors. Environ Sci Pollut Res. 19(3):858–870 (in Chinese).
- Mielke MS, Schaffer B. 2010. Photosynthetic and growth responses of Eugenia uniflora L. seedlings to soil flooding and light intensity. Environ Exp Bot. 68(2):113–121.
- Mittler R. 2006. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 11(1):15–19.
- Nejat N, Mantri N. 2017. Plant immune system: crosstalk between responses to biotic and abiotic stresses the missing link in understanding plant defence. Curr Issues Mol Biol. 23:1–16.
- Parad GA, Zarafshar M, Striker GG, Sattarian A. 2013. Some physiological and morphological responses of Pyrus boissieriana to flooding. Trees. 27(5):1387–1393.
- Parolin P. 2009. Submerged in darkness: adaptations to prolonged submergence by woody species of the Amazonian floodplains. Ann Bot. 103(2):359–376.
- Parolin P, De Simone O, Haase K, Waldhoff D, Rottenberger S, Kuhn U. 2004. Central Amazon floodplain forests: tree survival in a pulsing system. Bot Rev. 70:357–380.
- Pasquale N, Perona P, Francis R, Burlando P. 2012. Effects of streamflow variability on the vertical root density distribution of willow cutting experiments. Ecol Eng. 40:167–172.
- Pereira JS, Kozlowski TT. 1977. Variation among woody angiosperms in response to flooding. Physiol Plant. 41(3):184–192.
- Pezeshki SR. 2001. Wetland plant responses to soil flooding. Environ Exp Bot. 46(3):299–312.
- Pezeshki SR, Anderson PH, Shields FD. 1998. Effects of soil moisture regimes on growth and survival of black willow (Salix nigra) posts (cuttings). Wetlands. 18(3):460–470.
- Pezeshki SR, Pardue JH, DeLaune RD. 1996. Leaf gas exchange and growth of flood-tolerant and flood-sensitive tree species under low soil redox conditions. Tree Physiol. 16(4):453–458.
- Radtke A, Mosner E, Leyer I. 2012. Vegetative reproduction capacities of floodplain willows-cutting response to competition and biomass loss. Plant Biol (Stuttg). 14(2):257–264.
- Schultz JC, Appel HM, Ferrieri A, Arnold TM. 2013. Flexible resource allocation during plant defense responses. Front Plant Sci. 4:324.
- Su XL, Zeng B, Lin F, Qiao P, Ayi QL, Huang WJ. 2016. How does long-term complete submergence influence sex ratio and resource allocation of a dioecious shrub, Salix variegata Franch.? Ecol Eng. 87:218–223.
- Timoney KP, Argus G. 2006. Willows, water regime, and recent cover change in the Peace-Athabasca Delta. Ecoscience. 13(3):308–317.
- Vidoz ML, Loreti E, Mensuali A, Alpi A, Perata P. 2010. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J. 63(4):551–562.
- Webb JA, Wallis EM, Stewardson MJ. 2012. A systematic review of published evidence linking wetland plants to water regime components. Aquat Bot. 103:1–14.
- Ye BB, Chu ZS, Wu AP, Hou ZY, Wang SR. 2018. Optimum water depth ranges of dominant submersed macrophytes in a natural freshwater lake. PLoS One. 13(3):e0193176.
- Zhang L, Wang SR, Li YP, Zhao HC, Qian WB. 2015. Spatial and temporal distributions of microorganisms and their role in the evolution of Erhai Lake eutrophication. Environ Earth Sci. 74(5):3887–3896.